Abstract
This study investigates how complex motor-cognitive activities are processed in the subthalamic nucleus (STN) and internal globus pallidum (GPi), as adverse neuropsychiatric effects may accompany deep brain stimulation (DBS), mainly in Parkinson’s disease (PD) and STN-DBS. Dystonia patients with GPi-DBS electrodes (n = 5) and PD subjects (n = 5) with STN-DBS electrodes performed two tasks: (1) copying letters; and (2) writing any letter other than that appearing on the monitor. The cognitive load of the second task was greater than that of the first. Intracranial local field potentials (LFPs) were analysed. A beta power decrease was the main correlate of the enhanced cognitive load during the second task in both structures, with a lateralization to the left side, mainly in the GPi. A gamma power increase linked with the increased cognitive activity was observed only in the STN. Differences were also observed in the theta and alpha bandpasses. Beta ERD reactivity seems to be essential during the processing of complex motor-cognitive tasks, increases with enhanced cognitive effort, and was observed in both the STN and GPi. Oscillatory reactivity to effortful cognitive processing in other frequency bands was less consistent, with differences between the studied nuclei. Lateralization of activity related to cognitive factors was observed mainly in the GPi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The subthalamic nucleus (STN) and internal globus pallidum (GPi) are common targets for deep brain stimulation (DBS) which is an effective long-term treatment for neurological patients with a variety of movement disorders. For some patients, the generally successful DBS may be accompanied by cognitive impairment and neuropsychiatric disorders, especially in the case of the STN in Parkinson’s disease (PD) patients (Saint-Cyr et al. 2000; Temel et al. 2006; Voon et al. 2006; Witt et al. 2008). GPi is used as DBS target in dystonia patients (Jankovic 2006; Kupsch et al. 2006; Vidailhet et al. 2013). In PD patients, similar motor improvements were observed with both STN and GPi DBS. There are some studies suggesting fewer adverse cognitive, behavioural, or neuropsychiatric events in GPi-DBS than in STN-DBS (Anderson et al. 2005; Videnovic and Metman 2008; Follett et al. 2010; Rouaud et al. 2010; Fasano and Deuschl 2012; Emre et al. 2014). A long-term multicentre study on bilateral STN and GPi-DBS reported the occurrence of cognitive decline in 23% of the STN patients and in 12% of the GPi patients 5–6 years after surgery (Moro et al. 2010). By contrast, other studies have not observed a significant difference in mood or cognition (Okun et al. 2009) except for depression that was significantly less frequent in patients with GPi DBS (Sako et al. 2014).
Intracranial recordings via DBS electrodes provide direct access to the deep brain nuclei and local field potentials (LFPs) analysis has contributed significantly to knowledge about the physiology of the basal ganglia and the pathophysiological changes associated with movement disorders, in particular PD. Synchronized oscillations in the beta and gamma frequencies have a key role in motor control in cortico-basal ganglia loops (Brown 2006). STN LFP activity in the beta bandpass is suppressed before self-paced voluntary movement as well as before cued movements (Williams et al. 2003; Alegre et al. 2005; Androulidakis et al. 2008; Oswal et al. 2012, 2013). Changes in beta activity are not linked only to the movement, but they are modulated by contextual factors like reward or cognitive complexity (Oswal et al. 2013). Synchronized low-frequency oscillations (5–13 Hz) in Parkinson’s disease increase after dopaminergic medication both in the STN and the GPi and are related to PD dyskinesias as well as to dystonic movements (Silberstein et al. 2003; Priori et al. 2004; Foffani et al. 2005; Marceglia et al. 2007; Barow et al. 2014). Their possible role in non-motor functions has been also documented (Fumagalli et al. 2011; Rosa et al. 2013).
The aim of our work was to study processing of cognitive activity in the basal ganglia and to compare neurophysiological changes during complex motor-cognitive processing in the STN and GPi.
Methods and materials
Subjects
Five dystonia patients implanted with GPi electrodes and five PD patients with STN targets participated in the study (see Table 1). UPDRS (Unified Parkinson’s Disease Rating Scale) and dystonia rating scales (Comella et al. 2003) were used for the evaluation of the current clinical condition. The recordings were performed in the postoperative period before the stimulator implantation and the system internalisation. All patients were considered appropriate candidates for DBS by the Commission for Neuromodulation Surgery in Brno. All subjects were informed about the nature of this study and gave their informed consent. The study received the approval of the local ethics committee. Before the operation, all subjects underwent a detailed neuropsychological examination that revealed no evidence of dementia.
Surgical procedure
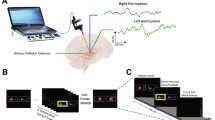
The stereotaxic frame used during the surgical procedure (electrode implantation) was the Leibinger open frame with the Praezis Plus software and the Talairach diagram. The STN coordinates used were in respect to the AC-PC (anterior commissure–posterior commissure) line: 12.0 mm laterally, 5.0 mm below, and 3.0 mm behind the midpoint of the AC-PC line. The GPi coordinates were: 20.0 mm laterally, 4.0 mm below, and 3.0 mm in front of the midpoint of the AC-PC line. The stimulation electrodes (Medtronic, Inc.) were implanted bilaterally into the targeted structure by stereotaxic MRI-guided technique under local anaesthesia (general anaesthesia in the GPi cases because of dystonia). The definitive electrode placement was confirmed by four microelectrode recordings. The motor part of the STN was identified by recording the specific patterns of neuronal activity and background activity, and by following motor responsiveness to intraoperative stimulation. Once the final target coordinates were determined, a permanent quadripolar DBS electrode (model 3389, with 1.5 mm contact length and 0.5 mm intercontact distance) was implanted. The electrode position was verified by the intraoperative use of fluoroscopy comparing the position of the microrecording electrodes trajectories with the definitive quadripolar macroelectrode trajectory. After surgery completion, CT scans under stereotactic conditions covering the entire length of the implanted electrodes were added. The series of images were reimported to the planning workstation and subsequently the coordinates were correlated with the real position of implanted electrodes. The change of electrode position is evident in the planning datasets so that the final electrode position can be evaluated without being burdened by artefacts caused by electrode material both in CT and MRI scans. Exact final electrode positions for each subject are presented in Fig. 1. In the immediate post-implantation period, the electrodes remained externalized. A special externalized cable enabled the intracranial EEG recording. The internalisation and the stimulator implantation were performed within a week after the positioning of the DBS electrodes.
Experimental protocol and recordings
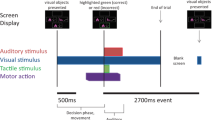
We used a visuomotor paradigm that we had employed in a previous study that investigated the neurophysiological correlates of increased task complexity and cognitive load on cortical structures and anterior nucleus of the thalamus in epilepsy surgery patients (Bočková et al. 2007, 2015). The visual stimuli were the letters of the alphabet presented in a random order on a monitor; there were 50 visual stimuli. Subjects performed two different visuomotor cognitive tasks. The first task was a simple cognitive task (Task 1)—copying letters from the monitor. The second task was a more complex cognitive task (Task 2)—writing a letter other than that which appeared on the monitor. The patients were instructed to write any letter other than the one on the monitor but not a letter that would immediately precede or follow that letter alphabetically. Patients received clear instructions and practised the task briefly before the recordings. They were asked to react immediately to the letter displayed on the monitor rather than engage in prolonged preparation and response planning.
The duration of the stimulus exposure was 200 ms; the interstimulus interval was 16 s.
Subjects reclined comfortably in the monitoring bed, in a quiet room, with a constant temperature. They were instructed to remain calm to keep their eyes fixed on the monitor, and to avoid unnecessary movements. The monitor was situated in the same place for all the subjects, 1.5 m in front of their eyes, at the end of the monitoring bed. Subjects wrote letters using an electrically connected pen. The paper the subjects wrote on was placed on a desk situated near their lower abdomen. The testing was visually supervised by the examiners and was also videotaped.
The intracerebral EEG signal was recorded by the EEG system TruScan 32 channel (Deymed Diagnostic, Alien Technic) and in subjects 4, 5 and 10 using the M&I EEG system because of EEG unit renovation. The recordings were monopolar, with a linked earlobe reference. The sampling rate was 1024 Hz with standard anti-aliasing filters before digitalization. In the trigger channel, the stimuli and motor reactions (pen-to-paper contact) were recorded, so that response onset time (RT) and duration of motor response could be monitored.
Data analysis
The data were processed and analysed off-line using ScopeWin and ScopeMat software. The data were segmented according to the stimulation trigger onset as we were mainly interested in the cognitive aspects of the task performance. The segments were visually inspected, and segments containing artificial signals or incorrect responses were removed. Between 20 and 50 segments were used for the final analysis. In each segment, the linear trend was eliminated. Bipolar montage evaluation was used to exclude the volume conduction from other structures, namely from the cortex or transsynaptic propagation along cortical-subcortical pathways (Wennberg and Lozano 2003, 2006) and confirm the local origin of the potentials. The bipolar power envelopes from two neighbouring contacts (contacts are presented in Fig. 1 for each subject) located within the targeted structure with eliminated phase-lock signals (subtraction of averaged trial) were computed in the determined frequency windows: theta 3–7 Hz, alpha 7–13 Hz, beta 13–35 Hz, and gamma (lower 35–80 Hz), using the Hilbert transform demodulation. The power envelopes were then averaged in single subject and in the GPi and STN subgroups of subjects to obtain grand averages (GA, see Figs. 3, 4). The statistical significance of ERS (event-related synchronization, power increase)/ERD (event-related desynchronization, power decrease) was analysed from the differences over trials between the mean power at baseline (1.6–0.1 s before stimuli) and the mean power in the 500 ms lasting segments. First segment starts at the 0 ms (stimuli position), second at 500 ms and last segment at 9500 ms position in trial. We used the non-parametric Wilcoxon rank sum (signed-rank) test for paired samples in each trial (Purcell et al. 2013) corrected for multiple comparisons across 20 moving segments. The differences between Task 1 and Task 2 were analysed with an unpaired t test; they are presented in Figs. 3, 4, and 6 (Kiebel et al. 2005) (see Fig. 2). Reaction times (RT) and motor response durations (defined from first pen touch to last pen liftoff) were also computed and correlated to the EEG changes; see Table 2, Figs. 5 and 6.
Example of beta ERD durations in one STN subject. Black curve Task 1, red curve Task 2. Red and black horizontal bars the ERS/ERD duration—statistical significance of differences between the mean power at the baseline region and the mean power in the window moving over the segment. Thick grey horizontal line the significant difference between Task 1 and Task 2
Results
We analysed oscillatory changes within the 3–80 Hz frequency ranges in five subjects with GPi electrodes and five subjects with STN electrodes. Figures 3 and 4 show GPi and STN grand averages in theta, alpha, beta, and gamma frequency ranges. In the theta bandpass, there were no significant differences between Task 1 and Task 2 observed in the two structures, except of higher activation (theta ERS) in Task 2 in the left STN. Alpha power ERD was observed on a comparable level in the STN during both tasks, in the GPi, a power decrease in the alpha frequency range was related only to Task 2. The most prominent oscillatory pattern related to the higher cognitive load was beta ERD in both structures. Beta ERD was higher and longer on both sides in the GPi. In the STN, the beta ERD was higher during Task 1 early after the stimulation, but lasted significantly longer during Task 2. Stronger gamma power ERS was found in the STN during Task 2; the opposite situation was found in the GPi. The increased beta ERD represents a pattern modification related to the increased cognitive complexity during Task 2, consistent with our previous recording studies from other brain structures (Bočková et al. 2007, 2015) and also described in a recent intracranial recording study in the subthalamic nucleus (Oswal et al. 2013).
Reaction times (RT) and motor response durations in each subject were computed and related to the beta ERD occurrence; see Table 2, Figs. 5 and 6 upper panel. Reaction times were markedly longer in Task 2 in all participants. This was not the case for the motor response duration, which was sometimes longer during Task 1 and sometimes during Task 2. The Task 2 related beta ERD was significantly longer in almost all cases. This prolonged activation was therefore not related to the longer motor performance, but to the cognitive processes. Figure 6 shows the differences between the two tasks in all subjects and grand averages. The differences between the tasks are presented in theta, alpha, beta, and gamma frequency ranges. There was a lateralization to the left side, mainly in the GPi in the beta frequency range, which was linked in this case with the enhanced cognitive load during Task 2.
Reaction times, motor responses, and beta ERD correlation. Boxplots of reaction times in ms from the left to the right: Mean reaction time, mean duration of reaction, duration of ERD for beta band power individually for left and right side. Significant difference (Wilcoxon, p ≤ 0.05) between Task 1 and Task 2 are marked by an asterisk
Task differences. The table introduces the temporal distribution of differences between tasks in GPi (left panel) and STN (right panel), and theta, alpha, beta, and gamma ranges in the 10-s interval after stimulation. Upper panel shows mean reaction time (point) and mean reaction duration (horizontal bar) for each of five GPI and five STN subjects. Black colour represents Task 1 and grey represents Task 2. Middle and bottom panels show Task 1 and Task 2 differences. Each horizontal bar represents a significant task difference in one subject. Grand averages (GA) are shown below each 5-patient block. Blue, grey, dark red, and light red horizontal bars represent durations of significant differences between Task 1 and Task 2: blue higher ERD during Task 2, grey higher ERD during Task 1, dark red higher ERS during Task 1, light red higher ERS during Task 2
Analysis with data segmented to the reaction onset (pen-to-paper contact)—see Figs. 7 and 8 was also performed to differentiate changes related only to movement performance from changes related to cognitive activity. The movement related activity starts immediately before and continue during motor reaction. Mean reaction time was significantly shorter than beta ERD duration—Table 2. Consequently, the higher beta power decrease can be observed not only immediately before the motor reaction onset, but significantly longer, i.e. 3–2 s before movement onset. This activity is therefore linked to increased cognitive load in Task 2 and demonstrates a true cognitive difference between Task 1 and Task 2.
Grand averages in GPi, movement onset cued analysis. Black curve Task 1, red curve Task 2. Thin black horizontal line statistical significance of the ERS/ERD, Task 1, thin red horizontal line statistical significance of the ERS/ERD, Task 2. Thick grey horizontal line significant differences between Task 1 and Task 2
Grand averages in STN, movement onset cued analysis. Black curve Task 1, red curve Task 2. Thin black horizontal line statistical significance of the ERS/ERD, Task 1. Thin red horizontal line statistical significance of the ERS/ERD, Task 2. Thick grey horizontal line significant differences between Task 1 and Task 2
In three of our PD patients, the recordings were performed in off as well as in on states. After levodopa intake, the beta power was slightly higher and prolongated, but without a statistical significance.
Discussion
In this study, we examined non-phase-locked event-related changes in oscillatory activities during a visuomotor task with two different levels of complexity. The power of oscillations may decrease (desynchronize) or increase (synchronize) during cognitive task performance. The main advantage of the event-related desynchronization/synchronization (ERD/S) methodology is the ability to distinguish between cortical inhibition and activation. We analysed power changes in the 2–80 Hz frequency range, i.e. within the theta, alpha, beta and gamma frequencies. The ERD of the alpha and beta rhythms has been interpreted as a correlate of activation, i.e. increased excitability of the cortex. The ERS in the alpha and lower beta bands has been interpreted as a correlate of a deactivation, i.e. cortical idling or active inhibition (Pfurtscheller 2001). Gamma band ERS is considered to be an elementary signal change with multiple functional correlates related to the information spread across brain networks (Basar-Eroglu et al. 1996; Schürmann et al. 1997; Crone et al. 1998; De Pascalis and Ray 1998; Pfurtscheller et al. 2003; Szurhaj et al. 2005; Ihara and Kakigi 2006).
We evaluated the human event-related EEG signal recorded via intracerebral depth electrodes.
Each electrode contained four 1.5 mm contacts with 0.5 mm intercontact distance. According to the postoperative CT scans and preoperative MRI scans fusion in the planning workstation, the real contacts positions were displayed. Contact pairs located directly in the targeted structures and the bipolar montages from these were used for the grand average analysis.
Our experimental protocol contained two tasks involving writing of single letters. The first task consisted of copying a letter from the screen; the second task required writing a different letter than that appearing on the screen. These two tasks are characterized by several shared cognitive components related to the writing of single letters. Specifically, the two tasks had in common the visual detection and reading of the letter on the screen and the preparation and execution of the writing movements. We supposed that attention and working memory were engaged to a comparable level in both experimental tasks, as the stimuli were the same in both conditions and the letters of the alphabet had to be maintained in working memory before and during motor execution. However, the second task was more complex and involved a higher cognitive load attributable to the additional requirement to perform several mental operations that are covered by the common term of executive function: the inhibition of an automatic (habitual) response (i.e. simply copying the letter shown on the screen) and the selection, planning, and execution of the alternative non-routine motor response based on a strategic memory search to retrieve a letter different from the one presented (Bočková et al. 2007). We therefore assumed that the most prominent difference between the two writing tasks is the higher cognitive effort and executive demand associated with the second task condition. The same experimental protocol was tested in studies with intracerebral recordings from the frontal, parietal and temporal cortices as well as from the anterior nucleus of the thalamus in patients with epilepsy. Consistent with the results reported here, the complex task elicited larger alpha and beta ERD than the simple task in several brain areas (Bočková et al. 2007, 2015).
Cognitive processes like decision making were reported to be linked with low-frequency (5–12 Hz) ERS in the STN (Marceglia et al. 2011). In this study, we did not observe any important changes within the low frequencies, except of higher theta ERS (which is known to be a correlate of activation) in Task 2 in the left STN. Activations in the alpha range (ERD) were present only during Task 2 in the GPi bilaterally. In contrast, there were similar activations during both tasks in the STN in the alpha frequency range. Higher gamma ERS was observed only in the STN bilaterally during Task 2. The increased cognitive demand produced mainly enhanced or prolonged local beta desynchronization in both structures; a lateralization to the left language-dominant hemisphere was observed mainly in the GPI; see Fig. 5.
Similar to the findings presented here are the results reported in another study from the STN. Perimovement activity was modified by cognitive factors; the beta power decreased while gamma power increased reciprocally (Oswal et al. 2013). The cognitive-motor integration required for successful execution of complex action sequences is mediated by basal ganglia-thalamocortical neural circuitry. In particular, the beta ERD appears to be a ubiquitous pattern reflecting complex cognitive activation in the brain. Consistent with this notion, the same oscillatory patterns were observed in frontal and temporal cortical structures and the anterior nucleus of the thalamus during performance of the cognitive-motor task used in this study (Bočková et al. 2007, 2015).
Differences between the STN and GPi, mainly the stronger lateralization in the GPi, and differences in the theta, alpha, and gamma oscillatory responses could be explained by different functional and anatomical connectivity of both structures (Manes et al. 2014). The STN is a basal ganglia input nucleus; the GPi is a major output nucleus of the BG-thalamocortical circuitry. The GPi, besides the excitatory inputs from the indirect pathway via the STN, receives inhibitory inputs from direct pathway striatal neurons, the thalamus, and brain stem nuclei (for review, see Nelson and Kreitzer 2014). The STN receives input in the circuitry from the indirect pathway as well as direct (hyperdirect) cortical input from the pericentral and prefrontal cortices (Haynes and Haber 2013). The cognitive role of the STN differs from the role of other structures within the BG circuitry; this difference is probably related to the function of the hyperdirect pathway. Based on direct depth electrode recordings, we suggested two ways that the STN may participate in cognitive activities. First, the STN is a relay nucleus that participates in processing cognitive and behavioural activities within the cortico-BG-thalamocortical loop. In addition to this known function, we suggested that the STN may (under the direct cortical control conveyed by the hyperdirect pathway) exert modulatory control over the output part of the cortico-BG-thalamocortical loop (Rektor et al. 2015). Difference in gamma reactivity between STN and GPi, i.e. higher gamma ERS linked with cognitive activity observed only in the STN might be related also to pedunculopontine nucleus (PPN) activity. This structure is connected with the STN and not with the GPI. PPN region has been studied as a novel target for DBS in PD. Improvement of levodopa-resistant motor symptoms like gait disorders, falls, freezing and postural imbalance was documented (Hamani et al. 2016; Mazzone et al. 2016; Fytagoridis et al. 2016). Individual PPN-DBS planning is required because of variability in the brainstem anatomy (Mazzone et al. 2013). The activation of PPN was linked with an increment in gamma band activity (Garcia-Rill 2015, Garcia-Rill et al. 2015, Urbano et al. 2016) and is proposed to participate in the process of preconscious awareness. Arousing stimuli simultaneously activate ascending projections of the PPN (Garcia-Rill et al. 2016).
Dopaminergic medication modifies the local field potentials in PD patients (Kühn et al. 2006; Androulidakis et al. 2007; Giannicola et al. 2010, 2013; Rodriguez-Oroz et al. 2011; Huebl et al. 2014). In three of our PD patients, we recorded the LFPs in both off and on states. Unfortunately, in the other two patients we could not record the on state because of time restrictions, and in one subject the quality of recording was not optimal. In general, oscillatory activity in the 8–35 Hz range is known to be suppressed after levodopa intake (Kühn et al. 2006; Giannicola et al. 2010). In this study, the beta power ERD slightly increased and was prolongated after levodopa intake in both the tasks, but without a statistical significance. Unfortunately, we could analyse ON state medication condition only in two subjects. Therefore, more data are needed to confirm our observation.
The main limitation of this study is that we recorded data from the STN in PD and from GPi in patients with dystonia. Differences in medication and disease pathophysiology may influence oscillatory patterns. Unfortunately, we did not have a sufficient number of GPi PD patients, as the STN is used as the primary target of choice for PD treatment at our centre. However, the main physiological oscillatory pattern related to the increased cognitive load, beta ERD enhancement, was observed in both structures regardless of the clinical diagnosis and treatment. Mainly, theta ERS is linked to abnormal involuntary movements, so we assume that dystonia should not greatly influence the beta frequency reactivity.
Conclusion
Beta ERD reactivity seems to be essential in processing complex motor-cognitive tasks. Beta increase with enhanced cognitive effort was observed in both the STN and GPi. Oscillatory reactivity to effortful cognitive processing in other frequency bands was less consistent with differences between the studied nuclei. The lateralization of activity related to cognitive factors was observed primarily in the GPi. It may be hypothesized that the differences in oscillatory patterns related to complex cognitive tasks in the GPi and in the STN is related to different anatomical and functional connectivity.
References
Alegre M, Alonso-Frech F, Rodríguez-Oroz MC, Guridi J, Zamarbide I, Valencia M, Manrique M, Obeso JA, Artieda J (2005) Movement-related changes in oscillatory activity in the human subthalamic nucleus: ipsilateral vs. contralateral movements. Eur J Neurosci 22:2315–2324
Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP (2005) Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol 62:554–560
Androulidakis AG, Kühn AA, Chen CC, Blomstedt P, Kempf F, Kupsch A, Schneider GH, Doyle L, Dowsey-Limousin P, Hariz MI, Brown P (2007) Dopaminergic therapy promotes lateralized motor activity in the subthalamic area in Parkinson’s disease. Brain 130(Pt 2):457–468
Androulidakis AG, Brücke C, Kempf F, Kupsch A, Aziz T, Ashkan K, Kühn AA, Brown P (2008) Amplitude modulation of oscillatory activity in the subthalamic nucleus during movement. Eur J Neurosci 27:1277–1284
Barow E, Neumann WJ, Brücke C, Huebl J, Horn A, Brown P, Krauss JK, Schneider GH, Kühn AA (2014) Deep brain stimulation suppresses pallidal low frequency activity in patients with phasic dystonic movements. Brain 137(Pt 11):3012–3024
Basar-Eroglu C, Struber D, Schurmann M, Stadler M, Basar E (1996) Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. Int J Psychophysiol 24:101–112
Bočková M, Chládek J, Jurák P, Halámek J, Rektor I (2007) Executive functions processed in the frontal and lateral temporal cortices: intracerebral study. Clin Neurophysiol 118:2625–2636
Bočková M, Chládek J, Jurák P, Halámek J, Stillová K, Baláž M, Chrastina J, Rektor I (2015) Complex motor-cognitive factors processed in the anterior nucleus of the thalamus: an Intracerebral Recording Study. Brain Topogr 28:269–278
Brown P (2006) Bad oscillations in Parkinson’s disease. J Neural Transm Suppl 70:27–30
Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T; Dystonia Study Group (2003) Rating scales for dystonia: a multicenter assessment. Mov Disord 18:303–312
Crone NE, Miglioretti D, Gordon B, Lesser R (1998) Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain 121:2301–2315
De Pascalis V, Ray WJ (1998) Effects of memory load on event-related patterns of 40-Hz EEG during cognitive and motor tasks. Int J Psychophysiol 28:301–315
Emre M, Ford PJ, Bilgiç B, Uç EY (2014) Cognitive impairment and dementia in Parkinson’s disease: practical issues and management. Mov Disord 29:663–672
Fasano A, Deuschl G (2012) Patients and DBS targets: is there any rationale for selecting them? Basal Ganglia 2:211–219
Foffani G, Ardolino G, Meda B, Egidi M, Rampini P, Caputo E, Baselli G, Priori A (2005) Altered subthalamo-pallidal synchronisation in parkinsonian dyskinesias. J Neurol Neurosurg Psychiatry 76:426–428
Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P; CSP 468 Study Group et al (2010) Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med 362:2077–2091
Fumagalli M, Giannicola G, Rosa M, Marceglia S, Lucchiari C, Mrakic-Sposta S, Servello D, Pacchetti C, Porta M, Sassi M, Zangaglia R, Franzini A, Albanese A, Romito L, Piacentini S, Zago S, Pravettoni G, Barbieri S, Priori A (2011) Conflict-dependent dynamic of subthalamic nucleus oscillations during moral decisions. Soc Neurosci 6:243–256
Fytagoridis A, Silburn PA, Coyne TJ, Thevathasan W (2016) Understanding the human pedunculopontine nucleus in Parkinson’s disease. J Neural Transm 123(7):769–774
Garcia-Rill E (2015) Waking and the reticular activating system in health and disease, 1st Edn. Elsevier, Netherlands
Garcia-Rill E, Luster B, D’Onofrio S, Mahaffey S (2015) Arousal, motor control, and parkinson’s disease. Transl Neurosci 6(1):198–207
Garcia-Rill E, Luster B, D’Onofrio S, Mahaffey S, Bisagno V, Urbano FJ (2016) Implications of gamma band activity in the pedunculopontine nucleus. J Neural Transm 123(7):655–665
Giannicola G, Marceglia S, Rossi L, Mrakic-Sposta S, Rampini P, Tamma F, Cogiamanian F, Barbieri S, Priori A (2010) The effects of levodopa and ongoing deep brain stimulation on subthalamic beta oscillations in Parkinson’s disease. Exp Neurol 226:120–127
Giannicola G, Rosa M, Marceglia S, Scelzo E, Rossi L, Servello D, Menghetti C, Pacchetti C, Zangaglia R, Locatelli M, Caputo E, Cogiamanian F, Ardolino G, Barbieri S, Priori A (2013) The effects of levodopa and deep brain stimulation on subthalamic local field low-frequency oscillations in Parkinson’s disease. Neurosignals 21:89–98
Hamani C, Lozano AM, Mazzone PA, Moro E, Hutchison W, Silburn PA et al (2016) Pedunculopontine nucleus region deep brain stimulation in Parkinson disease: surgical techniques, side effects, and postoperative imaging. Stereotact Funct Neurosurg 94(5):307–319
Haynes WI, Haber SN (2013) The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci 33:4804–4814
Huebl J, Spitzer B, Brücke C, Schönecker T, Kupsch A, Alesch F, Schneider GH, Kühn AA (2014) Oscillatory subthalamic nucleus activity is modulated by dopamine during emotional processing in Parkinson’s disease. Cortex 60:69–81
Ihara A, Kakigi R (2006) Oscillatory activity in the occipitotemporal area related to the visual perception of letters of a first/second language and pseudoletters. Neuroimage 29:789–796
Jankovic J (2006) Treatment of dystonia. Lancet Neurol 5:864–872
Kiebel SJ, Tallon-Baudry C, Friston KJ (2005) Parametric analysis of oscillatory activity as measured with EEG/MEG. Hum Brain Mapp 26:170–177
Kühn AA, Kupsch A, Schneider GH, Brown P (2006) Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci 23:1956–1960
Kupsch A, Benecke R, Müller J, Trottenberg T, Schneider GH, Poewe W et al (2006) Deep-Brain Stimulation for Dystonia Study Group. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 355:1978–1990
Manes JL, Parkinson AL, Larson CR, Greenlee JD, Eickhoff SB, Corcos DM, Robin DA (2014) Connectivity of the subthalamic nucleus and globus pallidus pars interna to regions within the speech network: a meta-analytic connectivity study. Hum Brain Mapp 35:3499–3516
Marceglia S, Bianchi AM, Baselli G, Foffani G, Cogiamanian F, Modugno N, Mrakic-Sposta S, Priori A, Cerutti S (2007) Interaction between rhythms in the human basal ganglia: application of bispectral analysis to local field potentials. IEEE Trans Neural Syst Rehabil Eng 15:483–492
Marceglia S, Fumagalli M, Priori A (2011) What neurophysiological recordings tell us about cognitive and behavioral functions of the human subthalamic nucleus. Expert Rev Neurother 11:139–149
Mazzone P, Sposato S, Insola A, Scarnati E (2013) The clinical effects of deep brain stimulation of the pedunculopontine tegmental nucleus in movement disorders may not be related to the anatomical target, leads location, and setup of electrical stimulation. Neurosurgery 73(5):894–906
Mazzone P, Vilela Filho O, Viselli F, Insola A, Sposato S, Vitale F, Scarnati E (2016) Our first decade of experience in deep brain stimulation of the brainstem: elucidating the mechanism of action of stimulation of the ventrolateral pontine tegmentum. J Neural Transm 123(7):751–767
Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J et al (2010) Longterm results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord 25:578–586
Nelson AB, Kreitzer AC (2014) Reassessing models of basal ganglia function and dysfunction. Annu Rev Neurosci 37:117–135
Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, Suelter M, Jacobson CE 4th, Wang X, Gordon CW Jr, Zeilman P, Romrell J, Martin P, Ward H, Rodriguez RL, Foote KD (2009) Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol 65:586–595
Oswal A, Litvak V, Sauleau P, Brown P (2012) Beta reactivity, prospective facilitation of executive processing, and its dependence on dopaminergic therapy in Parkinson’s disease. J Neurosci 32:9909–9916
Oswal A, Litvak V, Brücke C, Huebl J, Schneider GH, Kühn AA, Brown P (2013) Cognitive factors modulate activity within the human subthalamic nucleus during voluntary movement in Parkinson’s disease. J Neurosci 33:15815–15826
Pfurtscheller G (2001) Functional brain imaging based on ERD/ERS. Vision Res 41:1257–1260
Pfurtscheller G, Graimann B, Huggins JE, Levine SP, Schuh LA (2003) Spatiotemporal patterns of beta desynchronization and gamma synchronization in corticographic data during self-paced movement. Clinical Neurophysiolology 114:1226–1236
Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M et al (2004) Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Exp Neurol 189:369–379
Purcell BA, Schall JD, Woodman GF (2013) On the origin of event-related potentials indexing covert attentional selection during visual search: timing of selection by macaque frontal eye field and event-related potentials during pop-out search. J Neurophysiol 109:557–569
Rektor I, Bočková M, Chrastina J, Rektorová I, Baláž M (2015) The modulatory role of subthalamic nucleus in cognitive functions: a viewpoint. Clin Neurophysiol 126:653–658
Rodriguez-Oroz MC, López-Azcárate J, Garcia-Garcia D, Alegre M, Toledo J, Valencia M, Guridi J, Artieda J, Obeso JA (2011) Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain 134(Pt 1):36–49
Rosa M, Fumagalli M, Giannicola G, Marceglia S, Lucchiari C, Servello D, Franzini A, Pacchetti C, Romito L, Albanese A, Porta M, Pravettoni G, Priori A (2013) Pathological gambling in Parkinson’s disease: subthalamic oscillations during economics decisions. Mov Disord 28:1644–1652
Rouaud T, Dondaine T, Drapier S, Haegelen C, Lallement F, Péron J, Raoul S, Sauleau P, Vérin M (2010) Pallidal stimulation in advanced Parkinson’s patients with contraindications for subthalamic stimulation. Mov Disord 25:1839–1846
Saint-Cyr JA, Trépanier LL, Kumar R, Lozano AM, Lang AE (2000) Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain 123:2091–2108
Sako W, Miyazaki Y, Izumi Y, Kaji R (2014) Which target is best for patients with Parkinson’s disease? A meta-analysis of pallidal and subthalamic stimulation. J Neurol Neurosurg Psychiatry 85:982–986
Schürmann M, Basar-Eroglu C, Basar E (1997) Gamma responses in the EEG: elementary signals with multiple functional correlates. Neuro Rep 8:1793–1796
Silberstein P, Kuhn AA, Kupsch A et al (2003) Patterning of globus pallidus local field potentials differs between Parkinson’s disease and dystonia. Brain 126:2597–2608
Szurhaj W, Bourriez JL, Kahane P, Chauvel P, Mauguiere F, Derambure P (2005) Intracerebral study of gamma rhythm reactivity in the sensorimotor cortex. Eur J Neurosci 21:1223–1235
Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser-Vandewalle V (2006) Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Parkinsonism Related Dis 12:265–272
Urbano FJ, Luster BR, D’Onofrio S, Mahaffey S, Garcia-Rill E (2016) Recording gamma band oscillations in pedunculopontine nucleus neurons. J Vis Exp 14(115):e54685
Vidailhet M, Jutras MF, Roze E, Grabli D (2013) Deep brain stimulation for dystonia. Handb Clin Neurol 116:167–187
Videnovic A, Metman LV (2008) Deep brain stimulation for Parkinson’s disease: prevalence of adverse events and need for standardized reporting. Mov Dis 23:343–349
Voon V, Kubu C, Krack P, Houeto JL, Tröster AI (2006) Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Dis Suppl 14:S305–S327
Wennberg RA, Lozano AM (2003) Intracranial volume conduction of cortical spikes and sleep potentials recorded with deep brain stimulating electrodes. Clin Neurophysiol 114:1403–1418
Wennberg R, Lozano AM (2006) Restating the importance of bipolar recording in subcortical nuclei. Clin Neurophysiol 117:474–475
Williams D, Kühn A, Kupsch A, Tijssen M, van Bruggen G, Speelman H, Hotton G, Yarrow K, Brown P (2003) Behavioural cues are associated with modulations of synchronous oscillations in the human subthalamic nucleus. Brain 126:1975–1985
Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO et al (2008) Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol 7:605–614
Acknowledgements
This research has been financially supported by the Ministry of Education, Youth and Sports of the Czech Republic under the project CEITEC 2020 (LQ1601). The study was also supported by Grant AZV 16-33798A and has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 734718 (CoBeN). The technical part of this study was supported by Grant GA GACR P103/11/0933. The technical part of the study was also supported with institutional support RVO:68081731, MEYS CR (LO1212) together with EC (ALISI No. CZ.1.05/2.1.00/01.0017). We wish to thank prof. Novák and Ing. Říha for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bočková, M., Chládek, J., Jurák, P. et al. Oscillatory reactivity to effortful cognitive processing in the subthalamic nucleus and internal pallidum: a depth electrode EEG study. J Neural Transm 124, 841–852 (2017). https://doi.org/10.1007/s00702-017-1719-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-017-1719-6