Abstract
The region of the pedunculopontine tegmental nucleus (PPTg) has been proposed as a novel target for deep brain stimulation (DBS) to treat levodopa resistant symptoms in motor disorders. Recently, the anatomical organization of the brainstem has been revised and four new distinct structures have been represented in the ventrolateral pontine tegmentum area in which the PPTg was previously identified. Given this anatomical reassessment, and considering the increasing of our experience, in this paper we revisit the value of DBS applied to that area. The reappraisal of clinical outcomes in the light of this revisitation may also help to understand the consequences of DBS applied to structures located in the ventrolateral pontine tegmentum, apart from the PPTg. The implantation of 39 leads in 32 patients suffering from Parkinson’s disease (PD, 27 patients) and progressive supranuclear palsy (PSP, four patients) allowed us to reach two major conclusions. The first is that the results of the advancement of our technique in brainstem DBS matches the revision of brainstem anatomy. The second is that anatomical and functional aspects of our findings may help to explain how DBS acts when applied in the brainstem and to identify the differences when it is applied either in the brainstem or in the subthalamic nucleus. Finally, in this paper we discuss how the loss of neurons in brainstem nuclei occurring in both PD and PSP, the results of intraoperative recording of somatosensory evoked potentials, and the improvement of postural control during DBS point toward the potential role of ascending sensory pathways and/or other structures in mediating the effects of DBS applied in the ventrolateral pontine tegmentum region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pioneering models of neuronal pathways involved in Parkinson’s disease (PD) were based on blocks and wiring diagrams representing the major centers and connections in cortico-basal ganglia-cortical circuitry (DeLong 1990; Albin et al. 1995). These schematic representations were continuously updated as new findings on neuronal properties appeared, and, finally, brainstem nuclei were also considered to play a role in the proposed neuronal models (Wichmann and DeLong 2001; Braak and Del Tredici 2008). The loss of dopaminergic neurons that occurs in PD was hypothesized to disrupt the functional relationships in basal ganglia circuitry and, consequently, the activity of thalamic pathways that modulate the excitability of the cerebral cortex was thought to be considerably modified. With time, evidence was provided showing that increased irregular activity characterized neurons in the subthalamic nucleus (STN) and inner segment of the globus pallidus (GPi) in PD (Wichmann et al. 1994; Bergman et al. 1994). Thus, these structures were first considered as targets for deep brain stimulation (DBS) to treat PD motor symptoms.
The possibility of targeting other structures, in particular in the subrubral regions to the substantia nigra, was not considered feasible in the past because of the risk of damaging pontomesencephalic structures (Talairach et al. 1957). At the time, neurosurgeons had little experience with the stereotactic approach to pontomesencephalic nuclei, and the scientific literature was scanty, limited to one paper for DBS in pain (Young et al. 1992).
It has been in 2000 that Pahapill and Lozano, on the basis of animal and clinical studies, indicated the PPTg for potential surgery in parkinsonism. Following experimental studies in animals (Jenkinson et al. 2004; Takakusaki et al. 2004) the first implantations for PPTg DBS were then carried out (Mazzone et al. 2005a, b; Plaha and Gill 2005). However, the scant representation of the PPTg in human atlases existing at that time, and the different acronyms used by various authors: Tg.pd.po (Schaltenbrand and Wahren 1977), PPN (Lavoie and Parent 1994a; Pahapill and Lozano 2000; Zrinzo et al. 2008), PPTg (Olszewski and Baxter 1954; Paxinos and Huang 1995) caused some misunderstandings that were clarified as the number of implanted patients raised.
Both animal and human applications followed a rationale based on knowledge of cortico-basal ganglia circuitry existing at the time, and were also prompted by the suspicion that non-dopaminergic mechanisms could be involved in the pathogenesis of drug-resistant symptoms, such as gait and axial disturbances (Bonnet et al. 1987).
The debate concerning the functions of the PPTg was further complicated by different points of view expressed by some authors (Winn 2008; Gut and Winn 2015). Undoubtedly the PPTg is involved in arousal/attentional functions, as exhaustively discussed in a recent Garcia-Rill’s book (2015). But, as far as the motor nature of the PPTg, to settle things once and for all, there is undeniable evidence: it is part of the mesencephalic locomotor region (Skinner and Garcia-Rill 1984; Takakusaki et al. 2003; Karachi et al. 2012; Sherman et al. 2015), establishes relationships with basal ganglia (Edley and Graybiel 1983; Jackson and Crossman 1983; Sugimoto and Hattori 1984; Rye et al. 1987, 1988; Lee et al. 1988; Lavoie and Parent 1994a, b; Futami et al. 1995; Aravamuthan et al. 2007; Dautan et al. 2014), deep cerebellar nuclei and cerebral cortex (Hazrati and Parent 1992; Ruggiero et al. 1997; Muthusamy et al. 2007), sends fibers to brainstem neurons from which spinal cord-directed pathways originate (Rye et al. 1988; Skinner et al. 1990; Grofova and Keane 1991; Sherman et al. 2015), integrates vestibular, auditory, proprioceptive and sensorimotor signals (Krauthamer et al. 1995; Reese et al. 1995a, b; Kobayashi and Isa 2002; Aravamuthan and Angelaki 2012; Okada and Kobayashi 2013, 2014; Hong and Hikosaka 2014). Finally, a fMRI study showed activation of the PPTg in an imaginary walking task in health volunteers (Karachi et al. 2012) and activation of many presumed PPTg neurons intrasurgically recorded in PD patients have been reported to change their activity in relation to different aspects of locomotor imagery tasks and during voluntary and passive limb movements (Weinberger et al. 2008; Tattersall et al. 2014; Lau et al. 2015).
The results of the few groups who have performed PPTg DBS are not readily comparable because of the limited number of patients studied, the inclusion criteria, the surgical approaches, the postoperative methods used to evaluate clinical results, and the neuroradiological documentation of the implanted region provided in some studies, in which it is difficult to evaluate the real position of the stimulating contact pair with respect to anatomical landmarks and structures close to the PPTg.
Paxinos et al. (2012) recently attempted to harmonize the rodent and primate literature on the basis of anatomical and cytoarchitectonic homologies. The result is a profoundly modified terminology and spatial organization of tegmental structures compared with what it is reported in other atlases.
Given this innovative reorganization and terminology, it would be appropriate to carry out a revisitation of our results of PPTg DBS.
The revisitation that we present in this paper may help in clarifying a number of issues which have caused in the past some controversy, i.e. (1) the lack of appropriate representations of brainstem nuclei in currently available human stereotactic atlases, (2) the differences in clinical outcomes reported by different authors (Ferraye et al. 2010; Moro et al. 2010; Thevathasan et al. 2011, 2012; Khan et al. 2012; Schrader et al. 2013; Welter et al. 2015), and (3) the potential differences between the mechanism of action of DBS applied in the brainstem or in classical targets such as the STN. In our argumentation concerning the above issues, the precise location of the stimulating lead in the ventrolateral pontine tegmentum [by this term we mean a broader area that includes not only the pedunculopontine tegmental nucleus (PPTg) as formerly represented by Paxinos and Huang (1995), but also the new structures reported in their revision (2012) of the pontine tegmentum] and the neuronal loss that occurs in the brainstem both in PD and progressive supranuclear palsy (PSP) have a relevant role.
For a better understanding of our experience with brainstem DBS, we have organized this review according to four major headings dealing with surgery, patients and identification of the PPTg targeted area; correspondence between 3D modelling of brainstem nuclei and postoperative neuroradiology; effectiveness of PPTg DBS in improving postural control, and finally, usefulness of recording the somatosensory evoked potentials (SEPs) for correct targeting of the PPTg region.
Surgical procedure and reconsideration of the PPTg targeted area according to the revisitation of brainstem nuclei proposed by Paxinos and et al. (2012)
Our surgical procedures have been described in detail in a previous paper (Mazzone et al. 2013). In patients operated up to 2007 (Table 1, Group 1, early DBS), we performed bilateral implantations, targeting the PPTg and an additional nucleus chosen from traditional targets. i.e. either the GPi or the STN. Quadripolar electrodes (Medtronic®, Minneapolis, USA) were used, the 3389 for the PPTg and STN, and the 3387 for the GPi. In patients operated after 2007 (Table 1, Group 2, early DBS) we implanted only the PPTg in the side opposite to the most compromised hemisoma and/or considering the dominant hemisphere.
Up to now, we have implanted 39 leads in the PPTg in 32 patients. Of the 32 patients, 30 underwent surgery at the Stereotactic Unit of the A. Alesini CTO Hospital in Rome (ASL RM2) performed by the same neurosurgeon (PM), while two patients were implanted at the Stereotactic and Functional Neurosurgery Service, Medical School, Federal University of Goias, Goiânia, Goias, Brazil (by OFV) in strict accordance with the stereotactic procedures established in Rome. The procedure was approved by local ethics committees and all patients gave their informed consent to participation in the study. The selection criteria (relatively young age, absence of general medical disorders and cognitive impairment, absence of cerebrovascular diseases, prevalence of levodopa-refractory symptoms) were in agreement with those considered in the initial investigational application of PPTg DBS. In this regards, we want to stress once again the importance of considering eligible for PPTg DBS only patients in which a presurgical stereotaxically-based reconstruction of brain vessels guarantees to plan a lead trajectory with no risk of vessel damage. To give further safety to our surgery, we decided to avoid intraoperative micro-electrophysiological recordings of neuronal activity (IOMERS) after we realized that no useful firing pattern could be recorded to safely identify the nucleus, and considering also the risk of adverse hemorrhagic events that could be caused by penetrating the brain with the thin metal microelectrodes required by IOMERS.
Thus, we preferred to utilize the lead contacts for recording SEPs during surgery to be certain of the targeted site, as we will discuss in detail below. In such a way we have had no intraoperative adverse events and the procedure of implanting leads was not riskier in the PPTg than in other structures.
The clinical data and results have been detailed in previous papers (Mazzone et al. 2008, 2009, 2011, 2013, 2014). Since the six patients included in Group 1 have been largely described in a previous report (Stefani et al. 2007), in this review we focus mainly on results obtained for the 29 patients included in Group 2 (Table 1, late DBS), (26 males, 3 females; mean age: 61.2 ± 13.9 years; duration of disease: 9.8 ± 4.1 years; levodopa-equivalent daily dose: 950 ± 325 mg). The 29 patients in the Group 2 include the patients in which leads in traditional targets (GPi or STN) were removed and never replaced, thus maintaining only the PPTg stimulation.
Included in Group 2 there are also three recently implanted patients, one of whom implanted with an octapolar lead (Boston Scientific®, Marlborough, MA, USA).
The electrode implantation in the planned site was performed with the Maranello Stereotactic Apparatus (CLS Titanium, Forlì, Italy). The PPTg target planning was based on stereotactic angio-CT scans, and used the pontomesencephalic junction line (PMJ), the ventricular floor line (VFL), and the Obex as anatomical reference markers (Mazzone et al. 2013). Traditional stereotactic coordinates, standardized with respect to the line connecting the anterior and posterior commissures (AC–PC), were used for targeting the STN and the GPi.
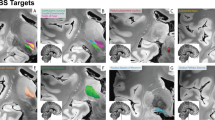
Since there was marked individual variability among patients in the height of the midbrain, the AC–PC line could be not employed in planning the insertion of the lead into the brainstem. Thus, we used individualized reference points that could easily be detected by using both angio-CT scans and the 2D Maranello stereotactic planning tool. Representative examples of stereotactic planning that was done with such reference landmarks are shown in Fig. 1a, b. Once the tip of the lead reached the planned target in the pons, intraoperative SEPs were recorded and analysed to correlate their waveforms with the position of the contacts of the lead and their distance from both the Obex and the Medial Lemniscus.
Representative examples of the 3D stereotactic planning. a Octapolar lead (red with black contacts) implantation according to the Paxinos et al. (2012) representation of brainstem nuclei, b quadripolar lead implantation according to Paxinos and Huang representation (1995). In the 2012 representation the region in which the PPTg pars compacta and pars disseminata b were earlier represented is populated by the isthmic reticular formation (isRt), the retroisthmic nucleus (RIs) and the pedunculotegmental nucleus. Note the larger extent of the cuneiform nucleus (CuN, green). The ventrolateral tegmental nucleus (VLTg), the Centrum Medianum-Parafascicularis Nucleus (CM-Pf, orange), the third ventricle (white) and the Medial Lemniscus (ML, yellow) are reported in both representations. The 2D elliptic yellow representation of the slice Tc 0 of the Schaltembrandt and Wahren’s brainstem atlas indicates the PMJ level. The midline and the Ventricular Floor Line (VFL) are represented by yellow crossed lines. The faint staining of structures in the 3D image makes it possible to appreciate the position of lead contacts with respect to the above mentioned structures
Figure 2 shows a comparison between planned and real X, Y, and Z coordinates. The values included in the figure allow the reader to appreciate the distance of the deepest contact from the specific reference points, i.e. the PMJ for the Z coordinate, the distance from the midline for the X coordinate, and the distance from the VFL for the Y coordinate. The differences between planned and actual coordinates concerning the zero contact were not significant for the X, Y and Z coordinates (X = 6.9 ± 0.7 vs 6.4 ± 1.8 mm; Y = 6.5 ± 1.2 vs 6.2 ± 1.5 mm; Z = 4.8 ± 2.7 vs 4.2 ± 1.6 mm, mean ± SD, one way-ANOVA followed a post hoc Newman-Keuls test, P > 0.05 all comparisons). These minimal differences confirm the high level of reliability of our planning method and stereotactic system.
Comparison between planned and real Z, X and Y coordinates (mean ± SD). The differences were not statistically significant (P > 0.05, one way ANOVA). The large SD of the realized Z coordinate was consistent with the high variability from patient to patient of the distal contact of the lead with respect to anatomical landmarks (PMJ and Obex)
After setting up the SEPs recording methods in patients in Group 1, SEPs were routinely recorded under general anaesthesia for patients included in Group 2, the PPTg being located in its surgical space medially with respect to the medial lemniscus, as illustrated in the former atlas of Paxinos and Huang (1995). Upon completing the SEP recordings, X-ray images were taken along the anteroposterior and laterolateral planes with the head of the patient in the stereotactic frame. In this way, it was possible to verify discrepancies between the planned and the actual coordinates by means of the 3P Maranello recalculation system software. The final position of the lead was further verified after surgery by MRI (Philips Gyroscan 0.1 T) (Fig. 3) in 28 patients, or by computerized tomography in three of the 31 implanted patients. Surgery was followed by a 15 day test period in which clinical evaluation of patients was carried out with the Unified Parkinson’s Disease Rating Scale (UPDRS) and with the Hoehn and Yahr scale during DBS OFF and DBS ON, in DRUGS OFF and DRUGS ON conditions (Table 2). Different active contact pairs were tested, and we varied the stimulation configuration, i.e. monopolar vs bipolar, continuous vs cyclic as detailed in a previous paper (Mazzone et al. 2013). For STN and GPi a high frequency of stimulation (160 Hz) was applied, whereas the PPTg area was stimulated at a low frequency (not higher than 40 Hz). The characteristics of stimulation and parameters settings are summarized in Table 3. No adverse events occurred either during or after surgery. In our 10 years’ experience with pontine DBS, 3 out of the 32 implanted patients died 7 years after surgery for supervening diseases, unrelated to both PPTg implantation and neurological diseases. Oscillopsias, trigeminal pain and urinary incontinence were never observed in either the postsurgical follow-up or under PPTg stimulation. The most common consequences of PPTg DBS were paresthesias in the hemisoma contralateral to the implanted side, which were felt by patients as stimulation was switched on or, for a while, when electrical parameters changed, particularly when the stimulation amplitude was increased. Within a short time, the paresthesias decreased and, finally, disappeared.
Representative axial (a, d), coronal (b, e) and sagittal (c, f) postoperative MRI slices in a patient implanted with a 3389 Medtronic quadripolar lead (a–c) and in a patient implanted with a Boston Scientific octapolar lead (d–f). The lead was located in the lateroventral region of the pontine tegmentum, as can be appreciated in the axial slices. The deepest lead contacts were clearly below the PMJ, as shown in the sagittal slices. Note the hypointense formation corresponding to the Medial Lemniscus, and the distance between the deepest contact and the Obex
We measured the distances between the contacts of the lead and surrounding structures, paying particular attention to the medial lemniscus, and correlated the distances with the electrical field that was generated by the stimulation (Fig. 4). We also considered the distance between the centre of each contact and the line perpendicular to the major axis of the following structures: the medial lemniscus, the superior cerebellar peduncle, the spinothalamic tract, and the mesencephalic tract of the fifth cranial nerve. By measuring these distances, we could estimate whether the representation of the electric field included structures that could cause phenomena reported by other authors, such as oscillopsias, incontinence or trigeminal pain, never observed in our practice. Given the spread of the electrical field, it is reasonable to hypothesize that, except for fibres responsible for paresthesias, there was no involvement of structures that could cause unwanted effects. In five patients from Group 1, the STN or GPi pulse generator was removed 3–4 years after implantation because of an infection in the subclavicular pocket hosting it. Interestingly, once the generator was removed, the patients, under sole PPTg DBS, still showed the benefits previously obtained using dual stimulation, and hence there was no need to implant a second generator for STN or GPI leads. This unexpected result strengthens the potential of using the PPTg as a target for DBS.
Real electrical field generated by a 3389 Medtronic lead contact (blue 1000 V/m; red 200 V/m; green 100 V/m). The dotted white lines represent the distance between the center of the contact and the center of specific anatomical structures surrounding the lead i.e. a Medial Lemniscus (ML, yellow), b spinothalamic tract (spth, orange), c mesencephalic tract of the fifth cranial nerve (not colored), d superior cerebellar peduncle (SCP, cyan). The position of these sensory pathways, which may be affected by the electrical field, may be inferred in the slice +33 of the Paxinos and Huang’s atlas (1995) which corresponds to the axial level of the PMJ in MRI. The pars compacta and pars disseminata of the PPTg are represented in violet and blue, respectively
We established the position of the leads by making reference to slices of the Paxinos and Huang’s brainstem atlas (1995) since it provided a more detailed representation of the PPTg region compared with other atlases, in which the PPTg region was partially represented or not reported at all. In that atlas the PPTg pars compacta (slices from +36 to +33) and pars dissipata (slices from +35 to +31) were represented in slices from +31 to +36 with respect to the Obex (Fig. 5a).
Comparison of brainstem nuclei in different Paxinos representations of brainstem nuclei. a Slice +33 according to the Paxinos and Huang’s atlas (1995): Pedunculopontine tegmental nucleus pars disseminata (PPTg, light blue), Pedunculopontine tegmental nucleus pars compacta (PPTg, gray), Cuneiform nucleus (CuN, green) and Medial Lemniscus (ML, yellow). b Slice +33 according to the Paxinos et al. (2012) revisitation of brainstem nuclei: isthmic reticular formation (isRt, dark blue), pedunculotegmental nucleus (PTg, gray), retroisthmic nucleus (RIs, blue), ML (yellow) and CuN (green). The superior cerebellar peduncle (SCP, cyan), the fourth ventricle (black) and the fourth cranial nerve provide landmarks in both representations. Note the differences in nomenclature, extent and size of different nuclei. The PPTg pars compacta and pars disseminata are no longer represented in the 2012 Paxinos et al. revisitation. c–e Averaged axial position of single contacts in 27 of the 32 patients we implanted according to the 2012 Paxinos revisitation. Contacts are indicated in white (contact 0), orange (contact 1), blue (contact 2) and black (contact 3). The slices +35 (c), +33 (d, corresponding to the PMJ) and +31 [in which the ventrolateral tegmental nucleus (VLTg) is represented] are the most important for the purpose of brainstem DBS. The numbers of contacts that were considered to estimate the mean values of x and y coordinates have been reported in each cylinder
We now need to reconsider the position of lead contacts according to the new description of brainstem nuclei conceived by Paxinos et al. (2012). In this representation the PPTg is no longer indicated as such, being replaced by three nuclear formations. The first is the pedunculotegmental nucleus (PTg), reported in slices from +31 to +36 from the Obex, which partially occupies the position in which the PPTg pars compacta was represented in the former atlas up to slice +33. The PPTg pars disseminata, as indicated in the previous atlas, should now correspond to two nuclear structures labelled as isthmic reticular formation (isRt, slices from +38 to +33) and retroisthmic nucleus (RIs, slices from +35 to +31). Moreover, the isRt in the new representation exceeds (slices +38 and +37) the previous rostral extent of the pars dissipata (Fig. 5b). The ventrolateral tegmental nucleus (VLTg, slices from +32 to +20), located in a ventrolateral position with respect to the RIs, is relevant to the revisitation of our work since the position of the active contact pair of the leads we implanted in 21 patients appears to be located deeply in the pons, virtually in correspondence with the VLTg (Fig. 5c–e). As a matter of fact, these structures (PTg, isRt, and RIs) were not present in the old atlas in the axial slices whereas the VLTg was reported in two slices, laterally to the inferior representation of the pars dissipata of the PPTg (slices +32 and +31).
According to the proposed new nomenclature and spatial organization of brainstem nuclei, most of the active contacts in our patients would be in the RIs and VLTg (Fig. 5c–e); in three patients they would have reached a rather lateral and deeper region of the VLTg (Fig. 6a, b).
a Three examples of the deeper position of the lead with respect to the axial extension of brainstem nuclei proposed by Paxinos and Huang (1995) and by Paxinos et al. (2012). b The lead was clearly located below the PMJ. The right panel shows the 3D representation of the lead, positioned in correspondence of the middle level of the VLTg according to the 1995 Paxinos and Huang’ representation, while the left panel refers to the Paxinos et al. revisitation (2012). c Left panels magnification of brainstem nuclei organization in the two Paxinos representations. c Right panels overlappings of the Tc 0 slice of Schaltembrand and Wahren atlas with the 3D brainstem nuclei anatomy based on the Paxinos and Huang (1995) (top); with the 3D anatomy based on the Olzesky and Baxter’s atlas (middle) and, finally, with the Schaltembrand and Wahren’s atlas (bottom). Abbreviations and colors as in Fig. 7b
All things considered, the 3D reconstruction that has been possible to obtain from the new slices is consistent with the reconstruction made by examining the old slices from +31 to +36. However, in the actual reconstruction the anatomical position of the contacts may be represented more accurately and it can be seen that in most cases the deepest contacts were inside the ventrolateral pontine tegmentum, while the highest contacts were localized in different structures, depending on the angles of the lead trajectory along the sagittal and frontal planes.
There is a caveat, however, to the revised anatomical organization by Paxinos et al. (2012). A previous study of the make-up of the PPTg showed that cholinergic, glutamatergic and GABAergic neurons of the rat PPTg are anteroposteriorly intermingled in both pars compacta and pars dissipata (Wang and Morales 2009). The pars compacta is equivalent to what they now refer to as PTg, while the pars dissipata appears to be equivalent to what they label RIs. This suggests that functionally, the cell populations may be anatomically spread but they represent the same functional entity. Similar studies need to be carried out in the human in order to determine if indeed cells in the PTg, isRt and RIs represent a functional entity or not.
As far as the cuneiform nucleus is concerned, in the new Paxinos’ atlas it is represented in its original position, dorsally to the PTg-isRt-RIs complex. Its axial representation do not extend beyond the PMJ, thus it lies in a position that never coincided with that of the most proximal contacts of our implanted leads. Hence, a direct involvement of the cuneiform nucleus in the effects we observed following PPTg DBS may be excluded. In this regard, it should be noted that the former concept expressed by Takakusaki et al. (2003, 2004), who ascribed to the cuneiform nucleus a major role in initiating gait and to the PPTg a major role in modulating skeletal muscle tone during locomotor movements in decerebrated cats, should not be interpreted as suggestive of two functionally separated structures since the axons of PPTg cholinergic axons give raise to widespread ascending projections in the upper brainstem (Dautan et al. 2014) and, in addition, these neurons may have 2–9 primary dendrites that extend for hundreds of microns (Reese et al. 1995c). Thus, further studies would be needed to elucidate if and how stimulation of the cuneiform nucleus may affect gait and posture in the absence of PPTg neurons and taking also into account the interspecies differences existing in the several pathways that may have a role in the effects of PPTg DBS (Alam et al. 2011).
In the future, a great help to answer to the problems presented above may come from the use of octapolar leads. In this context, the advantages offered by the implantation in the PPTg of an octapolar lead compared with a traditional quadripolar 3389 one may be better understood reexamining what it is represented in Fig. 1a. The eight contacts of the octapolar would allow to investigate the clinical effect of DBS applied in structures located at different heights above the +36 slice. In such a way a better discrimination of the effects of stimulating the cuneiform, subcuneiform and PPTg would be feasible and, in addition, it would be possible to stimulate simultaneously or separately thalamic nuclei, such as the parafascicular nucleus, and midbrain structures. Thus, the potentiality of the octapolar lead would offer a valuable tool in improving our possibility to intervene simultaneously in more than one structure along a single lead track.
The correspondence between 3D modelling of brainstem nuclei and postoperative neuroradiology
We established preoperatively the dimensions of the brainstem by volumetric MRI in order to measure brainstem anatomical parameters in each patient and obtain 3D-models of brainstem nuclei and sensory pathways. Rhinoceros® software ver. 3 SR4 was used for constructing the 3D representations from 29 out of the 32 patients and data were included in the MedicoCad planning navigational tool of the Maranello Stereotactic System. The same method was adopted for constructing 3D representations of the quadripolar and octapolar leads, in which the active contacts were included in actual size (Fig. 6b). The 3D reconstruction was first employed for planning and successively evaluating the postsurgical relationships between lead contacts and the PPTg representation. Figure 3 shows the postoperative control of a 3389 Medtronic (a–c) and of a Boston Scientific octapolar lead (d–f), respectively. The 3D reconstruction of brainstem nuclei that we provide was performed according to Paxinos’ revised anatomy of the PPTg area, as described above. An additional schema useful for both surgical planning and establishing lead position was obtained through a mixed 3D reconstruction that included anatomic representations reported in different atlases (Fig. 6c). The overlapping of PPTg-related slices from different atlases in the mixed 3D reconstruction makes it possible to minimize differences that occur in planning the implantation and in evaluating the actual position of the lead in the postoperative MRI (Fig. 6c). This 3D approach, on the one hand made it possible to visualize and evaluate the spatial correlations existing between the lead and the various nuclei reported in different atlases, on the other hand allowed us to evaluate the correlations of lead contacts with recorded SEPs. Moreover, the 3D reconstruction of the cerebral vascular system added safety to the surgical procedure giving the neurosurgeon the opportunity to avoid potential conflicts between the chosen lead trajectory and vessels.
Improved postural control in patients subjected to PPTg DBS
Most studies concerning the effect of PPTg DBS on motor control were based on subjective evaluation of traditional disability scales, and thus there is still a substantial lack of quantitative evaluation of motor parameters. We attempted to address this issue by objective analysis of oromandibular movements, gait, surface EMG and H-reflex (Pierantozzi et al. 2008; Caliandro et al. 2011; Mazzone et al. 2012, 2014). The bulk of results obtained from those studies clearly showed that under PPTg DBS patients improved their motor capabilities, and facilitation of hindlimb motoneurons occurred. These observations are in agreement with the clinical data discussed above, which support benefits in motor control by stimulating the region that, according to the Paxinos’ revision, should correspond to the ventrolateral pontine tegmentum. As far as postural stability is concerned, PD patients investigated in stabilometric tests have shown a reduction of the sway ellipse (SE) and total length of oscillation (TL) (Beuter et al. 2008; Blaszczyk and Orawiec 2011; Mancini et al. 2012; Panyakaew et al. 2015). The rigidity and lack of adequate postural control in these patients often resulted in falls, and under the eyes closed condition the postural control worsened. We are investigating static postural control in PD patients using a stabilometric platform, and the data collected to date support that improvement of postural stability may be achieved under PPTg DBS. The study is considering the reduction of falls in everyday life, the variations of SE and TL in the stabilometric test before and during stimulation and in the presence or absence of levodopa treatment.
So far, we have studied eight unilaterally implanted male PD patients (mean age 63.8 ± 10.4 years; disease duration: 12.0 ± 6.2 years; Hoehn and Yahr score: 3.7 ± 0.76) who showed severe signs of postural instability without tremor or diskynesia.
In explaining our data we must first consider that in PD patients SE and TL are reduced due to rigidity and lack of adequate postural control, which often result in falls, and under the eyes closed condition postural control worsens (Beuter et al. 2008; Blaszczyk and Orawiec 2011; Mancini et al. 2012; Panyakaew et al. 2015). Once treated with l-Dopa, patients showed an increase of SE and TL, and rigidity decreased although postural stability and falls continued to be critical. SE and TL were reduced by PPTg DBS compared to the l-Dopa effect but falls decreased, likely for a better postural control under PPTg DBS. In general, in the DBS OFF condition a significant increase of both SE and TL occurred when eyed were kept closed compared to what happened with eyes open. The reasons of this increase may be searched in a disruption of visual compensations coupled with the loss of vestibular and proprioceptive compensations of postural stability occurring in PD patients. PPTg DBS induced a decrease of SE both when eyes were kept open or close, without reaching level of statistical significance (ANOVA followed by Newman-Keuls test) (Fig. 7a). When measuring TL a statistical significant reduction occurred keeping eyes open and comparing DBS OFF and DBS ON conditions (Fig. 7b). Undoubtedly, the great variability from patient to patient in both SE and TL measurements with eyes closed may have greatly influenced the analysis. The large values of the calculated standard deviations may explain the absence of significance in the eyes closed condition comparing data collected in the presence or absence of PPTg DBS, thus further studies must be carried out to clarify this issue. An enlargement of the SE also occurred under drug administration but the difference with respect to the absence or presence of stimulation was not statistically significant. Overall, these results indicate that body oscillations were dampened by PPTg DBS, and the postural control was facilitated, without necessarily requiring a larger sway ellipse. Similar effects have been reported following STN DBS (Rocchi et al. 2002), but were probably mediated by mechanisms different from those that might have been directly triggered in the brainstem by PPTg DBS. Another evidence supporting that PPTg DBS may improve postural control comes from the analysis of the Romberg Index, calculated in the traditional manner [SE or TL (eye closed)/(eye opened) × 100] (Tjernstrom et al. 2014), thus expressed as percentage of SE or TL. In our patients the Romberg index was significantly improved during stimulation reaching values close to those obtained in normal subjects (Fig. 8).
Static balance under PPTg DBS. Under the closed eyes condition a large variability from patient to patient occurred in both Sway Ellipse area (SE) (a) and Total Length (TL) of oscillations (b) values, which were significantly higher compared to the open eyes condition. In general PPTg DBS showed a trend to reduce both SE and TL, but a level of statistical significance was reached only when the conditions of PPTg ON and PPTg OFF were compared when patients kept the eyes open
The values of the Romberg index (mm2) expressed as percentage of the Sway Ellipse area (SE) and of the Total Length (TL) of oscillations were significantly reduced in the eight patients studied in the presence of stimulation compared to the absence of stimulation (one way ANOVA followed by Newman-Keuls test; mean ± SD), approaching values recorded in normal subjects (horizontal rectangle)
A hypothesis to explain the improvement of postural control induced by stimulation of the ventrolateral pontine tegmentum in correspondence of the position occupied by the PPTg in the old atlas, might be based on the fact that rigidity is known to lessen under the action of dopaminergic drugs (Bejjani et al. 2000; Bartolic et al. 2005). Thus, the effects of ventrolateral pontine tegmentum stimulation might add to the levodopa action, acting on brainstem structures and neuronal network which are not controlled by dopamine (Bonnet et al. 1987; Rinne et al. 2008). A neuronal network involved in postural control that might be primarily affected by DBS in the ventrolateral pontine tegmentum could be the one that links the PPTg area to reticulospinal neurons (Garcia-Rill et al. 2001; Scarnati et al. 2011). In addition, the combined action of levodopa therapy and pontine DBS might facilitate transmission of ascending proprioceptive signals.
In this regard, it is worth noting that the PPTg, irrespective of its position in the former atlas and its presence in the ventrolateral pontine tegmentum, lies in a region bordered by three neuronal pathways in which sensory information that is critical for the control of posture during standing and walking: the medial lemniscus, the superior cerebellar peduncle and the spinothalamic tract.
The utility of SEPs for correct targeting of the PPTg, assessing postoperatively the real position of the lead and providing further insight into the DBS mechanism
The procedure for recording SEPs has been detailed in previous papers (Insola et al. 2012, 2014). Briefly, SEPs were evoked by median nerve stimulation during PPTg implantation in 24 out of the 32 patients enrolled in this study. None of them was affected by sensory deficits.
The neurophysiology of SEPs and the neurosurgery of the PPTg are strictly correlated because the morphology of SEPs waves consistently reflects the position in the brainstem of each contact. We recorded three types of waves in SEPs: biphasic (Type I), triphasic (Type II) and mixed (Type III) (Fig. 9). The spatial distribution of the different SEPs waves with respect to the anatomical landmarks we used for planning and successively assessing the position of the lead is reported in Fig. 10a, b. Type I waves were recorded by contacts located in lateral, anterior and dorsal positions with respect to the anatomical landmarks (Obex, VFL and PMJ); Type II waves could be recorded in medial, posterior and ventral sites, and, Type III waves were recorded in an intermediate position with respect to the sites in which Types I and II waves were found. In searching for a statistical correlation among the anatomical landmarks of the brainstem that we considered in each patient (the S3 distance, which corresponds to the height of midbrain; the PMJ position; the X, Y, and Z coordinates; the α and β angles of trajectories along the coronal and sagittal planes, respectively) (Mazzone et al. 2013) and the SEPs waveforms, we found a significant correlation between the three types of waves, the coordinates X, Z and the β angle. We performed a statistical analysis keeping in mind some of the above mentioned surgical parameters (distance from anatomical landmarks, coordinates and angles of trajectory) and the three types of waves detected. The first step was to search for a statistically significant difference between the waves using the surgical parameters. This was achieved by means of multivariate analysis of variance (MANOVA), after a previous Doornik-Hansen test for multivariate normality. After determining the capacity of the parameters to discriminate the waves, we carried out a non-parametric linear discriminant analysis which provided a predictive model for the wave type that was likely to be recorded given a specific set of surgical parameters. The conclusion drawn from this study is that from a surgical perspective intraoperative SEPs may be used as an effective tool to predict the final position of the leads, which in any case is always verified by postsurgical MRI.
The three types of SEPs waves recorded from the four contacts (PPTg 0–3) of the lead as it was inserted in the brainstem to target the PPTg. Monopolar and bipolar recordings are shown in columns a and b, respectively. Type I waves were recorded by contacts located in lateral, anterior and dorsal sites with respect to the Obex, VFL and PMJ. Type II waves were recorded by contacts located medial, posterior and ventral sites with respect to the anatomical landmarks. Type III waves were recorded in an intermediate position with respect to the sites in which Types I and II waves were found
SEPs waves may be predictive of the position of the stimulating lead in the brainstem. a Relationships between different types of SEPs waves with the position of the lead and active contact pairs with respect to the anatomical landmarks we used for planning and successively assessing the lead position. The white vertical bars represent the height of midbrain (S3 distance) while the gray bars indicate the axial extension of the PPTg according to the Paxinos and Huang atlas (1995). b Distribution of the different SEPs waves with respect to the PMJ, VLF and brainstem midline. Type I and type III waves were recorded from leads located dorsally and in a more anterior and lateral position with respect to the position in which Type II wave was found
Another aspect of SEPs that may help to elucidate how the DBS works in the brainstem concerns the modifications of the waveforms as stimulation progresses over time. We have so far evaluated SEP morphology following 3 months of continuous DBS in three patients. The results show that chronic pontine DBS abolished the late cortical components of the SEPs, which reappeared when DBS was discontinued for at least 20 h (Fig. 11). It is likely that this effect is related to the stimulation site, but further studies in a larger number of patients are required to validate this hypothesis. Moreover, the disappearance of the late cortical components suggests that DBS, when applied to pontine structures, might induce a remodeling of sensory afferents to thalamic nuclei and cerebral cortex through a polysynaptic mechanism. If so, the action of brainstem DBS on levodopa resistant signs might be mediated by its effect on ascending proprioceptive and somatosensory pathways as well as on projections toward the spinal cord.
Modification of the late cortical response in SEPs as DBS progresses. Trace 1 is a preoperative recording showing the presence of late cortical components (n50, p60 and n75). Note in trace 2 the disappearance of the late cortical components after long-term DBS. These components reappeared when DBS was discontinued for at least 20 h. Traces 3, 4, 5 and 6 show the SEPs waves recorded at different intervals after stopping DBS
Conclusions and perspectives
From the data discussed above, it appears that the most appropriate neurosurgical approach to intervene on brainstem structures requires to overcome some limits of traditional stereotactic neurosurgery. This means that new reference landmarks and planning methods, intra- and perioperative procedures under general anesthesia, and SEPs, rather than local analgesia and IOMERs, are required to be sure of the targeted structure and to ensure the best clinical outcome. In addition, in order to standardize methodology across different studies, the principle must be accepted that careful choice of stimulation parameters (e.g. low vs high frequency) must be based on the site to be stimulated and on specific symptoms that are featured in each patient.
The organization of structures contained in the midbrain and pons is not homogeneous, and the borders of nuclei in the ventrolateral pontine tegmentum are poorly defined; thus to target a precise and well-identified pontine structure it is crucial to establish the anatomy of the brainstem in each patient. In our practice we pay particular attention to the position of the PMJ, and to the anatomical and physiological relationships that the lead may have with the medial lemniscus. A key question concerning the stimulation of the former PPTg (or the complex PTg-isRt-RIs-VLTg, according to the Paxinos’ reorganization) is whether the final position of the lead tip must be merely considered as a constrained endpoint or whether we have to consider the ventrolateral pontine tegmentum in a broader sense, irrespective of the final location of the stimulating tip. The proposed new organization of brainstem nuclei validates some of our concepts about the meaning of the target but doubts over what may be considered as a target remain. In other words, the effects of PPTg DBS might not be site-specific but depend on the whole area in which the PPTg, or even the PTg-isRt-RIs-VLTg nuclei all together are located, where, in addition to poorly defined neuronal populations, descending and ascending axonal bundles also run.
Our clinical and instrumental data provide new insights and concepts into brainstem DBS, and confirm that the ventrolateral pontine tegmentum, which is enclosed between three major ascending sensory pathways, i.e. the medial lemniscus, the spinothalamic tract, and the cerebellar superior peduncle, is an optimal site for delivering DBS, particularly when levodopa resistant disabling gait and axial instability must be treated. We believe that the aforementioned area would be better indicated as a new region of application of DBS rather than a new target. This is because the latter term carries an anatomical meaning that fits better with lesional neurosurgery, rather than with functional neurosurgery or neuromodulation.
The anatomical proximity of the three sensory pathways to the stimulated site and adjacent structures may offer an alternative interpretation of the clinical effects of pontine DBS. Since neurons in the PPTg area degenerate in both PD and PSP (Hirsch et al. 1987; Jellinger 1988; Zweig et al. 1989; Braak et al. 2004), it appears odd to consider the effects of stimulation of the ventrolateral pontine tegmentum (including the PPTg) as the sole consequence of excitation or inhibition of cell bodies. Thus, it would be also reasonable to hypothesize a role of ascending sensory signals travelling in these pathways in the clinical effects induced by the stimulation of the ventrolateral pontine tegmentum. This hypothesis is also validated by the fact that an impaired functional integration of postural sensory signals occurs in patients with PD (Muller et al. 2013), as a possible consequence of impaired integrity of PPTg neurons and their thalamic efferents.
Furthermore, the DBS of the ventrolateral pontine tegmentum, regardless of the fine site of application, might also modulate the late cortical components of SEPs, that is, a polysynaptic neuronal action might be triggered leading to changes of cortical activity.
Finally, there are other aspects concerning the ventrolateral pontine tegmentum efferents or fibre systems passing through the region in which the PPTg is located, that deserve to be further investigated to explain the efficacy of DBS of the ventrolateral pontine tegmentum. We have already shown that brainstem descending pathways to motoneurons may be modulated by DBS of the ventrolateral pontine tegmentum (Pierantozzi et al. 2008; Scarnati et al. 2011). An additional issue is whether DBS modulates connections that DBS of the ventrolateral pontine tegmentum has with the cerebral cortex through the cerebellum. Indeed, diffusion tractography studies in normal subjects (Aravamuthan et al. 2007, 2008) have provided anatomical findings for the existence of a PPTg-cerebellum tract, and recent experimental studies have suggested that stimulation of both the PPTg and STN modulate the activity of cerebellar nuclei (Sutton et al. 2015; Vitale et al. 2016); thus, once activated, the cerebellum might influence the activity of the cerebral cortex independently of the dopaminergic machinery.
In the future, a great help to answer to the problems raised by stimulations of structures located at different heights above the +36 slice might come from the use of octapolar leads. In such a way, a better discrimination of the effects of stimulating the cuneiform, subcuneiform and PPTg would be feasible and, in addition, it would be possible to stimulate simultaneously or separately thalamic nuclei, such as the parafascicular nucleus, and midbrain structures.
Overall, given the body of data discussed above, neurostimulation should no longer be considered to act on the site in which it is applied, but should be seen as a procedure that may modulate complex neuronal networks, thus acting through polysynaptic mechanisms. Similar consideration were also formulated some years ago to explain the mechanism of action of STN DBS (Deniau et al. 2010).
In conclusion, the value of stimulation of the PPTg, or the newly defined ventrolateral pontine tegmentum, may be better appreciated considering that PD arises and progresses first in brainstem structures (Braak et al. 2004) sparing the STN neurons. In the light of this consideration, PPTg DBS should be seen as a procedure that may act through mechanisms different from those hypothesized to explain the effects of the STN DBS in PD, which are based on the survival of STN neurons.
References
Alam M, Schwabe K, Krauss JK (2011) The pedunculopontine nucleus area: critical evaluation of interspecies differences relevant for its use as a target for deep brain stimulation. Brain 134:11–23
Albin RL, Young AB, Penney JB (1995) The functional anatomy of disorders of the basal ganglia. Trends Neurosci 18:63–64
Aravamuthan BR, Angelaki DE (2012) Vestibular responses in the macaque pedunculopontine nucleus and central mesencephalic reticular formation. Neuroscience 223:183–199
Aravamuthan BR, Muthusamy KA, Stein JF, Aziz TZ, Johansen-Berg H (2007) Topography of cortical and subcortical connections of the human pedunculopontine and subthalamic nuclei. Neuroimage 37:694–705
Aravamuthan BR, Stein JF, Aziz TZ (2008) The anatomy and localization of the pedunculopontine nucleus determined using probabilistic diffusion tractography [corrected]. Br J Neurosurg 22(Suppl 1):S25–S32
Bartolic A, Pirtosek Z, Rozman J, Ribaric S (2005) Postural stability of Parkinson’s disease patients is improved by decreasing rigidity. Eur J Neurol 12:156–159
Bejjani BP, Gervais D, Arnulf I, Papadopoulos S, Demeret S, Bonnet AM, Cornu P, Damier P, Agid Y (2000) Axial parkinsonian symptoms can be improved: the role of levodopa and bilateral subthalamic stimulation. J Neurol Neurosurg Psychiatry 68:595–600
Bergman H, Wichmann T, Karmon B, DeLong MR (1994) The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 72:507–520
Beuter A, Hernandez R, Rigal R, Modolo J, Blanchet PJ (2008) Postural sway and effect of levodopa in early Parkinson’s disease. Can J Neurol Sci 35:65–68
Blaszczyk JW, Orawiec R (2011) Assessment of postural control in patients with Parkinson’s disease: sway ratio analysis. Hum Mov Sci 30:396–404
Bonnet AM, Loria Y, Saint-Hilaire MH, Lhermitte F, Agid Y (1987) Does long-term aggravation of Parkinson’s disease result from nondopaminergic lesions? Neurology 37:1539–1542
Braak H, Del Tredici K (2008) Cortico-basal ganglia-cortical circuitry in Parkinson’s disease reconsidered. Exp Neurol 212:226–229
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del TK (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134
Caliandro P, Insola A, Scarnati E, Padua L, Russo G, Granieri E, Mazzone P (2011) Effects of unilateral pedunculopontine stimulation on electromyographic activation patterns during gait in individual patients with Parkinson’s disease. J Neural Transm 118:1477–1486
Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Bolam JP, Gerdjikov T, Mena-Segovia J (2014) A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci 34:4509–4518
DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13:281–285
Deniau JM, Degos B, Bosch C, Maurice N (2010) Deep brain stimulation mechanisms: beyond the concept of local functional inhibition. Eur J Neurosci 32:1080–1091
Edley SM, Graybiel AM (1983) The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. J Comp Neurol 217:187–215
Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, Henry-Lagrange C, Seigneuret E, Piallat B, Krack P, Le Bas JF, Benabid AL, Chabardes S, Pollak P (2010) Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain 133:205–214
Futami T, Takakusaki K, Kitai ST (1995) Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci Res 21:331–342
Garcia-Rill E (2015) Waking and the reticular activating system in health and disease. Elsevier-Academic Press, Amsterdam
Garcia-Rill E, Skinner RD, Miyazato H, Homma Y (2001) Pedunculopontine stimulation induces prolonged activation of pontine reticular neurons. Neuroscience 104:455–465
Grofova I, Keane S (1991) Descending brainstem projections of the pedunculopontine tegmental nucleus in the rat. Anat Embryol (Berl) 184:275–290
Gut NK, Winn P (2015) Deep brain stimulation of different pedunculopontine targets in a novel rodent model of parkinsonism. J Neurosci 35:4792–4803
Hazrati LN, Parent A (1992) Projection from the deep cerebellar nuclei to the pedunculopontine nucleus in the squirrel monkey. Brain Res 585:267–271
Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F (1987) Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci USA 84:5976–5980
Hong S, Hikosaka O (2014) Pedunculopontine tegmental nucleus neurons provide reward, sensorimotor, and alerting signals to midbrain dopamine neurons. Neuroscience 282C:139–155
Insola A, Valeriani M, Mazzone P (2012) Targeting the pedunculopontine nucleus: a new neurophysiological method based on somatosensory evoked potentials to calculate the distance of the deep brain stimulation lead from the Obex. Neurosurgery 71:96–103
Insola A, Padua L, Mazzone P, Scarnati E, Valeriani M (2014) Low and high-frequency somatosensory evoked potentials recorded from the human pedunculopontine nucleus. Clin Neurophysiol 125:1859–1869
Jackson A, Crossman AR (1983) Nucleus tegmenti pedunculopontinus: efferent connections with special reference to the basal ganglia, studied in the rat by anterograde and retrograde transport of horseradish peroxidase. Neuroscience 10:725–765
Jellinger K (1988) The pedunculopontine nucleus in Parkinson’s disease, progressive supranuclear palsy and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 51:540–543
Jenkinson N, Nandi D, Miall RC, Stein JF, Aziz TZ (2004) Pedunculopontine nucleus stimulation improves akinesia in a Parkinsonian monkey. Neuroreport 15:2621–2624
Karachi C, Andre A, Bertasi E, Bardinet E, Lehericy S, Bernard FA (2012) Functional parcellation of the lateral mesencephalus. J Neurosci 32:9396–9401
Khan S, Javed S, Mooney L, White P, Plaha P, Whone A, Gill SS (2012) Clinical outcomes from bilateral versus unilateral stimulation of the pedunculopontine nucleus with and without concomitant caudal zona incerta region stimulation in Parkinson’s disease. Br J Neurosurg 26:722–725
Kobayashi Y, Isa T (2002) Sensory-motor gating and cognitive control by the brainstem cholinergic system. Neural Netw 15:731–741
Krauthamer GM, Grunwerg BS, Krein H (1995) Putative cholinergic neurons of the pedunculopontine tegmental nucleus projecting to the superior colliculus consist of sensory responsive and unresponsive populations which are functionally distinct from other mesopontine neurons. Neuroscience 69:507–517
Lau B, Welter ML, Belaid H, Fernandez VS, Bardinet E, Grabli D, Karachi C (2015) The integrative role of the pedunculopontine nucleus in human gait. Brain 138:1284–1296
Lavoie B, Parent A (1994a) Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. J Comp Neurol 344:210–231
Lavoie B, Parent A (1994b) Pedunculopontine nucleus in the squirrel monkey: cholinergic and glutamatergic projections to the substantia nigra. J Comp Neurol 344:232–241
Lee HJ, Rye DB, Hallanger AE, Levey AI, Wainer BH (1988) Cholinergic vs. noncholinergic efferents from the mesopontine tegmentum to the extrapyramidal motor system nuclei. J Comp Neurol 275:469–492
Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB (2012) Postural sway as a marker of progression in Parkinson’s disease: a pilot longitudinal study. Gait Posture 36:471–476
Mazzone P, Stanzione P, Lozano A, Sposato S, Scarnati E, Stefani A (2005a) Brain stimulation and movement disorders: Where are we going? In: Meglio M (ed) Proceedings of the 14th meeting of the World Society for Stereotactic and Functional Neurosurgery (WSSFN) Monduzzi, Bologna, Italy
Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, Stefani A (2005b) Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson’s disease. Neuroreport 16:1877–1881
Mazzone P, Sposato S, Insola A, Dilazzaro V, Scarnati E (2008) Stereotactic surgery of nucleus tegmenti pedunculopontine. Br J Neurosurg 22(Suppl 1):S33–S40
Mazzone P, Insola A, Sposato S, Scarnati E (2009) The deep brain stimulation of the pedunculopontine tegmental nucleus. Neuromodulation 12:191–204
Mazzone P, Sposato S, Insola A, Scarnati E (2011) The deep brain stimulation of the pedunculopontine tegmental nucleus: towards a new stereotactic neurosurgery. J Neural Transm 118:1431–1451
Mazzone P, Padua L, Falisi G, Insola A, Florio TM, Scarnati E (2012) Unilateral deep brain stimulation of the pedunculopontine tegmental nucleus improves oromotor movements in Parkinson’s disease. Brain Stimul 5:634–641
Mazzone P, Sposato S, Insola A, Scarnati E (2013) The clinical effects of deep brain stimulation of the pedunculopontine tegmental nucleus in movement disorders may not be related to the anatomical target, leads location, and setup of electrical stimulation. Neurosurgery 73:894–906
Mazzone P, Paoloni M, Mangone M, Santilli V, Insola A, Fini M, Scarnati E (2014) Unilateral deep brain stimulation of the pedunculopontine tegmental nucleus in idiopathic Parkinson’s disease: effects on gait initiation and performance. Gait Posture 40:357–362
Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, Lozano AM (2010) Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain 133:215–224
Muller ML, Albin RL, Kotagal V, Koeppe RA, Scott PJ, Frey KA, Bohnen NI (2013) Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease. Brain 136:3282–3289
Muthusamy KA, Aravamuthan BR, Kringelbach ML, Jenkinson N, Voets NL, Johansen-Berg H, Stein JF, Aziz TZ (2007) Connectivity of the human pedunculopontine nucleus region and diffusion tensor imaging in surgical targeting. J Neurosurg 107:814–820
Okada K, Kobayashi Y (2013) Reward prediction-related increases and decreases in tonic neuronal activity of the pedunculopontine tegmental nucleus. Front Integr Neurosci 7:36
Okada K, Kobayashi Y (2014) Fixational saccade-related activity of pedunculopontine tegmental nucleus neurons in behaving monkeys. Eur J Neurosci 40:2641–2651
Olszewski J, Baxter D (1954) Cytoarchitecture of the human brainstem. Lippincott, Philadelphia
Pahapill PA, Lozano AM (2000) The pedunculopontine nucleus and Parkinson’s disease. Brain 123:1767–1783
Panyakaew P, Anan C, Bhidayasiri R (2015) Visual deprivation elicits subclinical postural inflexibilities in early Parkinson’s disease. J Neurol Sci 349:214–219
Paxinos G, Huang XF (1995) Atlas of the human brainstem. Academic Press, San Diego
Paxinos G, Huang X, Sengul G, Watson (2012) Organization of brainstem nuclei. The human nervous system. Elsevier Academic Press, Amsterdam, pp 260–327
Pierantozzi M, Palmieri MG, Galati S, Stanzione P, Peppe A, Tropepi D, Brusa L, Pisani A, Moschella V, Marciani MG, Mazzone P, Stefani A (2008) Pedunculopontine nucleus deep brain stimulation changes spinal cord excitability in Parkinson’s disease patients. J Neural Transm 115:731–735
Plaha P, Gill SS (2005) Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. NeuroReport 16:1883–1887
Reese NB, Garcia-Rill E, Skinner RD (1995a) Auditory input to the pedunculopontine nucleus: II. Unit responses. Brain Res Bull 37:265–273
Reese NB, Garcia-Rill E, Skinner RD (1995b) Auditory input to the pedunculopontine nucleus: I. Evoked potentials. Brain Res Bull 37:257–264
Reese NB, Garcia-Rill E, Skinner RD (1995c) The pedunculopontine nucleus–auditory input, arousal and pathophysiology. Prog Neurobiol 47:105–133
Rinne JO, Ma SY, Lee MS, Collan Y, Roytta M (2008) Loss of cholinergic neurons in the pedunculopontine nucleus in Parkinson’s disease is related to disability of the patients. Parkinsonism Relat Disord 14:553–557
Rocchi L, Chiari L, Horak FB (2002) Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. J Neurol Neurosurg Psychiatry 73:267–274
Ruggiero DA, Anwar M, Golanov EV, Reis DJ (1997) The pedunculopontine tegmental nucleus issues collaterals to the fastigial nucleus and rostral ventrolateral reticular nucleus in the rat. Brain Res 760:272–276
Rye DB, Saper CB, Lee HJ, Wainer BH (1987) Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol 259:483–528
Rye DB, Lee HJ, Saper CB, Wainer BH (1988) Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol 269:315–341
Scarnati E, Florio T, Capozzo A, Confalone G, Mazzone P (2011) The pedunculopontine tegmental nucleus: implications for a role in modulating spinal cord motoneuron excitability. J Neural Transm 118:1409–1421
Schaltenbrand G, Wahren W (1977) Atlas for stereotaxy of the human brain. Thieme, New York
Schrader C, Seehaus F, Capelle HH, Windhagen A, Windhagen H, Krauss JK (2013) Effects of pedunculopontine area and pallidal DBS on gait ignition in Parkinson’s disease. Brain Stimul
Sherman D, Fuller PM, Marcus J, Yu J, Zhang P, Chamberlin NL, Saper CB, Lu J (2015) Anatomical location of the mesencephalic locomotor region and its possible role in locomotion, posture, cataplexy, and parkinsonism. Front Neurol 6:140
Skinner RD, Garcia-Rill E (1984) The mesencephalic locomotor region (MLR) in the rat. Brain Res 323:385–389
Skinner RD, Kinjo N, Henderson V, Garcia-Rill E (1990) Locomotor projections from the pedunculopontine nucleus to the spinal cord. Neuroreport 1:183–186
Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P (2007) Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 130:1596–1607
Sugimoto T, Hattori T (1984) Organization and efferent projections of nucleus tegmenti pedunculopontinus pars compacta with special reference to its cholinergic aspects. Neuroscience 11:931–946
Sutton AC, O’Connor KA, Pilitsis JG, Shin DS (2015) Stimulation of the subthalamic nucleus engages the cerebellum for motor function in parkinsonian rats. Brain Struct Funct 220:3595–3609
Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T (2003) Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience 119:293–308
Takakusaki K, Habaguchi T, Saitoh K, Kohyama J (2004) Changes in the excitability of hindlimb motoneurons during muscular atonia induced by stimulating the pedunculopontine tegmental nucleus in cats. Neuroscience 124:467–480
Talairach J, David M, Tornoux P, Korredor H, Kvasina T (1957) Atlas d’anatomie stereotaxique des noyaux gris centraux. Masson, Paris
Tattersall TL, Stratton PG, Coyne TJ, Cook R, Silberstein P, Silburn PA, Windels F, Sah P (2014) Imagined gait modulates neuronal network dynamics in the human pedunculopontine nucleus. Nat Neurosci 17:449–454
Thevathasan W, Coyne TJ, Hyam JA, Kerr G, Jenkinson N, Aziz TZ, Silburn PA (2011) Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery 69:1248–1253
Thevathasan W, Cole MH, Graepel CL, Hyam JA, Jenkinson N, Brittain JS, Coyne TJ, Silburn PA, Aziz TZ, Kerr G, Brown P (2012) A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain 135:1446–1454
Tjernstrom F, Bjorklund M, Malmstrom EM (2014) Romberg ratio in quiet stance posturography-test to retest reliability. Gait Posture
Vitale F, Mattei C, Capozzo A, Pietrantoni I, Mazzone P, Scarnati E (2016) Cholinergic excitation from the pedunculopontine tegmental nucleus to the dentate nucleus in the rat. Neuroscience 317:12–22
Wang HL, Morales M (2009) Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci 29:340–358
Weinberger M, Hamani C, Hutchison WD, Moro E, Lozano AM, Dostrovsky JO (2008) Pedunculopontine nucleus microelectrode recordings in movement disorder patients. Exp Brain Res 188:165–174
Welter ML, Demain A, Ewenczyk C, Czernecki V, Lau B, El HA, Belaid H, Yelnik J, Francois C, Bardinet E, Karachi C, Grabli D (2015) PPNa-DBS for gait and balance disorders in Parkinson’s disease: a double-blind, randomised study. J Neurol
Wichmann T, DeLong MR (2001) Basal ganglia circuits in movement and movement disorders. In: Kultas-Ilinsky K, Ilinsky IA (eds) Basal ganglia and thalamus in health and movement disorders. KluverAcademic/Plenum Publishers, New York, pp 11–25
Wichmann T, Bergman H, DeLong MR (1994) The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol 72:521–530
Winn P (2008) Experimental studies of pedunculopontine functions: are they motor, sensory or integrative? Parkinsonism Relat Disord 14(Suppl 2):S194–S198
Young RF, Tronnier V, Rinaldi PC (1992) Chronic stimulation of the Kolliker-Fuse nucleus region for relief of intractable pain in humans. J Neurosurg 76:979–985
Zrinzo L, Zrinzo LV, Tisch S, Limousin PD, Yousry TA, Afshar F, Hariz MI (2008) Stereotactic localization of the human pedunculopontine nucleus: atlas-based coordinates and validation of a magnetic resonance imaging protocol for direct localization. Brain 131:1588–1598
Zweig RM, Jankel WR, Hedreen JC, Mayeux R, Price DL (1989) The pedunculopontine nucleus in Parkinson’s disease. Ann Neurol 26:41–46
Acknowledgments
The authors wish to thank Prof. Edgar Garcia-Rill for his critical reading of the manuscript and suggestions. We are also grateful to Prof Francesco Masedu and Dr. Annamaria Capozzo, University of L’Aquila, for evaluating data and carrying out statistics concerning correlations between SEPs and anatomical landmarks, and Prof. Paolo Arena, DIEEI, University of Catania, for evaluating the electrical fields generated by DBS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical standard statement
All the procedures described in the present paper have been conducted according to ethical standards, approved by local ethical committees and patients gave their informed consent to participate to the described studies.
Rights and permissions
About this article
Cite this article
Mazzone, P., Vilela Filho, O., Viselli, F. et al. Our first decade of experience in deep brain stimulation of the brainstem: elucidating the mechanism of action of stimulation of the ventrolateral pontine tegmentum. J Neural Transm 123, 751–767 (2016). https://doi.org/10.1007/s00702-016-1518-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1518-5