Abstract
Advanced-stage Parkinson’s disease (PD) strongly affects quality of life (QoL). Continuous intraduodenal administration of levodopa (IDL) is efficacious, but entails high costs. This study aims to estimate these costs in routine care. 10 patients with advanced-PD who switched from oral medication to IDL were assessed at baseline, and subsequently at 3, 6, 9 and 12 months follow-up. We used the Unified PD Rating Scale (UPDRS) for function and 15D for Quality of Life (QoL). Costs were assessed using quarterly structured patient questionnaires and hospital registries. Costs per quality adjusted life year (QALY) were estimated for conventional treatment prior to switch and for 1-year treatment with IDL. Probabilistic sensitivity analysis was based on bootstrapping. IDL significantly improved functional scores and was safe to use. One-year conventional oral treatment entailed 0.63 QALY while IDL entailed 0.68 (p > 0.05). The estimated total 1-year treatment cost was NOK419,160 on conventional treatment and NOK890,920 on IDL, representing a cost of NOK9.2 million (€1.18 mill) per additional QALY. The incremental cost per unit UPDRS improvement was NOK25,000 (€3,250). Medication was the dominant cost during IDL (45 % of total costs), it represented only 6.4 % of the total for conventional treatment. IDL improves function but is not cost effective using recommended thresholds for cost/QALY in Norway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early Parkinson’s disease (PD) usually causes little disability if optimally treated but in later stages and fluctuating disease, both function and cost increase [5].
Motor fluctuations may be associated with fluctuating levodopa serum levels [19, 27]. Strategies to avoid fluctuations include use of longer acting oral medications and levodopa administration with shorter intervals [27, 31]. Other strategies include oral and transdermal prolonged release preparations and subcutaneous or intestinal drug administration [30]. Continuous subcutaneous administration of apomorphine and intraduodenal levodopa administration (IDL) are alternatives with proven efficacy in terms of reducing on–off fluctuations [2, 9]. Both are considered evidence-based alternatives [11]. Finally, deep brain stimulation, usually in the subthalamic nucleus (STN) is used [1, 9, 20]. Optimal choice of late stage PD therapy requires insight into the benefits and side effects of the different therapies, but also of the costs. While DBS implies high initial costs because of the device and surgery, IDL with Duodopa® is costly in the longer run because the drug has orphan status [32] and is priced accordingly. Two studies have been published on the cost effectiveness of intraduodenal levodopa therapy with widely diverging results [13, 15]. These studies, however, were based in part on trial data and modelling, and to our knowledge no studies of real life costs and outcomes have been published.
The aim of the present study was to estimate the costs and health consequences of replacing conventional oral treatment by IDL in late stage PD. We adopted a societal perspective, attempting to capture all costs and consequences. Because PD strongly influences patients’ quality of life, we used quality adjusted life years (QALYs) measured using the 15D instrument as the measure of health benefit [6].
Methods
Setting
One-year, prospective, open, clinical study of IDL treatment in the Neurology department of a University Hospital was conducted. We used the patients’ own quality of life and treatment costs prior to IDL as the control.
Patients
Inclusion criteria:
-
Clinical diagnosis of idiopathic PD.
-
Motor fluctuations despite optimized oral treatment.
-
Suitable for IDL treatment based on overall clinical assessment of severity of disease [Unified PD Rating Scale (UPDRS) and Hoehn and Yahr staging] and efficacy of previous treatment.
-
On–off registration supporting improvement of fluctuations by pilot IDL treatment as compared to oral treatment.
Exclusion criteria:
-
Severe dementia, confusion, psychosis or depression.
-
Contraindications against levodopa treatment.
Treatment protocol
Patients were clinically assessed as in-patients in the neurology department for possible IDL treatment. On–off registration was done over at least 24 h on current oral treatment. Efficacy of IDL treatment was then tested by infusion through a nasoduodenal tube. IDL administration was started at a dosage based on previous oral levodopa dose. Using continued on–off registration, the dosage was adjusted to achieve optimal on-time and minimize dyskinesia over the next few days. Once a stable optimal dose had been titrated, the nasoduodenal tube was removed and patients returned to their previous oral medication regime. Patients who showed improvement compared with the oral phase were offered long-term IDL treatment and an application for reimbursement similar to other PD drugs was submitted to the Norwegian Health Economics Administration (HELFO).
Patients were released from the hospital and readmitted after 4 weeks for insertion of a gastrostomy- and jejunal tube (J-PEG). This “PEG-week” was conducted using a procedure similar to that used during the test week. Patients remained hospitalized until the immediate postoperative phase was over and dosage was considered acceptable. Patients were released from the hospital and readmitted briefly after 1 month to check the general postoperative condition and at 3, 6, 9 and 12 months for functional assessment, on–off registration and dosage adjustment. After 12 months the patients left the study but were allowed to continue IDL treatment if they wished.
Quality of life (15D)
Patients filled in the 15 item, self-administered quality-of-life instrument 15D at baseline, admission to the PEG week and at the readmission time points. We translated 15D QoL data to a utility scale ranging from 0 to 1 by means of the algorithms published by Sintonen [22–24]. For missing data we used Last Observation Carried Forward (LOCF) for time points after the initiation of IDL treatment.
The change in quality adjusted life years (QALYs) from IDL treatment was estimated as the difference between the estimated QALY in the last year before IDL treatment based on the 15D value before inclusion in the study, and the first year on such treatment. The QALY was calculated as the area under the curve using the registered scores. Patient reported health care utilization and 15D data were collected using structured questionnaires. Patients were queried about any health care contacts, home nursing or stays in institutions as well as related travel costs. Information about length and reason for institutional stays were verified using individual patients’ hospital registries. Lost income for relatives due to care for patients at home was included as a health-related cost based on average wages in Norway during the same year.
Costs
Cost of medication was calculated based on actual prescriptions. All costs were measured in 2008 Norwegian Kroner (NOK). The total cost of PEG (NOK89,892—€11,566) was based on hospital accounts and included the cost of the nasoduodenal test period which was distributed across the expected duration of DLI treatment (4.2 years according to [18]). Other sources for cost calculations are given in Table 1. We included indirect costs based on patients self-reports.
Analysis
Descriptive data are presented as means and 95 % CI. For comparison of data on oral treatment and IDL, paired t tests were used with p < 0.05 as significant (Bonferroni corrected, as appropriate). Differences in total costs and QALYs were tested with bootstrapping.
Ethics
All participants gave written informed consent. The study was performed based on GCP and the Helsinki protocol. The relevant ethical, data handling and regulatory institutions approved the study procedures. The study was registered in the Clinical Trials registry (http://www.clinicaltrials.gov, Identifier: NCT00272688).
Results
Patients
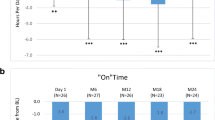
Patients’ characteristics are shown in Table 2. All 10 patients had both dyskinesia and off periods. For dyskinesia, six patients had a duration of 1–25 % waking hours, two patients 25–50 % and two more than 50 %. Off-period duration was 1–25 % for five patients, 25–50 % for four patients and >50 % for one patient. Nine patients participated according to protocol for the whole year while one withdrew consent for the study after 6 months, but chose to continue on IDL. The mean UPDRS scores as well as fluctuations improved compared to baseline (Fig. 1; Table 2). Total levodopa dosage with IDL treatment increased (Fig. 2). This dosage change occurred during the first 3 months and then remained stable. The optimal dosage of IDL determined during nasoduodenal testing was slightly lower than the oral levodopa equivalent dose at baseline (1,047 vs. 1,223 mg, p = 0.039––not significant compared to Bonferroni-adjusted significance limits).
UPDRS scores (lower, dashed line) and fluctuations (% time in near normal function/day as registered in on–off registration by trained nurses in the neurological ward—upper continuous line) at specific time points after baseline (0 time) before start of intraduodenal levodopa. Error bars are 95 % CI
Side effects/safety
The following side effects were seen during IDL: (1) technical/surgery related: six tube dislocations/leakage, three local pain around stoma/local chemical peritonitis not requiring treatment, two tube occlusions, two stoma infections/secretion from stoma; (2) possibly/probably medication related: hallucinations four times in same patient, three minor depressions, one diarrhoea, one leg cramps, one increased dyskinesia. There were three suspected serious adverse reactions (SUSARS) reported: (1) paranoid psychotic reaction during nasoduodenal testing (at night with pump off)––requiring temporary restraining of patient and antipsychotic medication; (2) atrial flutter, by cardiologist evaluated as probably unrelated to treatment, not requiring hospitalization but anticoagulation was initiated; (3) knotted intestinal tube requiring in-hospital stay overnight and new gastroscopy. None of the side effects led to termination of the IDL treatment. The patient who withdrew consent did so based on the general hassle of participating in the data collection of the study rather than on side effects.
Quality of life
The mean 15D score on oral treatment at baseline was 0.63 while it was on average 0.68 during 1 year of IDL implying 1-year QALYs of 0.63 and 0.68, respectively (Table 3). The difference was 0.047 (95 % CI 0.00063–0.097).
Costs
The mean 1-year cost per patient was NOK419,160 on oral treatment and NOK890,920 on IDL (Table 4), representing a mean incremental 1-year cost of NOK 471,760 (€60,697).
The distribution of costs across categories varied substantially with medication (levodopa) representing the largest IDL cost component (45 % of total), while non-medication health-related costs and indirect costs accounted for the largest proportion of oral treatment costs (54 and 39 %, respectively) (Table 5). Medications represented only 6.4 % of total costs of conventional oral treatment.
Cost effectiveness
With incremental cost of NOK471,760 per patient per year, and incremental QALY of 0.047, incremental cost per QALY was NOK9.2 million (€1.18 million). The incremental cost per unit improvement of the total UPDRS score was NOK25,228 (€3,246).
Sensitivity analysis
In probabilistic sensitivity analysis based on bootstrapping using the Norwegian recommended value of a QALY (NOK 500,000), the probability that IDL treatment is cost-effective is zero.
Due to the large variation of individual costs (Table 4), we also performed additional sensitivity analyses by removing the two patients with the highest and lowest cost increase, respectively. The results suggested incremental costs per QALY of NOK 6.4–21 million (€0.82–2.7 million).
Discussion
Main results
In this study of IDL, patients improved functional scores and had less motor fluctuations. Safety was acceptable, with side effects largely as expected in older patients with advanced-PD. The cost of IDL is high, and is not cost effective according to current Norwegian guidelines [21].
Methodological considerations
This study was an open, un-blinded, before–after study where patients were their own controls. A blinded study of this invasive treatment would have been impossible to perform. Patients who are considered for IDL treatment probably represent a selected group different from those who remain on oral treatment or have STN stimulation.
The 15D quality of life instrument was chosen because it is a multidimensional generic instrument that may capture improvement in QoL even in small patient groups [13, 22–24]. However, the instrument may miss some Parkinson-specific symptoms with a bearing on subjective QoL, especially related to fluctuations [10, 12]. The average improvement of 0.047 in 15D score is clinically relevant, but it is not statistically significant due to large variation across few patients. The improvement in UPDRS scores over time indicates that IDL leads to a real improvement of the patients’ lives. Though the 15D has been suggested to be a good, multidimensional score for comparing costs for different complex health states [25, 26], it was apparently not able to capture this improvement in a small patient group.
Retrospective recall of costs may be hampered by recall bias. To reduce recall time we asked patients to register their use of health care during the three weeks preceding each visit and then assumed the same cost level for the previous 3 months. However, costs associated with in-hospital stays were recorded directly from patient registers and we expect that they are relatively complete.
Discussion and comparison with results of others
To our knowledge, no previous long-term cost-utility study based on actual costs and outcomes has been published for IDL. Functional measures such as the UPDRS score have been used to assess cost effectiveness [16, 29]. However, this makes comparisons with non-Parkinson diseases impossible. Other studies have based estimations on shorter term QoL assessments extrapolated into the future and standard costs assumed to be associated with the patients [13], or on modelling based on QoL (EQ-5D) levels associated with patients with different levels of PD function [15]. These approximations may lead to loss of information regarding individual patients. Our study may be regarded as a supplement to the above-mentioned studies. There are probably two reasons why we arrive at a much higher cost per QALY than other studies with the exception of the study by Kristiansen and co-workers. First, we used 15D which may result in more “conservative” QALY gains, and second, our 1-year study does not capture future cost savings from less use of nursing home, etc. It should be noted, however, that there is little direct evidence of such savings.
Comparison with other treatments
Until now, the choice between IDL, DBS and continuous apomorphine treatments has been more dependent on the expertise of the various treatment centres than on evidence-based comparisons between them. There are no randomized, head-to-head comparisons of these treatment methods. A non-randomized comparison of the functional efficacy of IDL, STN stimulation and apomorphine pump indicated that both IDL and STN stimulation produced motor improvement, while apomorphine gave inadequate motor control [7]. The alternative strategies are all associated with high costs. In Europe, the estimate of the annual cost of an average PD patient is between €10,000 and €20,000 while that of an advanced-phase patient may be as high as €30,000 [14]. For STN, initial procedure-related costs have been estimated at €10,000–37,000 [8, 16, 28, 29]. Apomorphine medication costs have been estimated at between €13,500 and €73,000–91,000 per year [4, 17]. For IDL, levodopa medication costs alone are approximately €50,000 per year.
There are no direct cost-effectiveness comparisons between IDL, DBS and apomorphine infusion. However, studies have compared DBS or apomorphine infusion with conventional oral therapy. A study based on probabilistic decision modelling found an incremental cost-effectiveness ratio of $49,194 (€37,630) per QALY gained for DBS vs. best per oral medication. QoL improvement over 18 % was regarded as probably cost effective (<$50,000/QALY) [28]. A recent study used the EQ-5D for QALY based estimations of cost effectiveness of DBS and found a cost-effectiveness ratio of €34,389 per QALY with a QALY improvement from 0.54 to 0.76 suggesting cost effectiveness [29]. However, excluding two patients on apomorphine pump treatment from the control group in this study increased the cost-effectiveness ratio to €62,148 per QALY, indicating the importance of the cost of expensive medication and the sensitivity to high costs for few individuals in the analyses [29].
Our results for IDL are similar to a Swedish study that also used the 15D instrument and reported a cost-effectiveness ratio of SEK6.1 million (€645,500) per QALY [13]. A Markov model from the UK, suggested a cost per QALY for IDL of £36,000 (€41,000) using EQ-5D [15]. [8, 16, 29] It is clear that the results of cost-effectiveness studies depend critically on methods, not least for measuring QoL.
Policy implications
In Norway, the Directorate of Health has suggested that society should be willing to pay NOK300,000 to NOK800,000 per good life year (QALY) [21]. The cost per QALY in this study is well beyond that threshold. This raises the question of whether society should be willing to pay more for orphan drugs than other treatment [3]. Measuring individual costs of an advanced-PD patient group, as we have here, is expected to give large variations in costs of care and it is obvious that such variations are larger in a smaller patient group. However, our results represent real measured costs in Norway of consecutive patients treated based on the established clinical indications and sensitivity analyses suggest them to be valid despite these variations. Such individual variations need to be considered by policy makers and associated ethical challenges, such as which patient groups may be eligible for IDL, whether there should be a maximum permissible individual cost and how IDL should be paid for, should be discussed further.
There is little doubt, however, that IDL may substantially improve patients’ lives.
References
Benabid AL (2003) Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol 13:696–706
Colzi A, Turner K, Lees AJ (1998) Continuous subcutaneous waking day apomorphine in the long term treatment of levodopa induced interdose dyskinesias in Parkinson’s disease. J Neurol Neurosurg Psychiatry 64:573–576
Desser AS, Gyrd-Hansen D, Olsen JA, Grepperud S, Kristiansen IS (2010) Societal views on orphan drugs: cross sectional survey of Norwegians aged 40 to 67. BMJ 341:c4715
Dodel R, Eggert KM, Singer MS, Eichhorn TE, Pogarell O, Oertel WH (1998) Costs of drug treatment in Parkinsons disease. Mov Disord 13:249–254
Dodel RC, Berger K, Oertel WH (2001) Health-related quality of life and healthcare utilisation in patients with Parkinson’s disease: impact of motor fluctuations and dyskinesias. Pharmacoeconomics 19:1013–1038
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL (2007) Methods for the economic evaluation of health care programmes. Oxford University Press, Oxford
Elia AE, Dollenz C, Soliveri P, Albanese A (2012) Motor features and response to oral levodopa in patients with Parkinson’s disease under continuous dopaminergic infusion or deep brain stimulation. Eur J Neurol 19:76–83
Fraix V, Houeto JL, Lagrange C, Le PC, Krystkowiak P, Guehl D, Ardouin C, Welter ML, Maurel F, Defebvre L, Rougier A, Benabid AL, Mesnage V, Ligier M, Blond S, Burbaud P, Bioulac B, Destee A, Cornu P, Pollak P (2006) Clinical and economic results of bilateral subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatry 77:443–449
Goetz CG, Poewe W, Rascol O, Sampaio C (2006) Eidence based medical review update: pharmacological and surgical treatments of Parkinsons disease: 2001 to 2004. Mov Disord 20:523–539
Haapaniemi TH, Sotaniemi KA, Sintonen H, Taimela E (2004) The generic 15D instrument is valid and feasible for measuring health related quality of life in Parkinson’s disease. J Neurol Neurosurg Psychiatry 75:976–983
Horstink M, Tolosa E, Bonuccelli U, Deuschl G, Friedman A, Kanovsky P, Larsen JP, Lees A, Oertel W, Poewe W, Rascol O, Sampaio C (2006) Review of the therapeutic management of Parkinson’s disease. Report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES). Part II: late (complicated) Parkinson’s disease. Eur J Neurol 13:1186–1202
Isacson D, Bingefors K, Kristiansen IS, Nyholm D (2008) Fluctuating functions related to quality of life in advanced Parkinson disease: effects of duodenal levodopa infusion. Acta Neurol Scand 118:379–386
Kristiansen IS, Bingefors K, Nyholm D, Isacson D (2009) Short-term cost and health consequences of duodenal levodopa infusion in advanced Parkinson’s disease in Sweden: an exploratory study. Appl Health Econ Health Policy 7:167–180
Lindgren P, von Campenhausen S, Spottke E, Siebert U, Dodel R (2005) Cost of Parkinsons disease in Europe. Eur J Neurol 12:68–73
Lowin J, Bergman A, Chaudhuri KR, Findley LJ, Roeder C, Schifflers M, Wood E, Morris S (2011) A cost-effectiveness analysis of levodopa/carbidopa intestinal gel compared to standard care in late stage Parkinson’s disease in the UK. J Med Econ 14:584–593
Meissner W, Schreiter D, Volkmann J, Trottenberg T, Schneider GH, Sturm V, Deuschl G, Kupsch A (2005) Deep brain stimulation in late stage Parkinson’s disease: a retrospective cost analysis in Germany. J Neurol 252:218–223
Meissner W, Trottenberg T, Klaffke S, Paul G, Kühn AA, Arnold G, Einhäupl K-M, Kupsch A (2001) Apomorphinterapi versus tiefe Hirnstimulation. Nervenarzt 72:924–927
Nyholm D, Klangemo K, Johansson A (2012) Levodopa/carbidopa intestinal gel infusion long-term therapy in advanced Parkinson’s disease. Eur J Neurol
Olanow CW, Agid Y, Mizuno Y, Albanese A, Bonucelli U, Damier P, DeYebenes J, Gershanik O, Guttman M, Grandas F et al (2004) Levodopa in the treatment of Parkinson’s disease: current controversies. Mov Disord 19:997–1005
Pahwa R, Factor SA, Lyons KE, Ondo WG, Gronseth G, Bronte-Stewart H, Hallett M, Miyasaki J, Stevens J, Weiner WJ (2006) Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality standards subcommittee of the American Academy of Neruology. Neurology 66:983–995
Saelensminde K (2007) Helseeffekter i samfunnsøkonomiske analyser (Eng: Health effects in society economic analyses). Sosial og helsedirektoratet (The Norwegian directorate of health), Oslo (IS-1435)
Sintonen H (1994) The 15D-measure of health-related quality of life. I. Reliability, validity and sensitivity of its health state descriptive system. National Centre for Health Program Evaluation, Melbourne
Sintonen H (2001) The 15D instrument of health-related quality of life: properties and applications. Ann Med 33:328–336
Sintonen H (2012) The homepage of the 15D instrument: http://www.15d-instrument.net/15d. Terraventum
Stavem K (1999) Reliability, validity and responsiveness of two multiattribute utility measures in patients with chronic obstructive pulmonary disease. Qual Life Res 8:45–54
Stavem K, Bjornaes H, Lossius MI (2001) Properties of the 15D and EQ-5D utility measures in a community sample of people with epilepsy. Epilepsy Res 44:179–189
Stocchi F, Olanow CW (2004) Continuous dopaminergic stimulation in early and advanced Parkinson’s disease. Neurology 62:S56–S63
Tomaszewski KJ, Holloway RG (2001) Deep brain stimulation in the treatment of Parkinson’s disease: a cost-effectiveness analysis. Neurology 57:663–671
Valldeoriola F, Morsi O, Tolosa E, Rumia J, Marti MJ, Martinez-Martin P (2007) Prospective comparative study on cost-effectiveness of subthalamic stimulation and best medical treatment in advanced Parkinson’s disease. Mov Disord 22:2183–2191
Verhagen Metman L, Gillespie M, Farmer C, Bibbiani F, Konitsiosis S, Morris M, Shill H, Bara-Jimenez W, Mouradian MM, Chase TN (2001) Continuous transdermal dopaminergic stimulation in advanced Parkinsons disease. Clin Neuropharmacol 24:163–169
Widner H (2003) Strategies to modify levodopa treatment. Adv Neurol 91:229–236
Willis M, Persson U, Zoellner Y, Gradl B (2010) Reducing uncertainty in value-based pricing using evidence development agreements: the case of continuous intraduodenal infusion of levodopa/carbidopa (Duodopa(R)) in Sweden. Appl Health Econ Health Policy 8:377–386
Acknowledgments
Halldis Nedrebø and Hilde Slorafoss are gratefully acknowledged for help and support of the patients and data collection. Harri Sintonen is acknowledged for permission to use the 15D instrument and valuation algorithms. The study was performed with the help of an unspecified study grant from Solvay pharma which is gratefully acknowledged. Katarina Engman, Umbilicus Nordica AB, Umeå, Sweden is acknowledged for excellent monitoring of the study.
Conflicts of interest
Dr Lundqvist reports a study grant from Solvay Pharma during the conduct of the study and grants and personal fees from Abbvie (present manufacturer of the treatment studied) outside the study. Dr Sønbø Kristiansen reports collaboration with the manufacturer of Duodopa® about 10 years ago, and later with Abbvie on other products. Dr Reiertsen reports lecture fees from Abbvie outside the present work. There are no other conflicts of interests reported by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lundqvist, C., Beiske, A.G., Reiertsen, O. et al. Real life cost and quality of life associated with continuous intraduodenal levodopa infusion compared with oral treatment in Parkinson patients. J Neurol 261, 2438–2445 (2014). https://doi.org/10.1007/s00415-014-7515-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7515-4