Abstract
Cognitive adverse effects were reported after the deep brain stimulation (DBS) of the anterior nucleus of the thalamus (AN) in epilepsy. As the AN may have an influence on widespread neocortical networks, we hypothesized that the AN, in addition to its participation in memory processing, may also participate in cognitive activities linked with the frontal neocortical structures. The aim of this study was to investigate whether the AN might participate in complex motor–cognitive activities. Three pharmacoresistant epilepsy patients implanted with AN–DBS electrodes performed two tasks involving the writing of single letters: (1) copying letters from a monitor; and (2) writing of any letter other than that appearing on the monitor. The cognitive load of the second task was increased. The task-related oscillatory changes and evoked potentials were assessed. Local event-related alpha and beta desynchronization were more expressed during the second task while the lower gamma synchronization decreased. The local field event-related potentials were elicited by the two tasks without any specific differences. The AN participates in cognitive networks processing complex motor–cognitive tasks. Attention should be paid to executive functions in subjects undergoing AN–DBS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deep brain stimulation (DBS) of different brain targets has been successfully used to treat various neurological and psychiatric disorders. Since the SANTE Study (Fisher et al. 2010) was published, DBS of the anterior nucleus of the thalamus (AN) has become a promising method of treating refractory epilepsy. For some patients, especially those with advanced Parkinson’s disease (PD), generally successful DBS may be accompanied by cognitive impairment and neuropsychiatric disorders (Saint-Cyr et al. 2000; Herzog et al. 2003; Anderson et al. 2005; Temel et al. 2005, 2006; Voon et al. 2006; Witt et al. 2008; Ballanger et al. 2009; Okun et al. 2009). That is because the basal ganglia, particularly the subthalamic nucleus, which is the most common stimulation target, participate in the cognitive neuronal circuits (Parent and Hazrati 1995; Baunez and Robbins 1999; Graybiel 1997; Aron et al. 2007; Benarroch 2008; Baláž et al. 2008, 2010, Bočková et al. 2011). Little is known about the involvement of AN in cognitive activities in human subjects. It is probable that AN–DBS produces memory changes. A recent experimental study in rats (Hamani et al. 2010) as well as studies in human subjects (Fisher et al 2010) showed that high frequency electrical stimulation of AN could decrease performance in memory paradigms. This may be explained by the position of the AN within the Papez circuit. The AN may also have an influence on widespread neocortical networks, particularly in the frontal lobe. The AN projects to the cingulate gyrus, which has well-documented roles in self-regulation and executive cognition (Jones et al. 2006), with further projections to wide regions of the neocortex (Zhong et al. 2011). The AN is also a key structure in the intrathalamic pathways. We hypothesized that the AN, in addition to its participation in memory processing, may also participate in other cognitive activities, namely in those that are processed in networks comprising the frontal neocortical structures. For these reasons, we focused on complex motor–cognitive tasks as it is known that willed and automatic actions are controlled at different levels depending on the degree of task difficulty and complexity (Norman and Shalice 1986; Luu and Tucker 2002).
We recorded electrical activity linked with cognitive functions via DBS electrodes implanted in the AN. Intracranial recording studies provide direct access to the subcortical structures. The aim of this study was to investigate whether the AN might participate in processing higher order cognitive activities. If so, AN–DBS might lead to adverse effects in the studied domains, and attention should be paid to these functions in human subjects undergoing AN–DBS. We have not seen any published data regarding the higher order cognitive functions related to the AN or in patients with AN–DBS.
Materials and Methods
Subjects
Three pharmacoresistant epilepsy patients with DBS macroelectrodes implanted in the AN target participated in the study (see Table 1). The recordings were performed in the postoperative period before the stimulator implantation and the system internalisation. Two patients had a previous surgical intervention and all patients had undergone unsuccessful vagal nerve stimulation treatment; the device was finally explanted. All patients were indicated for the DBS surgery by the Commission for Neuromodulation Surgery of the Brno Epilepsy Centre. All the subjects were informed about the character of this study and gave their informed consent. The study received the approval of the local ethics committee. Before the operation, all subjects underwent a detailed neuropsychological examination (Wechsler adult intelligence scale, Wechsler memory scale III, Stroop test, Rey-osterrieth complex figure test, Verbal fluency test, Boston naming test, Hamilton anxiety scale, Montgomery-asberg depression rating scale). The examination showed no signs of dementia. Patients intellect ranged from the population average to borderline deficiency. All patients had a slight to moderate impairment of executive functions, diminished quality of performance was particularly in psychomotor speed and attentional functions. Basic symbolic functions were without specific disorders. Mnestic abilities varied from average to mild impairment.
Surgical Procedure
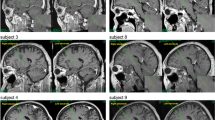
The stereotactic frame used during the surgical procedure was the Leibinger ceramic open frame with the Praezis Plus software and the Talairach and Schaltenbrand Bailey atlases. The initial coordinates for the anterior thalamic nucleus as related to the AC–PC (anterior commissure–posterior commissure) line centre were 0–2 mm anterior to the midpoint, 5.5 mm laterally, and 10–12 mm above AC–PC line. The entry point for the electrode was planned at the proximity of coronal suture. The final target was modified according to local anatomy, and particular attention was paid to the safe distance of the trajectory from thalamostriate vein and choroid plexus. All four electrode contacts were planned to be inside thalamic structures. The stimulation leads (Medtronic, Inc.) were implanted bilaterally into the targeted structure by stereotactic MRI-guided technique under local anaesthesia. Intraoperative micro recordings to guide lead placement were employed. Intraoperative stimulation was used to test possible adverse effects. Once the final target coordinates were defined, a permanent quadripolar DBS electrode (model 3389, with 1.5 mm contact length and 0.5 mm intercontact distance) was implanted. The electrode position was verified by the intraoperative use of fluoroscopy comparing the position of the micro recording electrodes trajectories with the definitive quadripolar macro electrode trajectory. After surgery completion, CT scans under stereotactic conditions covering the entire length of the implanted electrodes were added. The series of images were reimported to the planning workstation and subsequently the coordinates were correlated with the real position of implanted electrodes (as shown in Fig. 1). The change of electrode position is evident in the planning data sets so that the final electrode position can be evaluated without being burdened by artefacts caused by electrode material both in CT and MRI scans. The positions of the electrodes and their contacts in the brain were also later verified using post-placement magnetic resonance imaging with electrodes in situ.
The real electrode and all contacts positions are shown for both sides as defined by the fusion of postoperative CT with preoperative MRI scans in the planning workstation. MRI projections are along the electrode trajectory. An image from the Talairach atlas (Talairach et al. 1967) in approximately same position is shown. Contacts 4 are placed in the borderline between the AN and the lateral ventricle. Contacts 3 are all placed in the AN. Contacts 2 are localized in the AN or on the border between the AN and the DMN of the thalamus. Contacts 1 are all located in the DMN of the thalamus
Experimental Protocol and Recordings
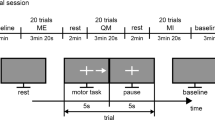
We used a complex visuomotor paradigm with an increased load of complex cognitive functions that we had used in an earlier work for studying the frontal, parietal, and temporal cortices (Bočková et al. 2007). The visual stimuli were the letters of the alphabet presented in a random order on a monitor. Subjects performed two different visuomotor cognitive tasks. The first task was a simple cognitive task (task 1)—copying letters from the monitor. The second task was a more complex cognitive task (task 2)—writing a letter other than that which appeared on the monitor. The patients were instructed to write any letter other than the one on the monitor but not a letter that would immediately precede or follow that letter alphabetically. Patients received clear instructions and practised the task briefly before the recordings. They were asked to react immediately to the letter displayed on the monitor, and not to pre-prepare their responses.
The duration of the stimulus exposure was 200 ms; the interstimulus interval was 16 s.
Subjects reclined comfortably in the monitoring bed, in a quiet room, with a constant temperature. They were instructed to remain calm, to keep their eyes fixed on the monitor, and to avoid unnecessary movements. The monitor was situated in the same place for all the subjects, 1.5 m in front of their eyes, at the end of the monitoring bed. Subjects wrote letters using an electrically connected pen. The paper the subjects wrote on was on a desk situated near their lower abdomen. The testing was visually supervised by the examiners and was also videotaped.
The intracerebral EEG signal was recorded by the M&I EEG system. The recordings were monopolar, with a linked earlobe reference. The sampling rate was 20 kHz with standard anti-aliasing filters before digitalization. The signal was thereafter off-line filtered in the band pass up to 300 Hz and downsampled to 1 kHz. The filter was based on Fourier transformation. In the trigger channel, the stimuli and reactions (pen-to-paper contact) were recorded, so the response onset time could be monitored.
Data Analysis
The data were processed and analysed off-line using ScopeWin and ScopeMat software. The data were first segmented according to the stimulation trigger onset. The segments were visually inspected, and segments containing artificial signals or mistaken responses were removed. In each segment the linear trend was eliminated.
Time frequency analysis (TFA) (Akay 2000) with eliminated phase-lock signals (subtraction of averaged trial) was used to determine the event related desynchronization/synchronization (ERD/S) in 2–80 Hz frequency ranges (see Fig. 3). TFA produces a matrix in which each row represents the over trials averaged signal power envelopes in a 4 Hz frequency band width (x-axis represents time; y-axis represents frequency). The frequency step between two rows was 1 Hz. The signal power envelope within rows was evaluated by Hilbert transform demodulation. One matrix thus contains, in the vertical axis, power envelopes for the frequencies 2–6, 3–7, and up to 76–80 Hz, with 75 rows in total. The horizontal axis of the matrix represents the time intervals from 4 s before stimulation to 12 s after simulation. In the baseline-normalized TFA matrix, ERS is represented by positive values (red) and ERD by negative values (blue). Normalization with a baseline was done according the equation:
PWbaseline—mean power from baseline, PW(t)—instantaneous power. The limitation of ±100 of normalized values is used. The scale in the TFA matrix in the figures is +100, −100. The +100 value (red) means a doubling of instantaneous power with respect to the baseline region; the −100 value (blue) means a drop by half. This procedure provides a comparable colour interpretation of ERS and ERD.
The statistical significance of ERS/ERD was analysed from the differences over trials between the mean power at baseline and the mean power in the window moving over segment. Statistical significance is presented in a matrix where the significant changes to baseline are dark red, light red, dark blue, and light blue. Dark red identifies a power increase when P < 0.01, light red a power increase when P < 0.05, dark blue a power decrease when P < 0.01, and light blue a power decrease when P < 0.05. White regions of this matrix identify non-significant changes to baseline.
To identify differences between stimuli in time–frequency interpretation, we computed an inter-stimulus statistical significance matrix—Fig. 2, right panel. Each point of the matrix represents the significance of differences between tasks in the corresponding time and frequency position. In each time–frequency position, we tested the differences of two vectors that represent the power envelope value in the appropriate frequency range and time position over trials from task1 and task2. We used a non-paired t test to determine significance. In Fig. 2, dark grey represents P < 0.01 and light grey P < 0.05. The significance is after correction, as the same data are used two times.
ERD/ERS in 2–80 Hz frequency range and intervals −4 and 12 s before and after stimuli expressed by TFA, in bipolar montage analysis. The TFA with statistical significance to baseline region (baseline region is determined by green vertical lines) is shown. Red identifies a power increase (synchronization), blue a power decrease (desynchronization). On the top both TFA power and TFA with statistical significance in subject 1 are presented. Local field lower gamma ERS can be observed during both tasks with writing single letters in subjects 1 and 2, weaker during task 2. Specific higher activation—alpha and beta ERD—related to increased complexity appeared during task 2 in all subjects: in subjects 1 and 2 on the left side and in subject 3 on both sides. Left panel task 1, middle panel task 2, right panel task 2–task 1 difference (Color figure online)
Event-related potentials (ERPs) were given in averaged segments filtered in 0.1–40 Hz bandwidths. The baseline interval was determined as 1,600–100 ms prior to stimuli. The statistical significance of ERPs to baseline was analysed in a similar way as for ERS/ERD, analysing the differences over trials between the mean computed during the baseline and the mean computed inside the moving interval with a length of one-third of baseline. Non-parametric Wilcoxon rank sum (signed rank) test for paired samples was used. The ERP were considered as significant when P < 0.05, indicated by black rectangles in Fig. 3.
ERPs in bipolar montage analysis. The black rectangle defines the area up to 1 s after stimulus, in which statistically significant differences to baseline can be observed. In subject 1, local phase reversals (generated in contacts L3 and R4—highlighted in black fields) are shown; in subject 2, local amplitude gradients are shown (with the maximum in contacts L4 and R4—highlighted in black fields, except on the right side during task 2, where no significant changes were found)
Bipolar montage evaluation was used to exclude the volume conduction from other structures, namely from the cortex or transsynaptic propagation along cortical-subcortical pathways (Wennberg and Lozano 2003, 2006) and confirm the local origin of the potentials. Contacts in the thalamus were placed very close together. Any EEG signal from the common reference was eliminated by a bipolar montage. Even minor bipolar montage activity displays the origins of detected activity in the AN.
Results
As the first step, we analysed oscillatory changes. Figure 2 shows TFA with statistical significance where a local AN alpha and beta ERD was more expressed during task 2 and comprised a wider frequency range on the left side in subject 1 and 2, and on both sides in subject 3. This represents a pattern modification related to the increased complexity during task 2, which is known from our previous study (Bocková et al. 2007) and was described in a recent intracranial recording study in the subthalamic nucleus (STN) (Oswal et al. 2013). This ERD started around 500 ms in subjects 1 and 3. It started after 2 s in subject 2. Because the reaction times started after 1,500 ms, we assume the later ERD in subject 2 also related to the task performance (see Table 2). We observed these oscillatory changes in the left sided electrodes in all patients, and in patient 3 also in the right sided electrode. Whether this lateralization has a physiological significance, e.g. a relation to the lateralization of the reading and writing to the left hemisphere, remains to be examined on a larger population of tested subjects.
In addition, we found significant lower gamma (around 30–40 Hz) ERS during both tasks in subjects 1 and 2. The gamma power reciprocally also slightly decreased while the alpha and beta power decreased with the higher complexity during the task 2.
As the second step we analysed ERPs. Figure 3 displays ERPs in bipolar montage analysis where we observed AN area local field evoked potentials on both the left and right sides in two subjects during both visual cognitive motor tasks with writing single letters, without any specific differences between the tasks. An early peak around 100–200 ms, probably corresponding to the N2 component known from scalp recordings, was followed by a slow wave ERP lasting between 300 and 750 ms, peaking at 300–500 ms. As the slow wave formed a plateau, the exact latency of the peak could only be estimated approximately. In the bipolar montage, subject 1 displayed phase reversal (generated in contacts L3 and R4) and subject 2 an amplitude gradient (with the maximum in contacts L4 and R4) in the AN area with both tasks. This indicates the involvement of the AN area in cognitive processes that are common to both tasks, i.e. the detection and reading of the letter on the screen and the preparation and the execution of writing. ERPs in subject 3 were not analysable because of prominent slow spontaneous activity of unknown origin with a frequency around 2 Hz that disturbed the evoked potentials (on the other hand, oscillations on frequencies higher than 2 Hz remained analysable).
Discussion
We studied the non-phase-locked event-related changes in the oscillatory activities, as well as phase-locked cognitive event-related electrical activity, i.e. ERPs. In the first case, the power of oscillations may decrease (desynchronize) or increase (synchronize). The main advantage of the ERD/S methodology in comparison to ERPs is the ability to distinguish between cortical inhibition and activation. We analysed power changes in the 2–80 Hz frequency range, i.e. within the theta, alpha, beta, and low and high gamma frequencies. The ERD of the alpha and beta rhythms has been interpreted as a correlate of activation, i.e. increased excitability of the cortex. The ERS in the alpha and lower beta bands has been interpreted as a correlate of a deactivation, i.e. cortical idling or active inhibition (Pfurtscheller 2001). Gamma band ERS seems to be an elementary signal change with multiple functional correlates related to the information spread across brain networks (Basar-Eroglu et al. 1996; Schürmann et al. 1997; Crone et al. 1998; De Pascalis and Ray 1998; Pfurtscheller et al. 2003; Szurhaj et al. 2005; Ihara and Kakigi 2006).
Our experimental protocol contained two tasks with writing single letters. The first task consisted of copying a letter from the screen; the second task required writing a different letter than that appearing on the screen. These two tasks are characterized by several common aspects linked with the writing of single letters. The two tasks had in common: visual detection and reading of the letter on the screen and the preparation and execution of writing. We supposed the attention and working memory load to be engaged on a comparable level in both of the tasks in our experimental protocol as the stimuli were same for both tasks—the letters of the alphabet had to be kept in working memory before and during the task performance. The second task was more complex and less automated and demanded a higher cognitive load which comprised several components, including a higher demand on long-term memory (retrieval of the letters of the alphabet) and several acts that are covered by the common term of executive function: the inhibition of automatic (habitual) responses and the selection, planning, and execution of the chosen response. We assumed that the most prominent difference between the tasks is the higher complexity in the second task. The same experimental protocol was tested in a study with intracerebral recordings from the frontal, parietal and temporal cortices. In several frontal and temporal neocortical areas, the complex task elicited larger alpha and beta ERD than the simple task (Bočková et al. 2007).
As AN–DBS may affect frontal lobe as well as temporal lobe epilepsy, we assumed that cortical regions that had been activated by our tasks could be in functional relation with the AN. AN stimulation produces EEG changes in the frontal and temporal cortex (Kerrigan et al. 2004) and inhibits frontal and temporal lobe seizures.
In this study, the enhanced cognitive demand in task 2 led to an enhanced desynchronization of the oscillations in the AN area in all tested subjects. A large alpha and beta ERD appeared during the complex task in contrast with no significant changes or weak ERD during the simple task. A reciprocal lower gamma ERS decreased with the enhanced complexity. Similar findings were reported in a study from the subthalamic nucleus in PD patients. Peri movement activity was modified by cognitive factors; the beta power decreased while gamma power increased reciprocally (Oswal et al. 2013). We observed gamma power reduction during task 2. Both structures displayed the same desynchronization in the lower frequencies, which is probably a correlate of a mental effort. While the degree of difference between the oscillatory changes elicited by the two tasks varied across the subjects, the general pattern could be detected in all subjects (see Fig. 2). Subject 1 had the largest difference between reaction times in the two tasks. That is probably because in this subject, the increased mental effort in the more complex task was clearly apparent and was reflected by the alpha and beta ERD enhancement. In subject 2, there was no difference in reaction times during the two tasks and the task 2 reaction time was the shortest within the group. We believe that in this case the mental effort increase was the lowest and was reflected by a weak ERD. This subject was the youngest and had the highest score in the presurgical IQ test. Brighter individuals might be characterized by lower and topographically more differentiated brain activation (alpha band ERD) than less intelligent individuals (Neubauer et al. 2006). The later latency of the ERD onset for subject 2 might be explained by the fact that this ERD is just a fragment of the ERD generally observed in the other subjects, probably active stimulus–response remapping (Oswal et al. 2013). Each subject can have an individual strategy for processing complex cognitive tasks. In this case, the ERD started after the movement onset, while in the other two subjects it started before the movement. The ERD in subject 2 was also related to the complex cognitive task performance, as the general pattern of gamma ERS reduction/alpha and beta ERD increase was also present in this subject. Subject 3 had the longest reaction times, and there was a clear difference in reaction times between the tasks with appropriate ERD changes linked to high mental effort.
We also observed cognitive activity related evoked potentials in two subjects (in subject 3 the ERPs were not analysable due to strong slow external activity that disturbed the phase-locked changes in the thalamus). Steep amplitude gradients and phase reversals were observed between neighbouring contacts L3–L4 and R3–R4 (the ERPs are shown in Fig. 3). These evoked potentials and the low gamma ERS were observed during both tasks of our experimental protocol and probably reflect common processes, e.g. attention, working memory, and reading and writing letters. It confirms that the AN participates in processing complex cognitive visuomotor tasks.
The ERD/ERS pattern is interpreted in general as a thalamocortical mechanism to facilitate focal activation and information processing (focal ERD) by simultaneous deactivation or inhibition of other cortical areas (surround ERS) with the goal of optimizing the energy demand in task-related cortical areas (Pfurtscheller 2006). There is no reason to speculate that the ERD/ERS in the AN should represent another function than it does in the cortex. The second task related occurrence of alpha–beta ERD indicates that the AN participates actively in the cortico-subcortical network processing cognitive functions. The AN stimulation might influence complex cognitive functions via pathways connecting the AN with the prefrontal cortex.
We evaluated the human event-related EEG signal recorded via intracerebral depth electrodes.
Each electrode contained four 1.5 mm contacts with 0.5 mm intercontact distance. Not all contacts were inserted directly to the anterior thalamus; some were placed in its proximity. According to the postoperative CT scans and post-placement MRI, mainly L3 and R3 contacts were placed in the area that anatomically corresponds to the anterior thalamus. The local ERD sources were observed in L2–L3 and R2–R3 montages, i.e. directly in the AN. In subject 2, we observed the local generation of ERD during task 2 on the left side in more distal contacts between L1–2. Contact L1 was located in the dorsal medial nucleus (DMN) so this activity could have been generated in the AN as well as in the DMN. As we observed the origin of this LFP in the AN in the other subjects, we suggest that the ERD in subject 2 more probably also originated in the AN. A possible bias might be due to the small size of the AN from which we could record activity produced in the neighbouring structures. In another study, the verification of DBS electrode placement indicated that structures other than the AN were also stimulated, namely the thalamic nuclei latero-polaris, reticulatus polaris, ventro-oralis internus, and campus Forelii (Osorio et al. 2007). We assume that we recorded mainly from the AN area but some influence of neighbouring thalamic structures cannot be excluded.
Intracerebral recordings are performed on epileptic patients, and we cannot fully exclude the influence of the pathological process and of drugs on the recorded electrical activity. The depth electrodes are submerged in the brain tissue and record from their immediate vicinity. The data are thus obtained directly from subcortical structures, which are inaccessible by scalp measurement. It is not possible to record from the deep brain structures other than in patients with diseases affecting the brain. On the other hand, no interictal epileptiform activity has been recorded in the human AN. We should be careful in interpreting our data in the light of these limitations.
More clinical and experimental studies focused on the possible impact of the high frequency stimulation of the AN on cognitive functions are needed in order to find optimal stimulation parameters with clinical effect and minimalise possible cognitive adverse effects.
Conclusion
The anterior thalamus and frontal and temporal areas participate in cognitive networks processing complex visuomotor tasks. Therefore AN stimulation could influence performance of higher mental cognitive operations. More attention should be paid to complex cognitive functions in subjects undergoing AN–DBS.
References
Akay M (2000) Time frequency and wavelets in biomedical signal processing. Wiley, New York
Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP (2005) Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol 62(4):554–560
Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA (2007) Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27(14):3743–3752
Baláž M, Rektor I, Pulkrábek J (2008) Participation of the subthalamic nucleus in executive functions: an intracerebral recording study. Mov Disord 23(4):553–557
Baláž M, Srovnalová H, Rektorová I, Rektor I (2010) The effect of cortical repetitive transcranial magnetic stimulation on cognitive event-related potentials recorded in the subthalamic nucleus. Exp Brain Res 203(2):317–327
Ballanger B, van Eimeren T, Moro E, Lozano AM, Hamani C, Boulinguez P, Pellecchia G, Houle S, Poon YY, Lang AE, Strafella AP (2009) Stimulation of the subthalamic nucleus and impulsivity: release your horses. Ann Neurol 66(6):817–824
Basar-Eroglu C, Struber D, Schurmann M, Stadler M, Basar E (1996) Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. Int J Psychophysiol 24(1):101–112
Baunez C, Robbins TW (1999) Effects of transient inactivation of the subthalamic nucleus by local muscimol and APV infusions on performance on the five-choice serial reaction time task in rats. Psychopharmacology 141(1):57–65
Benarroch EE (2008) Subthalamic nucleus and its connections: anatomic substrate for the network effects of deep brain stimulation. Neurology 70(21):1991–1995
Bočková M, Chládek J, Jurák P, Halámek J, Rektor I (2007) Executive functions processed in the frontal and lateral temporal cortices: intracerebral study. Clin Neurophysiol 118(12):2625–2636
Bočková M, Chládek J, Jurák P, Halámek J, Baláž M, Rektor I (2011) Involvement of the subthalamic nucleus and globus pallidus internus in attention. J Neural Transm 118(8):1235–1245
Crone NE, Miglioretti D, Gordon B, Lesser R (1998) Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain 121(12):2301–2315
De Pascalis V, Ray WJ (1998) Effects of memory load on event-related patterns of 40-Hz EEG during cognitive and motor tasks. Int J Psychophysiol 28(3):301–315
Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, SANTE Study Group et al (2010) Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51:899–908
Graybiel AM (1997) The basal ganglia and cognitive pattern generators. Schizophr Bull 23(3):459–469
Hamani C, Dubiela FP, Soares JC, Shin D, Bittencourt S, Covolan L, Carlen PL, Laxton AW, Hodaie M, Stone SS, Ha Y, Hutchison WD, Lozano AM, Mello LE, Oliveira MG (2010) Anterior thalamus deep brain stimulation at high current impairs memory in rats. Exp Neurol 225(1):154–162
Herzog J, Reiff J, Krack P, Witt K, Schrader B, Müller D, Deuschl G (2003) Manic episode with psychotic symptoms induced by subthalamic nucleus stimulation in a patient with Parkinson’s disease. Mov Disord 18(11):1382–1384
Ihara A, Kakigi R (2006) Oscillatory activity in the occipitotemporal area related to the visual perception of letters of a first/second language and pseudoletters. Neuroimage 29(3):789–796
Jones BF, Barnes J, Uylings HB, Fox NC, Frost C, Witter MP, Scheltens P (2006) Differential regional atrophy of the cingulate gyrus in Alzheimer disease: a volumetric MRI study. Cereb Cortex 16(12):1701–1708
Kerrigan JF, Litt B, Fisher RS, Cranstoun S, French JA, Blum DE, Dichter M, Shetter A, Baltuch G, Jaggi J, Krone S, Brodie M, Rise M, Graves N (2004) Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia 45(4):346–354
Luu P, Tucker DM (2002) Self-regulation and the executive functions: electrophysiological clues. In: Zani A, Proverbio AM (eds) The cognitive electrophysiology of mind and brain. Academic Press, San Diego, pp 199–223
Neubauer AC, Fink A, Grabner RH (2006) Sensitivity of alpha band ERD to individual differences in cognition. Prog Brain Res 159:167–178
Norman DA, Shalice T (1986) Attention to action: willed and automatic control of behaviour. In: Davidson RJ, Schwartz GE, Shapiro D (eds) Consciousness and self- regulation. Plenum, New York, pp 1–18
Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F et al (2009) Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol 65(5):586–595
Osorio I, Overman J, Giftakis J, Wilkinson SB (2007) High frequency thalamic stimulation for inoperable mesial temporal epilepsy. Epilepsia 48(8):1561–1571
Oswal A, Litvak V, Brücke C, Huebl J, Schneider GH, Kühn AA, Brown P (2013) Cognitive factors modulate activity within the human subthalamic nucleus during voluntary movement in Parkinson’s disease. J Neurosci 33(40):15815–15826
Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev 20(1):128–154
Pfurtscheller G (2001) Functional brain imaging based on ERD/ERS. Vis Res 41(10):1257–1260
Pfurtscheller G (2006) The cortical activation model (CAM). Prog Brain Res 159:19–27
Pfurtscheller G, Graimann B, Huggins JE, Levine SP, Schuh LA (2003) Spatiotemporal patterns of beta desynchronization and gamma synchronization in corticographic data during self-paced movement. Clin Neurophysiol 114(7):1226–1236
Saint-Cyr JA, Trépanier LL, Kumar R, Lozano AM, Lang AE (2000) Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain 123(10):2091–2108
Schürmann M, Basar-Eroglu C, Basar E (1997) Gamma responses in the EEG: elementary signals with multiple functional correlates. NeuroReport 8(7):1793–1796
Szurhaj W, Bourriez JL, Kahane P, Chauvel P, Mauguiere F, Derambure P (2005) Intracerebral study of gamma rhythm reactivity in the sensorimotor cortex. Eur J Neurosci 21(5):1223–1235
Talairach J, Szikla G, Tournoux P, Prosalentis A, Bordas-Ferrer M, Covello J, Jacob M, Mempel H, Suser P, Bancaud J (1967) Atlas d´Anatomie Stereotaxique du Telencephale. Masson, Paris
Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V (2005) The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol 76(6):393–413
Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser-Vandewalle V (2006) Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Park Relat Disord 12(5):265–272
Voon V, Kubu C, Krack P, Houeto JL, Tröster AI (2006) Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord 14(S14):S305–S327
Wennberg RA, Lozano AM (2003) Intracranial volume conduction of cortical spikes and sleep potentials recorded with deep brain stimulating electrodes. Clin Neurophysiol 114(8):1403–1418
Wennberg R, Lozano AM (2006) Restating the importance of bipolar recording in subcortical nuclei. Clin Neurophysiol 117(2):474–475
Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO et al (2008) Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol 7(7):605–614
Zhong XL, Yu JT, Zhang Q, Wang ND, Tan L (2011) Deep brain stimulation for epilepsy in clinical practice and in animal models. Brain Res Bull 85(3):81–88
Acknowledgments
The study was supported by CEITEC. *CZ.1.05/1.1.00/02.0068*. The technical part of this study was supported by Grant GA GACR P103/11/0933. The technical part of the study was also supported by the project Application Laboratories of Advanced Micro technologies and Nanotechnologies, CZ.1.05/2.1.00/01.0017, co-funded by the Operational Programme ‘Research and Development for Innovations’, the European regional development fund, and the state budget. We wish to thank prof. Brázdil, prof. Kuba, prof. Novák and ing. Říha for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bočková, M., Chládek, J., Jurák, P. et al. Complex Motor–Cognitive Factors Processed in the Anterior Nucleus of the Thalamus: An Intracerebral Recording Study. Brain Topogr 28, 269–278 (2015). https://doi.org/10.1007/s10548-014-0373-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-014-0373-7