Abstract
Ageing is associated with a reduction in muscle carnosine (β-alanyl-l-histidine), but there are no data on the changes specifically in type I and type II muscle fibres. Given the higher carnosine content of type II fibers, changes observed in whole muscle may be secondary to a shift in fibre composition. Carnosine, β-alanine, histidine, taurine, and citrate synthase (CS) and glycogen phosphorylase (Phos), were measured in pools of single muscle fibres from freeze-dried muscle biopsies of vastus lateralis of nine elderly sedentary subjects (65–80 years) with osteoarthritis of the knee and undergoing total knee replacement, and nine young moderately active healthy subjects (20–35 years). Fibres were characterised as type I or II by myosin ATPase activity. Carnosine was 53.2% lower in type II fibres of older subjects resulting in an estimated 7% (and most probably still higher) decline in intracellular physico-chemical buffering capacity. Younger subjects showed higher CS activities in type I and higher Phos activities in type II fibres. These differences were less apparent in elderly subjects. Possible causes for the change in the carnosine content are reduced physical activity, reduced meat intake, or the result of progressive denervation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carnosine (β-alanyl-l-histidine), a dipeptide with a pKa of 6.83, is an effective hydrogen ion (H+) buffer over the physiological pH range. Species exposed to prolonged periods of hypoxia show the highest muscle contents of carnosine and related histidine-dipeptides (Abe 2000; Crush 1970; Suyama et al. 1970). Maintenance of intracellular pH will be important to normal muscle function including that of the elderly. Carnosine has also been implicated in cell ageing by its ability to act as an antioxidant (Boldyrev et al. 1993), as a sacrificial peptide preventing glycation (Hipkiss et al. 1995), and in preventing the formation of protein-protein cross links by reacting with protein–carbonyl groups (Hipkiss 2000). However, whilst these properties may be important to cells maintained in culture, such effects have not been demonstrated in muscle. Only the function of carnosine as a pH buffer is undisputed resulting as it does from a pKa of 6.83 of the imidazole ring of carnosine. Carnosine exhibits a 1.5–2 (humans) to 5 (equines)-fold difference in concentration between types I and II muscle fibres, being highest in the latter (Dunnett and Harris 1995; Harris et al. 1998; Hill et al. 2006). The influence of age on the muscle content has been investigated only once in humans (Stuerenburg and Kunze 1999), with a further study performed in rats (Johnson and Hammer 1993a). Measurements were performed on whole muscle, mixed-fibre samples. Although a reduction in carnosine with age was identified, this could have been secondary to an age-induced shift in fibre population towards increased type I fibres. Furthermore, the control subjects in the human study demonstrated symptoms of neuromuscular disease, and therefore unlikely to be representative of a normal population.
Of the two precursors to carnosine, the high concentration of histidine in muscle relative to its Km (16.8 μM) (Horinishi et al. 1978) with carnosine synthetase (Enzyme Commission: 6.3.2.11) suggests that this is unlikely to be limiting to synthesis. In contrast, β-alanine occurs in only very low concentrations in muscle relative to its km of 1300–2300 μM with carnosine synthetase (Kish et al. 1978; Ng and Marshall 1978; Skaper et al. 1973), and consequently is more likely to be limiting (Harris et al. 2006).
Taurine is not involved in carnosine synthesis, but shares the same transporter as β-alanine for uptake into muscle (Komura et al. 1996). Taurine is found in equally high concentrations as carnosine in muscle (Harris et al. 2006) exhibiting the opposite distribution, being highest in type I fibres (Dunnett and Harris 1995; Harris et al. 1998). Suggested roles for taurine include osmoregulation (Cuisiner et al. 2002), regulation of Ca2+ homeostasis during muscle contraction (Steele et al. 1990) and effects on chloride (Cl) conductance and membrane excitability (De Luca et al. 1994). Changes in muscle taurine could have important implications for the reported age-related decline in neuromuscular function (Hakkinen et al. 1998).
In the present investigation, possible changes in carnosine and taurine with age were examined in pools of muscle fibres obtained by muscle biopsy or open surgery, and characterised by staining for myosin ATPase activity. To further define the biochemical characteristics of muscle fibres, which could change with ageing, fibre pools were also assayed for glycogen phosphorylase (Phos) and citrate synthase (CS). Ageing is associated with changes in muscle enzyme activities including decreases in mitochondrial enzyme-activities (Trounce et al. 1989). Other studies have provided conflicting results of the changes in muscle. One study showed an age-related increase of Phos (Johnson and Hammer 1993b), whilst another no change (Orlander et al. 1978). Similar results have been reported for the oxidative marker, CS (Aniansson et al. 1981; Coogan et al. 1992).
One possible factor contributing to such differences is the analysis of whole muscle rather than single or pools of similar fibres. This will be a particular problem where there are two fold or more differences between fibres, e.g. carnosine, and where there is selective loss in one or other fibre type (Sjostrom et al. 1992) with ageing. Comparative changes in whole muscle will overlook changes in different fibre types unique in their biochemical and metabolic properties. We have attempted to overcome this in the present study by assaying muscle fibre pools.
Materials and methods
Subjects
Older subjects (n = 9) (Table 1) were patients undergoing total knee joint arthroplasty for knee osteoarthritis. They were otherwise healthy as indicated by physical examination and blood biochemistry. Younger subjects (n = 9) were not specifically trained but regularly participated in one or more sports. Ethical approval for the study was first obtained from the Ethics Committees of the Grampian Health authority, Hamilton Health Sciences and the University of Chichester.
Muscle sampling
Muscle biopsies from younger subjects were taken from the mid portion of the vastus lateralis (Bergstrom 1962). Samples were obtained from the distal vastus lateralis from older subjects during open surgery for joint arthroplasty. Part of each sample was set aside for histochemistry, whilst the remainder was frozen in liquid nitrogen and stored until freeze-dried. Histochemistry was performed only on samples obtained from the older subjects.
Duplicate 12 μm cross-sections of samples were cut using a refrigerated cryostat, mounted on glass slides, and characterised for type I and II muscle fibres by staining for myosin ATPase activity at pH 9.6 following pre-incubation at pH 4.3 (Brooke and Kaiser 1970). A 1% solution of toluidine monochromatic dye was applied prior to examination by light microscopy to assist identification of fibre type and number. Fibres were counted on a digitised photograph over a field comprising 128–240 fibres.
Individual fibres were dissected from freeze-dried samples. From each fibre, three fragments were cut and mounted on separate slides and then characterised as type I or II by staining for myosin ATPase activity as before. The remaining larger portions were then pooled, with respect to fibre type, and weighed using a quartz fish pole balance (Lowry and Passoneau 1972) (minimal pool weights >5 μg).
Biochemical analysis
Fibre pools were extracted in glass vials with 100 μl methanol–0.4M borate buffer (75:25,v/v, pH 9.65) (2). β-Alanine, carnosine, histidine and taurine were measured by HPLC following pre-column derivitisation with 1 mg ml-1 ortho-phthaldialdehyde containing 20 μl ml−1 mercaptopropionic acid (Dunnett and Harris 1995; Dunnett and Harris 1997).
A separate pool of five fibres was homogenised in 0.1 ml ice-cold reagent (50 mM KH2PO4 –50 mM K2HPO4, pH 7.5) for analysis of CS and Phos enzyme activities (Sewell et al. 1994). Phos was assayed at pH 7.00, 340 nm in a system comprising (final concentrations) 100 mM imidazole; 10 mM KH2PO4; 2 mM magnesium acetate; 0.1 mM EDTA; 2 mM AMP; 70 mM glycogen; 1 mM NAD; 8.5 μM glucose 1,6-bisphosphate; 1 mM dithiothreitol; 500 mg l−1 bovine serum albumin; 1 unit ml−1 phosphoglucomutase; 2 units ml−1 glucose 6-phosphate dehydrogenase. CS was assayed at pH 8.00, 412 nm in a system comprising (final concentrations) 100 mM glycylglycine; 1 mM EDTA; 0.2 mM dithiobisnitrobenzoate (DTNB); 0.1 mM acetylCoA; 0.63 mM oxaloacetate. Assays were run in semi-microcuvettes over 60 min or more, against identical controls but lacking homogenate.
Statistical Analysis
Possible differences between carnosine, taurine, histidine, Phos and CS between type I and II muscle fibres were examined by t-test for paired data. Differences between fibre means between age groups (young versus elderly) were investigated by Welch’s modified t-test for data with unequal variances.
Results
Coefficients of variation for the methods used in the assay of β-alanine, carnosine, histidine, taurine, Phos (type I and II fibres) and CS (type I and II fibres) activities, were (when assayed on the same day): 2.08, 1.14, 4.49, 1.93, 5.09 (I) & 3.69 (II), and, 6.45 (I) & 5.60 (II) %, respectively; and, (when assayed on separate days): 5.04, 3.30, 4.23, 2.92, 9.09 (I) & 3.81 (II), and, 6.62 (I) & 7 .77 (II) %, respectively.
The mean proportion of type I fibres in elderly subjects was 64.1 ± 5.2%. Data on younger subjects were not obtained in the current study. However, data from a parallel group of young men sampled at the same time showed a type I fibre population of c. 45% in the vastus lateralis (Carter et al. 2001; Yasuda et al. 2005).
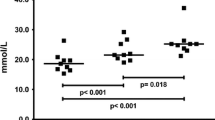
Carnosine was significantly lower in type I compared to type II muscle fibres in younger subjects (Fig. 1 and Table 2). The mean within subject difference between fibre types in younger subjects was 18.6 ± 11.5 mmol kg−1 dm (P < 0.001). The concentration in type II fibres was significantly lower in elderly compared to younger subjects (P < 0.02), such that the content in both fibre types in elderly subjects were approximately the same, and comparable to that in type I fibres of younger subjects. There was no apparent difference in fibre contents due to gender (Fig. 1). Based on the fibre compositions given above for young and elderly subjects, the estimated whole (mixed fibre) muscle carnosine concentrations were 24.2 and 16.1 mmol kg−1 dm, respectively, an apparent reduction in the muscle carnosine content of 33.5% in the elderly.
β-Alanine in both fibre types was below the limit of detection. Histidine was lower in type I fibres of younger subjects compared to type II (P < 0.001). Elderly subjects showed an increase in histidine in type I fibres compared to younger subjects, the mean concentration in these fibres being of the same order as found in type II fibres, i.e. 5.5 and 5.4 mmol kg−1 dm, respectively.
Taurine showed the opposite distribution to carnosine and histidine in younger subjects, being higher in type I fibres (Table 2 and Fig. 2). The mean within subject difference between fibre types in younger subjects was 25.7 ± 20.5 mmol.kg−1 dm (P = 0.006). This distinction was lost in elderly subjects with the concentration in type I fibres increasing and that in type II decreasing.
Higher CS activities were recorded in type I compared to type II fibres in younger subjects (Table 2 and Fig. 3). The mean within subject difference between fibre types in younger subjects was 23.0 ± 16.6 mmol (kg dm)−1 min−1 (P = 0.002). The distinction was again lost in elderly subjects with a 48.8% increase in CS activity in type II fibres. The difference in CS in type II fibres between age groups was significant (P = 0.016). Phos showed the opposite fibre distribution to CS in younger subjects with highest activities in type II fibres (Table 2 and Fig. 4). The mean within subject difference between fibre types in younger subjects was 18.1 ± 11.5 mmol (kg dm)−1 min−1 (P = 0.027). There was no significant difference in Phos activities between fibre types in elderly subjects.
Significant inverse correlations in young were found between carnosine and CS, and taurine and Phos (Table 3, Figs. 5 and 6), and in elderly subjects between taurine and Phos. Taurine was positively correlated with CS in younger subjects.
Discussion
Carnosine concentrations in type II muscle fibres were reduced by a mean of 53.2% in elderly subjects with osteoarthritis of the knee. However, no change was observed in type I fibres. In the study of Stuerenburg and Kunze (1999), age-related losses of carnosine of 37% from whole muscle of rats and of 63% in human skeletal muscle were recorded. The greater decline in carnosine observed by these authors, compared to the 53.2% measured in type II fibres in this study (and much less than the estimated 33.5% decline at the whole muscle level), may reflect that the older subjects in the previous study had motor neurone disease (Stuerenburg and Kunze 1999). This condition induces alpha motor unit loss and subsequent muscle denervation.
The higher carnosine concentration found in type II fibres of younger subjects, as well as the opposite distribution of taurine, is consistent with earlier findings in humans of Harris et al. (1998) and Hill et al. (2006), as are also the mean concentrations of both in the two fibre populations. Still earlier studies have established a similar fibre distribution of carnosine and taurine in muscle fibres from horses (Dunnett and Harris 1995) and camels (Dunnett et al. 1997). Similarly, higher activities of CS in type I fibres and higher activities of Phos in type II fibres have previously been reported (Harris et al. 1976).
The present study points to a decline in carnosine concentrations in elderly subjects with osteoarthritis of the knee. Using the Henderson–Hasselbach equation, this would equate to a decline in the specific contribution of carnosine to muscle buffering capacity also of 53.2% in type II muscle fibres over the pH range 6.5–7.1 (Table 2), although just 7% when other sources of buffering are taken into consideration. This calculation is based on an assumed non-carnosine buffering capacity between pH 6.5 and 7.1 of 74 mmol H+ kg−1 dm (Harris et al. 1990) determined by acid titration of muscle homogenates. However, this is almost certainly an over-estimate of the non-carnosine buffering capacity. As a result these calculations probably under-estimate the importance of carnosine as an intracellular buffer and thus, also, the effect of the decline in muscle carnosine seen in the elderly.
The maintenance of the carnosine concentration in type I muscle fibres (Table 2) may result from proportionately greater use of type I fibres with increasing age. In support of this, type I fibre cross sectional area has been shown to be unaffected by age (Aniansson et al. 1986), whilst type II fibre cross sectional area appears more susceptible to the effects of disuse, ageing and denervation (Johnson and Hammer 1993b, Essen-Gustavsson and Borges 1986).
The decline in skeletal carnosine in type II fibres of elderly subjects seen in this study, and previously at the whole muscle level in humans and rats (Stuerenburg and Kunze 1999; Johnson and Hammer 1993b), may reflect changes in the hydrolysis or synthesis of carnosine. Lenney et al (1982) suggested that carnosinase (EC.3.4.13.3) activity is increased with ageing, leading to increased degradation of carnosine and its related histidine analogues. However, a decline in muscle carnosine may equally be related to decreased expression of the transporter for β-alanine as a precursor to carnosine, or a decrease in carnosine synthetase. The concentration of β-alanine was below the limit of detection of the assay used (c.<0.1 mmol kg−1 dm) in samples from both young and elderly subjects.
An adequate supply of histidine is necessary for maintenance of intramuscular carnosine concentrations, although its concentration is not thought to be limiting to synthesis (Harris et al. 2006; Dunnett and Harris 1999). The present study showed a significant elevation of histidine in type I fibres with increasing age, but no difference in their carnosine content. On the other hand, we observed a decrease with age in the carnosine content in type II fibres, although the histidine content was the same as in younger subjects.
Ageing did not significantly affect muscle fibre taurine contents (Table 2), despite a trend towards higher contents in type II fibres. Airaksinen et al (1990) previously reported increases in the taurine content of mixed muscle with age, although this could equally result from a shift in fibre composition towards type I. Airaksinen et al (1990) recruited subjects between the ages of 22 and 57 years, and therefore they would be expected to show little or no neuronal loss, which occurs mainly after the age of 60 years. Their normal group of subjects similarly may not have been representative, since all 48 exhibited symptoms suggestive of neuromuscular disorders. The subjects in the present study are representative of an aged population, likely to display characteristic denervation and demyelineation (65–80 years). Data from the present study showed a 52.5% increase in type II muscle fibre taurine content with age, but given the large variation in taurine concentrations, this was not statistically significant (Table 2).
The distribution pattern of taurine and carnosine is such that both are preferentially concentrated into specific fibre types. In young individuals, taurine demonstrates a higher affinity for type I fibres, and carnosine for type II fibres consistent with its role as a proton buffer. Huxtable (1992) suggested that chronic denervation would lead to a decrease in intracellular taurine and its concentration in muscles has been related to neural input, especially in type II fibres (Landon 1982), as shown also by Airaksinen et al (1990). Studies in rats and mice, however, demonstrate the inverse relationship to that predicted (Iwata and Baba 1985), and may represent biochemical or morphological differences between species.
Assay of enzymic activities revealed a significant difference between elderly and younger subjects in type II CS activity. Citrate synthase is considered a flux-limiting enzyme influencing skeletal muscle oxidative capacity, and any change in its activity with age is likely to have an effect on overall mitochondrial function (Trounce et al. 1989). The present study indicates an age-related increase in type II fibres (48.6%), but no change in type I. Despite a 32.5% increase in Phos activity in type I fibres in elderly subjects, higher activities were again noted in type II compared with type I fibres. The higher activity in type II fibres, particularly in younger subjects is consistent with previous work (Harris et al. 1976; Schantz and Henriksson 1987). The present data suggest a greater variation with ageing in the enzymic distribution and ratio of these enzymes between fibre types.
In conclusion, the present study points to an age-induced reduction in muscle carnosine content, mainly in type II fibres. A decline in the level of carnosine would inevitably reduce the physico-chemical buffering capacity of the muscle cells contributing, in theory, to a decline in anaerobic exercise capacity. However, whether any such impairment of intracellular buffering is compensated for by changes in other muscle constituents, or would be sufficient to impact on daily activity, is unknown. Although age per se may be one of the influences affecting metabolites measured in this study, we cannot exclude possible effects arising from changes in diet and nutritional status (Harris et al. 2006), and exercise habits (Parkhouse et al. 1985; Tallon et al. 2005).
References
Abe H (2000) Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry (Mosc) 65:757–765
Airaksinen EM, Paljarvi L, Partanen J, Collan Y, Laakso R, Pentikainen T (1990) Taurine in normal and diseased human skeletal muscle. Acta Neurol Scand 81:1–7
Aniansson A, Grimby G, Hedberg M, Krotkiewsky M (1981) Muscle morphology enzyme activity and muscle strength in elderly men and women. Clin Physiol 1:73–86
Aniansson A, Hedberg G, Henning GB, Grimby G (1986) Muscle morphology, enzymatic activity, and muscle strength in elderly men. Muscle Nerve 9:585–591
Bergstrom J (1962) Muscle electrolytes in man determined by neutron activation analysis on needle biopsy specimens: A study in normal subjects, kidney patients and patients with chronic diarrhoea. Scand J Clin Lab Invest 14:Suppl 68
Boldyrev AA, Koldobski A, Kurella E, Maltseva V, Stvolinski S (1993) Natural histidine-containing dipeptide carnosine as a potent hydrophilic antioxidant with membrane stabilizing function. A biomedical aspect Mol Chem Neuropathol 19:185–192
Brooke MH, Kaiser KK (1970) Muscle fibre types: how many and what kind. Arch Neurology 23:369–379
Carter SL, Rennie CD, Hamilton SJ, Tarnopolsky MA (2001) Changes in skeletal muscle in males and females following endurance training. Can J Physiol Pharmacol 79:386–92
Coogan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO (1992) Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol Biol Sci 46B: 71–76
Crush KG (1970) Carnosine and related substances in animal tissues. Comp Biochem Physiol 34:3–30
Cuisinier C, Michotte De Welle J, Verbeeck RK, Poortmans JR, Ward R, Sturbois X, Francaux M (2002) Role of taurine in osmoregulation during endurance exercise. Eur J Appl Physiol 876:489–95
De Luca A, Tricarico S, Pierno D, Camerino C (1994) Aging and chloride channel regulation in rat fast-twitch muscle fibres. Pflugers Arch 427:80–85
Dunnett M, Harris RC (1995) Carnosine and Taurine contents of different fibre types in the middle gluteal muscle of the Thoroughbred horse. Equine Vet J Suppl 18:214–217
Dunnett M, Harris RC (1997) High-performance liquid chromatographic determination of imidazole dipeptides, histidine, 1-methylhistidine and 3-methylhistidine in equine and camel muscle and individual muscle fibres. J Chromat B Biomedical Applications 688:47–55
Dunnett M, Harris RC (1999) Influence of oral ß-alanine and L-histidine supplementation on the carnosine content of the gluteus medius. Equine Vet J Suppl 30:499–504
Dunnett M, Harris RC, Soliman MZ, Suwar AAS (1997) Carnosine, anserine and taurine contents in individual fibres from the middle gluteal muscle of the camel. Res Vet Sci 62:213–216
Essen-Gustavsson B, Borges O (1986) Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta Physiol Scand 126:107–114
Hakkinen K, Newton RU, Gordon SE (1998) Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J Gerontol Biol Sci 53:B415-B423
Harris RC, Dunnett M, Greenhaff PL (1998) Carnosine and taurine contents in individual fibres of human vastus lateralis muscle. J Sport Sci 16:639–643
Harris RC, Essen B, Hultman E (1976) Glycogen phosphorylase activity in biopsy samples and single muscle fibres of musculus quadriceps femoris of man at rest. Scand J Clin Lab Invest 36:521-526
Harris RC, Marlin DJ, Dunnett M, Snow DH, Hultman E (1990) Muscle buffering capacity and dipeptide content in the thoroughbred horse, greyhound dog and man. Comp Biochem Physiol 97A: 249–251
Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA (2006) The absorption of orally supplied ß-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30:279–289
Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA (2006) Influence of β-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity Amino Acids (Epub ahead of print Jul 28)
Hipkiss AR (2000) Carnosine and protein carbonyl groups: a possible relationship. Biochemistry (Mosc) 65:771–778
Hipkiss AR, Michaelis J, Syrris P (1995) Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett 371:81–85
Horinishi H, Grillo M, Margolis FL (1978) Purification and characterization of carnosine synthetase from mouse olfactory bulbs. J Neurochem 31:909–919
Huxtable RJ (1992) The physiological actions of taurine. Physiol Rev 72:101–142
Iwata H, Baba A (1985) Specific increases of taurine in denervated skeletal muscle. Prog Clin Biol Res 179:397–405
Johnson P, Hammer JL (1993a) Histidine dipeptide levels in ageing and hypertensive rat skeletal and cardiac muscles. Comp Biochem Physiol 103B:981–984
Johnson P, Hammer JL (1993b) Cardiac and skeletal muscle enzyme levels in hypertensive and ageing rats. Comp Biochem Physiol 104B:63–67
Kish SJ, Perry TL, Hansen S (1978) Regional distribution of homocarnosine-carnosine synthetase and homocarnosinase in human brain. J Neurochem 32:1629–1636
Komura J, Tamai I, Senmaru M, Terasaki T, Sai Y, Tsuji A (1996) Sodium and chloride ion-dependent transport of beta-alanine across the blood-brain barrier. J Neurochem 67:330–335
Landon DN (1982) Skeletal muscle – normal morphology, development and innervation. In: Mastaglia FL, Walton J (eds) Skeletal muscle pathology, Churchill, Livingstone, London, pp 1–87
Lenney JF, George RP, Weiss AM, Kucera CM, Chan PW, Rinzler GS (1982) Human carnosinase, and activation by cadmium. Clin Chem Acta 123:221–231
Lowry OH, Passoneau JV (1972) A flexible system of enzymatic analysis. Academic Press, New York, pp 291
Ng RH, Marshall FD (1978) Regional and subcellular distribution of homocarnosine-carnosine synthetase in the central nervous system. J Neurochem 30:187–190
Orlander J, Kiesling KJ, Larsson L, Karlsson J, Aniansson A (1978) Skeletal muscle metabolism and ultrastructure in relation to age in sedentary men. Acta Physiol Scand 104:249–261
Parkhouse WS, McKenzie DC, Hochachka PW, Ovalle WK (1985) Buffering capacity of deproteinized human vastus lateralis muscle. J Appl Physiol 58:14–17
Schantz PG, Henriksson. J (1987) Enzyme levels of the NADH shuttle systems: measurements in isolated muscle fibres from humans of differing physical activity. Acta Physiol Scand 129:505–515
Sewell DA, Harris RC, Marlin DJ (1994) Skeletal muscle characteristics in 2 year-old race-trained thoroughbred horses. Comp Biochem Physiol 108:87–96
Sjostrom M, Lexell J, Downham DY (1992) Differences in number and fibre type proportion within fascicles: A quantitative morphological study of whole vastus lateralis muscle from childhood to old age. Anatom Rec 234:183–189
Skaper SD, Das S, Marshall FD (1973) Some properties of a homocarnosine-carnosine synthetase isolated from rat brain. J Neurochem 21:1429–1445
Steel DS, Smith GL, Miller DJ (1990) The effects of taurine on Ca2+ uptake by the sarcoplasmic reticulum and Ca2+ sensitivity of chemically skinned rat heart. J Physiol 422:499–511
Stuerenburg HJ, Kunze K (1999) Concentrations of free carnosine (a putative membrane-protective antioxidant) in human muscle Biopsies and rat muscles. Archiv Gerontol Geriatrics 29:107–113
Suyama M, Suzuki T, Maruyama M., Saito K (1970) Determination of carnosine, anserine and balanine in the muscles of animals. Bull Jap Soc Sci Fish 36:1048–1053
Tallon MJ, Harris RC, Boobis L, Fallowfield J, Wise JA (2005) The carnosine content of vastus lateralis is elevated in resistance trained bodybuilders. J. Strength Condit Res 19:725–729
Trounce I, Byrne E, Marzuki S (1989) Decline in skeletal muscle mitochondrial respiration chain function with ageing. Lancet 2:44–45
Yasuda N, Glover EI, Phillips SM, Isfort RJ, Tarnopolsky MA (2005) Sex-based differences in skeletal muscle function and morphology with short-term limb immobilization. J Appl Physiol 99:1085–1092
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tallon, M.J., Harris, R.C., Maffulli, N. et al. Carnosine, taurine and enzyme activities of human skeletal muscle fibres from elderly subjects with osteoarthritis and young moderately active subjects. Biogerontology 8, 129–137 (2007). https://doi.org/10.1007/s10522-006-9038-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-006-9038-6