Abstract
The TANDEM investigation was carried out in 17 Italian Movement Disorder centers on behalf of a joint initiative of neurologist members of the Italian Academy for Parkinson’s disease and Movement Disorders (LIMPE-DISMOV Academy) and gastroenterologist members of the Italian Society of Digestive Endoscopy (SIED) to evaluate the efficacy and tolerability of levodopa-carbidopa intestinal gel (LCIG) in patients with advanced Parkinson's disease (PD) in routine medical care. Motor scores in “ON” and OFF” state (UPDRS-III), complications of therapy (UPDRS-IV), activities of daily living, sleep disorders and quality of life were evaluated at baseline and at two follow-up assessments (FUV1 and FUV2) within the initial 12-month LCIG treatment. In 159 patients (55% males) with a mean age of 69.1 ± 6.6 years and a diagnosis of PD since 13.6 ± 5.5 years, the UPDRS-III total score (in “OFF”) decreased from baseline (45.8 ± 13.2) to FUV1 (41.0 ± 17.4; p < 0.001) and FUV2 (40.5 ± 15.5; p < 0.001), the UPDRS-IV total score decreased from baseline (8.8 ± 2.9) to FUV1 (5.1 ± 3.4; p < 0.001) and FUV2 (5.5 ± 3.2; p < 0.001). The percentage of patients exhibiting freezing, dystonia, gait/walking disturbances, falls, pain and sleep disorders was significantly reduced. Twenty-eight device complications were reported and 11 (6.9%) patients prematurely terminated the study. LCIG after 12-month treatment led to sustained improvement of time spent in “OFF”, complications of therapy, PD-associated symptoms and sleep disorders. LCIG tolerability was consistent with the established safety profile of LCIG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder, characterized by resting tremor, rigidity and bradykinesia. These motor symptoms depend upon striatal dopamine denervation and are ameliorated by dopamine replacement therapy (Wirdefeldt et al. 2011).
Levodopa (LD) is still the most effective symptomatic drug for PD treatment (Wirdefeldt et al. 2011). Long-term oral LD administration, however, may be associated with the development of disabling motor complications (Wirdefeldt et al. 2011; Antonini et al. 2018a, b, c; Fabbrini et al. 2007) that negatively impact activities of daily living (ADL). Erratic gastric emptying, central pharmacodynamic mechanisms and pulsatile levodopa plasma levels are among the key factors for motor fluctuations (Antonini et al 2010; Contin and Martinelli 2010; Nutt 2008).
In recent years, PD has been increasingly associated with a variety of non-motor symptoms (NMS) (Kalia and Lang 2015; Chaudhuri et al. 2011) which contribute to an increasing overall morbidity, significantly impair quality of life (QoL) (Antonini et al. 2018a, b, c) and increase health care costs, particularly in the advanced stages of the disease (Lundqvist et al. 2014; Palhagen et al. 2016).
Levodopa-carbidopa intestinal gel (LCIG) allows continuous drug delivery of levodopa-carbidopa directly to the upper intestine, thus providing more stable plasma levels than oral therapy (Nyholm et al 2003, 2013), reducing motor (Palhagen et al 2016, Antonini et al. 2013, Olanow et al 2014, Slevin et al. 2015, Fernandez et al. 2015, Antonini et al. 2017) and non-motor complications (Antonini et al. 2018a, b, c; Reddy et al. 2012; Chaudhuri et al. 2013) and improving QoL (Antonini et al. 2018a, b, c; Lundqvist et al. 2014).
The TANDEM (MulTidisciplinary Approach in loNg-term DEvice use for Medication infusion) investigation was a joint collaboration between neurologists and gastroenterologists in 17 Italian PD care centres with the objective to evaluate the effectiveness of LCIG in advanced PD patients in routine medical care and to verify whether the LCIG drug/device system was well-tolerated. Motor fluctuations, associated PD symptoms, ADL and QoL were assessed during a 12-month treatment.
Patients and methods
Study design
The TANDEM investigation included 159 patients affected by advanced PD at 17 Movement Disorders centers already treated with LCIG based on clinician judgment and according to SPC. The efficacy and safety of LCIG treatment in routine medical care were retrospectively collected at baseline and prospectively assessed and two follow-up visits (FUV1 and FUV2) within the first 12 months following PEG-J placement.
Patients
Demographic and clinical features of consecutive patients treated with LCIG in a routine health care setting were retrospectively collected in a database between November 2014 and November 2017. This observational research was approved by local ECs and all patients provided written informed consent for recording and analysing their clinical data during routine medical care.
Effectiveness assessments
Motor scores were assessed by means of the UPDRS part-III scale in “ON” and “OFF” condition. Complications of pharmacological therapy were obtained by the UPDRS-IV. Presence of dystonia, pain, gait and walking disturbances, falls, early morning and nocturnal akinesia were evaluated by the investigators, while ADL was assessed by the UPDRS part-II. Considering the observational nature of this investigation, the questionnaires related to the QoL, NMS and cognitive function were collected only if they were previously recorded in the patient’s hospital clinical chart, mainly the Parkinson’s Disease Questionnaire of 8 items (PDQ-8) or 39 items (PDQ-39) or by the Non-Motor Symptoms Scale (NMSS). Cognitive function was detected, if available, by means of the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA), when routinely applied by the participating centers. A detailed listing of device complications reported by the gastroenterologists will be reported in a further publication.
Timing
For each subject, scales, if available, were retrospectively collected at baseline (BL), corresponding to the time of discharge from hospital following PEG-J placement, and at two follow-up prospective assessments, performed 1–5 months (FUV1) and 6–12 months (FUV2) after BL, respectively. Previous PD treatments were considered as well.
Statistical analysis
Data were summarized using descriptive techniques such as measures of central tendency (i.e., median or mean) and measures of variability (i.e., standard deviation or interquartile range) for continuous variables and absolute and relative frequencies for categorical variables.
Mean values of UPDRS part-III score in “OFF” and “ON” time, UPDRS part-IV, UPDRS part-II, and PDQ-8 or PDQ-39 scores at the three time points were estimated using mixed linear regression models, including time points as covariates. A mixed logistic regression was applied to evaluate the percentage of patients affected by PD symptoms at different time points.
Mixed regression models are a subject-specific model and are a method of choice for analysing longitudinal data.
All analyses were performed using Stata version 13.0.
Results
Patients (N = 159; 55% males) had a mean age of 69.1 ± 6.6 years and a PD duration of 13.6 ± 5.5 years. The previous oral PD treatments used before LCIG therapy were LD (98%), dopamine agonists (79%), catechol-O-methyl-transferase (COMT) inhibitors (41%) and monoamine oxidase B (MAO-B) inhibitors (54%) (Table 1).
Clinical baseline characteristics of patients are listed in Table 1. The mean baseline scores of the MMSE, the MoCA and the NMSS, available for a very small sample, were 24.7 ± 3.0 (N = 24), 20.6 ± 3.3 (N = 5) and 70.0 ± 38.6 (N = 18), respectively, and no changes were observed at the FUV1 and FUV2.
Reasons for switching from oral PD treatment to LCIG infusion were motor fluctuations in 143 (90%) patients, prolonged “OFF” time in 105 (66%) and dyskinesia in 86 (54%) (Table 1).
The LCIG infusion parameters (morning dose, infusion duration and dosing, dose and number of extra doses) did not change at the FUV1 and FUV2 compared to BL (Table 2). Oral PD treatment administered concomitantly to LCIG was used only in a few patients at BL [oral night-time LD (N = 8), dopamine agonists (N = 6) and MAO-B inhibitor (N = 6)] and remained unchanged at FUV1 and FUV2, except for oral night-time LD which was administered to 22 and 15 patients at FUV1 and FUV2, respectively (Table 2).
Effectiveness
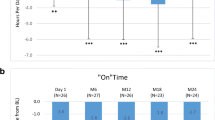
The mean UPDRS part-III score in “OFF” time significantly decreased from BL (45.8 ± 13.2) to FUV1 (41.0 ± 17.4; − 11%, p < 0.001) and to FUV2 (40.5 ± 15.5; − 12%, p < 0.001) (Fig. 1). The mean UPDRS part-IV score significantly decreased from BL (8.8 ± 2.9) to FUV1 (5.1 ± 3.4; − 42%; p < 0.001) and to FUV2 (5.5 ± 3.2; − 38%; p < 0.001) (Fig. 1). The mean PDQ-39 score decreased from BL (36.7 ± 2 0.2; N = 34) to FUV1 (29.0 ± 21.5; N = 24; − 21%; ns) and to FUV2 (27.7 ± 18.4; N = 24; − 24%; ns). The mean PDQ-8 (N = 61 at BL) and the mean UPDRS-II scores (N = 61 at BL) remained unchanged. The UPDRS part-III (“OFF” and “ON” time) and UPDRS part-IV were performed as routine assessments at all participating sites, while the UPDRS part-II scale, the PDQ-8, PDQ-39 questionnaires and NMSS were used at some sites but only in a limited number of patients (Table 1).
Motor fluctuations and complication of therapy: UPDRS-III “On”, UPDRS-III “Off” and UPDRS-IV ADL total scores at baseline (start of LCIG treatment with PEG-J treatment) at FUV1 and FUV2. Asterisks represent statistical significance (**p ≤ 0.001) compared to baseline. Numbers indicated in brackets represent the number of observations at each visit. TANDEM population (N = 159), number of observations indicated in brackets. UPDRS Unified Parkinson's Disease Rating Scale
Similarly to the improvement of motor symptoms, the percentage of patients affected by associated PD non-motor symptoms significantly decreased at FUV1 and FUV2 vs. BL. In particular, pain (p = 0.035 and p = 0.002), freezing of gait (p < 0.001 and p < 0.001), dystonia (p = 0.02 and p = 0.001), falls (p < 0.001 and p = 0.005) and gait and walking disturbances (p < 0.001 and p < 0.001) were significantly reduced at FUV1 and FUV2. Early morning, nocturnal akinesia and mood swings decreased significantly at FUV1 (p = 0.002, p = 0.010 and p = 0.011, respectively), when compared to BL (Table 3).
The proportion of patients suffering from sleep disorders was also markedly reduced at FUV1 (p = 0.017) and FUV2 (p = 0.007) compared to BL (Table 3). Changes of the incidence of different patterns of sleep disorders were not significant at each follow-up assessment compared to BL (Table 3).
Safety
During the data collection period, neurologists reported 28 complications (Table 4). The most frequent were stoma granuloma (N = 6), occlusion of internal percutaneous endoscopic jejunal (PEJ) tubes due to kinking/knotting (N = 6) and other J-tube occlusions (N = 5). Out of 11 patients in whom premature termination of LCIG was reported, 9 were related to the decision of the patients or physicians to interrupt treatment for poor compliance/efficacy, one was due to a device complication and one to an adverse event (Table 4). Seven deaths were reported due to sepsis (N = 1), acute meningoencephalitis (N = 1), and unknown reason (N = 5), all considered not related to treatment.
Discussion
The TANDEM investigation is the largest Italian joint initiative of neurologists and gastroenterologists for retrospective and prospective data collection deriving from routine clinical care assessing motor functions, specific PD-associated symptoms and sleep disorders in advanced PD patients treated with LCIG. The web-based electronic system allowed both, neurologists and gastroenterologists, to collect multidisciplinary, homogeneous data and to assess the effectiveness of LCIG and tolerability of the drug/device system in routine clinical practice (Fig. 2).
Quality of life: PDQ-8, PDQ-39 and ADL total scores at baseline (start of LCIG treatment with PEG-J treatment) at FUV1 and FUV2. Asterisks represent statistical significance (p ≤ 0.05) compared to baseline. Numbers indicated in brackets represent the number of observations at each visit. PDQ-8 8-item Parkinson’s Disease Questionnaire, PDQ-39 39-item Parkinson’s Disease Questionnaire, BL baseline (discharge from hospital post-PEG-J placement), FUV1 follow-up visit 1, FUV2 follow-up visit 2
Here, we report the clinical outcomes assessed by the neurologists on motor fluctuations, PD-associated symptoms, ADL and QoL.
LCIG led to marked improvement of motor symptoms during the 12-month treatment period. Consistent with previous studies, we did not find a significant change in the UPDRS-III score in ON time (Sensi et al 2014; Zibetti et al. 2014). Differently from previously published studies, a significant improvement in both follow-up assessments was reported for UPDRS-III in OFF time. It is worth to note that in the recently published GLORIA study a significant change of UPDRS-III score in ON condition was observed after 18 and 24 months of LCIG treatment (Antonini et al. 2017). Moreover, Zibetti et al. (2013a; b) showed that PD symptoms, particularly axial symptoms, deteriorated significantly over a 3-year LCIG treatment only in advanced PD patients with a diagnosis of probable dementia.
We found that the mean UPDRS-IV score was significantly reduced by 42% and 38% at the first and second follow-up visit, respectively. A similar magnitude of reduction was reported in a large recently published Italian observational GREENFIELD study (Lopiano et al. 2019).
In our investigation, a significant reduction in the frequency of PD-associated symptoms such as freezing, dystonia, falls, gait and walking disturbances and overall pain was reported at each FUV and enabled patients to increase their mobility. Moreover, the reduction of the frequency of axial symptoms is consistent with the recently published data from a retrospective study on 32 advanced PD patients, where it was shown that LCIG has a favourable effect on freezing of gait (FoG) and on freezing refractory to oral therapy (Rispoli et al 2018; Cossu et al. 2015; Zibetti et al. 2018).
The assessment of sleep disorders at BL revealed that 62% of the patients suffered from PD-associated symptoms and that sleep disorders were significantly reduced (p = 0.007) within the 12 months of LCIG treatment. Since sleep disorders had a high prevalence in PD patients with a significant impact on patient’s QoL (Ondo 2014), the marked reduction in the frequency of sleep disorders, observed in our investigation, represents an important finding. These findings are relevant if we consider that recently it has been shown that LCIG improves the quality of sleep, inducing a less fragmented sleep pattern, as assessed by polysomnography after 6 months of treatment (Zibetti et al. 2013a, b; 2017).
LCIG was administered in the majority of patients as monotherapy and the dose remained stable during the 12 months of treatment period. Only few patients received concomitant additional oral anti-PD treatments such as dopamine agonists, COMT or MAO-B inhibitors. This is an important finding in line with a sub-analysis of the GLORIA registry which showed that 57% of the patients starting LCIG in monotherapy remain stable in monotherapy at 24 months and they experienced the fewest discontinuations, adverse drug reactions (ADRs), and serious ADRs compared to patients in polytherapy (Poewe et al 2019). This issue is to be taken into account considering that it has been reported that a poorer compliance and reduced adherence to therapy was frequently associated with polypharmacy and drug regimen complexity with consequent worsening of symptoms control (Grosset et al 2005; Daley et al. 2012; Malek and Grosset 2015; Poewe et al 2019). In other studies, the proportion of patients with LCIG monotherapy was substantially similar (Zibetti et al. 2013a, b; 2014).
Oral night-time levodopa was administered to only few patients (5%) at baseline and was the only concomitant PD treatment administered at FUV1 and FUV2 to slightly more patients (14% and 9%, respectively). This percentage of nocturnal use of oral LD is substantially similar to that reported in the GREENFIELD study ranging from 23 to 26% (Lopiano et al 2019), while in another study reporting marked improvement of nocturnal sleep, the proportion of patients using oral bed-time LD was substantially higher (64%) (Zibetti et al. 2017).
We showed no significant changes in ADL (UPDRS-II), in QoL (PDQ-8 and PDQ-39) and the NMSS. Likely, this was due to the very low number of observations at baseline visit which further decreased at follow-up assessments. Investigations conducted in routine medical care may bear this bias, since participating centres assessments were routinely performed during medical care. In our investigation, some centres used PDQ-8 while others used PDQ-39 assessment and only a few centres used NMSS.
Other limitations of this routine medical care investigation were the open-label design of the study, the lack of a control group, and the absence of a systematic collection and analysis of adverse events like in a clinical study setting.
The cooperation between neurologists and gastroenterologists is crucial for a sustained and well-managed LCIG treatment, during the PEG-J positioning, throughout the short-term post-positioning phase and the long-term maintenance period, to ensure optimal functioning, a low rate of complications and a good tolerability of the drug/device system.
In this investigation, the rate of premature discontinuations was low (6.9%) compared to that reported in another multi-national LCIG investigation, where the dropout rate after the 12 months of follow-up was twice as high (14.5%) (Antonini et al. 2015). A slightly higher rate of discontinuation (9.5%) within the 1st year of LCIG treatment was reported in an Italian retrospective, multicentre survey conducted by 60 PD specialists including 905 patients (Sensi et al. 2017). The high percentage of patients remaining on treatment in the short- and long-term follow-up was attributed to careful follow-up and appropriate patient and caregivers training as well as to the effectiveness of LCIG treatment. Therefore, LCIG infusion in Italy is managed in a uniform manner at a clinical, practical and organizational level.
Neurologists reported 28 device related complications during the observational period.
Our investigation provided important 'real-life' clinical evidence on the use of LCIG drug/system for advanced PD patients and demonstrated continuous care and hand-to-hand collaboration between neurologists, gastroenterologists and care teams.
In conclusion, LCIG treatment in advanced PD patients led to significant and sustained reduction of time spent in “OFF” and a significant improvement in motor complications and sleep disorders. The safety results were consistent with the previously established safety profile.
Limitations and strengths
In this observational investigation, due to the retrospective assessment of subjects already in treatment with LCIG, many baseline observations were missing, particularly QoL or NMS questionnaires and scales. Therefore, the questionnaires with baseline assessment were available only in a very limited sample and this could have influenced the significance of the differences between baseline and follow-up measurements. This suggests that a standard procedure for PD-associated symptoms collection has not yet been established among centers and that it would be important for the clinicians to always have wider diffusion and standardization in the usage of the tools and questionnaires for the clinical assessment of PD patients along the disease progression. Nevertheless, the common access of neurologists and gastroenterologists of the same center to the subjects’ clinical data and nevertheless the increased cooperation between gastroenterologists and neurologists in each center, the involvement of other specialistic figures, like neuropsychologists or nutritionists, could have represented a real multidisciplinary approach to patient management. Since this was an observational investigation and LCIG was implemented in routine care, the outcomes, even if in the absence of a true control group, may be considered representative of real-world clinical practice for the holistic care of PD patients. The lack of instruments to detect the improvement related to the multidisciplinary intervention and the related patient’s satisfaction is another limitation of this investigation and this should be further assessed in the future.
Change history
03 June 2020
The original version of this article unfortunately contained a mistake. Alfredo Berardelli and Giovanni Introduction
References
Antonini A, Nitu B (2018) Apomorphine and levodopa infusion for motor fluctuations and dyskinesia in advanced Parkinson disease. J Neural Transm. https://doi.org/10.1007/s00702-018-1906-0
Antonini A, Chaudhuri KR, Martinez-Martin P, Odin P (2010) Oral and infusion levodopa-based strategies for managing motor complications in patients with Parkinson’s disease. CNS Drugs 24:119–129
Antonini A, Odin P, Lopiano L, Tomantschger V, Pacchetti C, Pickut B, Gasser U, Calandrella D, Mancini F, Zibetti M, Minafra B, Bertaina I, Deyn P, Cras C, Wolf E, Spielberger S, Poewe W (2013) Effect and safety of duodenal levodopa infusion in advanced Parkinson’s disease: a retrospective multicenter outcome assessment in patient routine care. J Neural Transm 120:1553–1558
Antonini A, Yegin A, Preda C, Bergmann L, Poewe W (2015) Global long-term study on motor and non-motor symptoms and safety of levodopa-carbidopa intestinal gel in routine care of advanced Parkinson's disease patients: 12-month interim outcomes. Parkinsonism Relat Disord 21:231–235
Antonini A, Poewe W, Chaudhuri KR, Jech R, Pickut B, Pirtošek Z, Szasz J, Valldeoriola F, Winkler C, Bergmann L, Yegin A, Onuk K, Barch D, Odin P, On behalf of the GLORIA study co-investigators (2017) Levodopa-carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord 45:13–20
Antonini A, Moro E, Godeiro C, Reichmann H (2018a) Medical and surgical management of advanced Parkinson's disease. Mov Disord 33:900–908
Antonini A, Robieson WZ, Bergmann L, Yegin A, Poewe W (2018b) Age/disease duration influence on activities of daily living and quality of life after levodopa-carbidopa intestinal gel in Parkinson's disease. Neurodegener Dis Manag 8(3):161–170
Antonini A, Stoessl AJ, Kleinman LS, Skalicky AM, Marshall TS, Sail KR, Onuk K, Odin P (2018c) Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson's disease: a multi-country Delphi-panel approach. Curr Med Res Opin 20:1–11. https://doi.org/10.1080/03007995.2018.1502165
Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P (2011) Parkinson's disease: the non-motor issues. Parkins Rel Disord 17:717–723
Chaudhuri KR, Rizos A, Sethi KD (2013) Motor and non-motor complications in Parkinson’s disease: an argument for continuous drug delivery? J Neural Transm 120:1305–1320
Contin M, Martinelli P (2010) Pharmacokinetics of levodopa. J Neurol 257:253–261
Cossu G, Ricchi V, Pilleri M, Mancini F, Murgia D, Ricchieri G, Mereu A, Melis M, Antonini A (2015) Levodopa-carbidopa intrajejunal gel in advanced Parkinson disease with "on" freezing of gait. Neurol Sci 36(9):1683–1686. https://doi.org/10.1007/s10072-015-2234-x
Daley DJ, Myint PK, Gray RJ, Deane KH (2012) Systematic review on factors associated with medication non-adherence in Parkinson's disease. Parkinsonism Relat Disord 18(10):1053–1061
Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG (2007) Levodopa induced dyskinesias. Mov Disord 22:1379–1389
Fernandez HH, Standaert DG, Hauser RA, Lang AE, Fung VSC, Klostermann F, Lew MF, Odin P, Steiger M, Yakupov EZ, Chouinard S, Suchowersky O, Dubow J, Hall CM, Chatamra K, Robieson WZ, Benesh JA, Espay AJ (2015) Levodopa-carbidopa intestinal gel in advanced Parkinson's disease: final 12-month, open-label results. Mov Disord 30:500–509
Grosset KA, Bone I, Grosset DG (2005) Suboptimal medication adherence in Parkinson's disease. Mov Disord 20(11):1502–1507
Kalia LV, Lang AE (2015) Parkinson disease. Lancet Neurol 386:896–912
Lopiano L, Modugno N, Marano P, Sensi M, Meco G, Solla P, Gusmaroli G, Tamma F, Mancini F, Quatrale R, Zangaglia R, Bentivoglio A, Eleopra R, Gualberti G, Melzi G, Antonini A (2019) Motor and non-motor outcomes in patients with advanced Parkinson's disease treated with levodopa/carbidopa intestinal gel: final results of the GREENFIELD observational study. J Neurol 266(9):2164–2176
Lundqvist C, Beiske AG, Reiertsen O, Kristiansen IS (2014) Real life cost and quality of life associated with continuous intraduodenal levodopa infusion compared with oral treatment in Parkinson patients. J Neurol 261:2438–2445
Malek N, Grosset DG (2015) Medication adherence in patients with Parkinson's disease. CNS Dugs 29:47–53
Nutt JG (2008) Pharmacokinetics and pharmacodynamics of levodopa. Mov Disord 23:S580–S584
Nyholm D, Askmark H, Gomes-Trolin C, Knutson T, Lennernäs H, Nyström C, Aquilonius SM (2003) Optimizing levodopa pharmacokinetics: intestinal infusion versus oral sustained- release tablets. Clin Neuropharmacol 26:156–163
Nyholm D, Odin P, Johansson A, Chatamra K, Locke C, Dutta S, Othman A (2013) Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease patients. AAPS J 15:316–323
Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ, Pritchett Y, Chatamra K, Benesh J, Lenz RA, Antonini A (2014) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13:141–149
Ondo WG (2014) Sleep/wake problems in Parkinson’s disease: pathophysiology and clinicopathologic correlations. J Neural Transm 121(Suppl 1):S3–13. https://doi.org/10.1007/s00702-014-1239-6
Palhagen SE, Sydow O, Johansson A, Nyholm D, Holmberg B, Widner H, Dizdar N, Linder J, Hauge T, Jansson R, Bergmann L, Kjellander S, Marshall TS (2016) Levodopa-carbidopa intestinal gel (LCIG) treatment in routine care of patients with advanced Parkinson's disease: an open-label prospective observational study of effectiveness, tolerability and healthcare costs. Parkinsonism Relat Disord 29:17–23
Poewe W, Bergmann L, Kukreja P, Robieson WZ, Antonini A (2019) Levodopa-carbidopa intestinal gel monotherapy: GLORIA registry demographics, efficacy, and safety. J Parkinsons Dis 9(3):531–541
Reddy P, Martinez-Martin P, Rizos A, Martin A, Faye GC, Forgacs I, Odin P, Antonini A, Chaudhuri KR (2012) Intrajejunal levodopa versus conventional therapy in Parkinson disease: motor and nonmotor effects. Clin Neuropharmacol 35(5):205–207
Rispoli V, Andreasi Golfrè N, Penna G, Preda F, Contini E, Sensi M (2018) Levodopa/carbidopaintestinal gel infusion therapy: focus on gait and balance. Mov Disord Clin Pract. https://doi.org/10.1002/mdc3.12640
Sensi M, Preda F, Trevisani L, Contini E, Gragnaniello D, Capone JG, Sette E, Golfre-Andreasi N, Tugnoli V, Tola MR, Quatrale R (2014) Emerging issues on selection criteria of levodopa carbidopa infusion therapy: considerations on outcome of 28 consecutive patients. J Neural Transm 121(6):633–642
Sensi M, Cossu G, Mancini F, Pilleri M, Zibetti M, Modugno N, Quatrale R, Tamma F, Antonini A (2017) Which patients discontinue? Issues on levodopa/carbidopa intestinal gel treatment: Italian multicentre survey of 905 patients with long-term follow-up. Parkinsonism Relat Disord 38:90–92. https://doi.org/10.1016/j.parkreldis.2017.02.020
Slevin JT, Fernandez HH, Zadikoff C, Hall C, Eaton S, Dubow J, Chatamra K, Benesh J (2015) Long-term safety and maintenance of efficacy of levodopa-carbidopa intestinal gel: an open-label extension of the double-blind pivotal study in advanced Parkinson's disease patients. J Parkinsons Dis 5:165–174
Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J (2011) Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol 26:S1–S58
Zibetti M, Merola A, Ricchi V, Marchisio A, Artusi CA, Rizzi L, Montanaro E, Reggio D, De Angelis C, Rizzone M, Lopiano L (2013a) Long-term duodenal levodopa infusion in Parkinson's disease: a 3-year motor and cognitive follow-up study. J Neurol 260:105–114
Zibetti M, Rizzone M, Merola A, Angrisano S, Rizzi L, Montanaro E, Cicolin A, Lopiano L (2013b) Sleep improvement with levodopa/carbidopa intestinal gel infusion in Parkinson disease. Acta Neurol Scand 127(5):e28–32. https://doi.org/10.1111/ane.12075
Zibetti M, Merola A, Artusi CA, Rizzi L, Angrisano S, Reggio D, De Angelis C, Rizzone M, Lopiano L (2014) Levodopa/carbidopa intestinal gel infusion in advanced Parkinson's disease: a 7-year experience. Eur J Neurol 21:312–318
Zibetti M, Romagnolo A, Merola A, Priano L, Montanaro E, Angrisano S, Tribolo A, Cicolin A, Lopiano L (2017) A polysomnographic study in Parkinsonian patients treated with intestinal levodopa infusion. J Neurol 264(6):1085–1090. https://doi.org/10.1007/s00415-017-8491-2
Zibetti M, Angrisano S, Dematteis F, Artusi CA, Romagnolo A, Merola A, Lopiano L (2018) Effects of intestinal levodopa infusion on freezing of gait in Parkinson disease. J Neurol Sci 385:105–108
Acknowledgements
The EDC system used to collect clinical data and the statistical analysis was provided by Informa Srl, Italy, upon request of the Italian Society Accademia LIMPE-DISMOV and of the Italian Society of Digestive Endoscopy (SIED). The authors would like to acknowledge the following non-author co-workers for their contributions: Laura Timelli for the statistical analysis of data. Stefania Tagliente, Department NESMOS, “Sapienza” University, Sant’Andrea Hospital, Roma;Elena Caputo, Valeria Lucches,Marco V. Rossi, Neurology Unit, Miulli Hospital, Acquaviva delle Fonti (BA), Gaia Donata Oggioni, Parkinson’s Disease and Movement Disorders Centre, Neurology Unit, University of Piemonte Orientale, Novara, Debora Lanni, Istituto Neurologico Mediterraneo IRCCS "Neuromed", Pozzilli, Maurizio Zibetti, Department of Neuroscience, University of Turin, Francesco Bove, Maria Gabriella Vita and Maria Elena Riccioni (Fodazione Policlinico Universitario A. Gemelli IRCCS), Romeand Antonio De Mase and Rosa De Micco, Department of Medical, Surgical, Neurological, Metabolic and Aging Sciences, University of Campania, “Luigi Vanvitelli”for data collection.
Funding
The medical writing/editing and publication management of this publication was provided by Urs E. Gasser, ClinResearch Ltd, Switzerland, funded by AbbVie Srl Italy. AbbVie Srl Italy also funded database software management and educational meetings”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Antonini received honorarium from AbbVie and has received compensation for consultancy and speaker-related activities from AbbVie, UCB, Lundbeck, Bial, Neuroderm, Boehringer Ingelheim, Mundipharma and Zambon. N. Modugno has received compensation for speaker related activities from AbbVie and UCB Pharma. M. Sensi and has received compensation for speaker related activities from AbbVie. L. Lopiano has received honoraria for consulting services and symposia from AbbVie, UCB, DOC, Zambon, Lundbeck and Bial. F. Mancini received compensation for consultancy and speaker-related activities from AbbVie and Zambon. G. Cossu received honoraria for consulting services and symposia from AbbVie. P. Solla, received honoraria for participation in advisory boards from AbbVie. F.E. Pontieri received honoraria for participation in advisory boards from AbbVie, and speaker and/or consulting honoraria from AbbVie, Lundbeck, FB Health, Bial and Zambon. N. Tambasco received speaker honoraria from Lundbeck and AbbVie. A. Tessitore received speaker and/or consulting honoraria from Lundbeck, AbbVie, UCB, Allergan and Almirall. G. Abbruzzese received speaker honoraria from AbbVie and participated in an Advisory Board for Zambon. A. Berardelli reported no disclosures. I. Stroppa received honoraria for participation in advisory boards from Abbie and speaker and/or consulting honoraria from AbbVie. A. Stefani received honoraria for consulting services and symposia from AbbVie. G. Fabbrini received honoraria for consulting services from AbbVie. AR. Bentivoglio received honoraria for participation in advisory board from Merz and AbbVie and has received compensation for speaker-related activities from AbbVie, Allergan, Bial, Ipsen, Lundbeck, Neuroderm, UCB and Zambon. C. Comi received honoraria for symposia from AbbVie and Zambon. B. Minafra received speaker honoraria from Boston Scientific, Medtronic and Chiesi. G. Riboldazzi received honoraria for consulting services and symposia from AbbVie. F. Tamma received honoraria for consulting services and symposia from AbbVie. D. Melchionda received honoraria for consulting services from AbbVie. T. Martino received honoraria for consulting services from AbbVie.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Antonini, A., Abbruzzese, G., Berardelli, A. et al. The TANDEM investigation: efficacy and tolerability of levodopa-carbidopa intestinal gel in (LCIG) advanced Parkinson’s disease patients. J Neural Transm 127, 881–891 (2020). https://doi.org/10.1007/s00702-020-02175-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-020-02175-1