Abstract
Type B monoamine oxidase (MAO-B) is proposed to be involved in the pathogenesis of neurodegenerative disorders, such as Parkinson’s disease, through oxidative stress and synthesis of neurotoxins. MAO-B inhibitors, rasagiline and selegiline [(−)deprenyl], protect neuronal cells by direct intervention in mitochondrial death signaling and induction of pro-survival Bcl-2 and neurotrophic factors. Recently, type A MAO (MAO-A) was found to mediate the induction of anti-apoptotic Bcl-2 by rasagiline, whereas MAO-A increases in neuronal death and also serves as a target of neurotoxins. These controversial results suggest that MAO-A may play a decisive role in neuronal survival and death. This paper reports that rasagiline and selegiline increased the mRNA, protein and catalytic activity of MAO-A in SH-SY5Y cells. Silencing MAO-A expression with small interfering (si)RNA suppressed rasagiline-dependent MAO-A expression, but MAO-B overexpression in SH-SY5Y cells did not affect, suggesting that MAO-A, not MAO-B, might be associated with MAO-A upregulation. Rasagiline reduced R1, a MAO-A specific repressor, but selegiline did not. Mithramycin-A, an inhibitor of Sp1 binding, and actinomycin-D, a transcriptional inhibitor, reduced the rasagiline-dependent upregulation of MAO-A mRNA, indicating that rasagiline induced MAO-A transcriptionally through R1-Sp1 pathway, whereas selegiline by another non-defined pathway. These results are discussed in relation to the role of MAO-A and these MAO-B inhibitors in neuronal death and neuroprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monoamine oxidase [monoamine: oxygen oxidoreductase (deaminating), EC 1.4.3.4, MAO] is a major catabolism enzyme of monoamine neurotransmitters and classified as types A and B, according to the substrate specificity and the sensitivity to distinct inhibitors (Shih et al. 1999). Type B MAO (MAO-B) has been proposed to be involved in the pathogenesis of Parkinson’s disease (PD) through increased generation of reactive oxygen species (ROS) from oxidation of monoamine substrates and the oxidative synthesis of neurotoxins from precursors, such as 1-methyl-4-phenyl-pyridinium ion (MPP+) from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Selegiline [(−)deprenyl, N-(phenylisopropyl)-N-methylpropargylamine] was the first MAO-B inhibitor that protected neuronal cells from MPP+ cytotoxicity (Heikkila et al. 1985). Selegiline and another MAO-B inhibitor, rasagiline [N-propargyl-1(R)-aminoindan], are now applied as adjunct of L-DOPA therapy or monotherapy in PD (Riederer et al. 2004; Ebadi et al. 2006; Youdim et al. 2006; Bortolato et al. 2008). These MAO-B inhibitors protect neuronal cells in cellular and animal models of neurodegenerative disorders, through suppression of mitochondrial apoptotic signaling and upregulation of anti-apoptotic Bcl-2 and Bcl-xL and pro-survival neurotrophic factors, including glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) (Akao et al. 2002; Maruyama et al. 2004; Naoi and Maruyama 2010; Naoi et al. 2011; Maruyama and Naoi 2012). Inhibition of MAO activity is not considered as the sole mechanism underlying neuroprotective function of MAO-B inhibitors (Finberg and Youdim 2002).
In contrast, the expression of type A MAO (MAO-A) was upregulated in apoptosis induced by withdrawal of nerve growth factor (NGF) or serum (De Zutter and Davis 2001; Ou et al. 2006a). An endogenous neurotoxin, N-methyl(R)salsolinol bound to MAO-A and induced apoptosis in SH-SY5Y cells (Yi et al. 2006). In staurosporine-induced apoptosis, MAO-A activity was post-transcriptionally upregulated, in SH-SY5Y cells, resulting in increased ROS production (Fitzgerald et al. 2007). Vice versa, hydrogen peroxide increased MAO activity in human brain homogenates (Konradi et al. 1986). A selective MAO-A inhibitor, clorgyline [N-methyl-N-propargyl-3(2,4-dichlorophenoxy)-propylamine], prevented the apoptosis induced by NGF depletion and also reduced MPTP neurotoxicity (De Girolamo et al. 2001). Clorgyline inhibits MAO-A activity and reduces ROS production, which is proposed to account for its neuroprotective function. These results suggest that MAO-A may be an apoptogenic gene.
On the other hand, we found that MAO-A mediated the induction of anti-apoptotic Bcl-2 expression by rasagiline and silencing MAO-A expression with short interfering (si)RNA suppressed the induction (Inaba-Hasegawa et al. 2012). In cDNA microarray study on the gene induction in SH-SY5Y cells, rasagiline increased MAO-A, suggesting the involvement of MAO-A also in the neuroprotective function of rasagiline (Naoi et al. 2007).
In this paper, we investigated the effects of rasagiline and selegiline on the mRNA, protein and enzymatic activity of MAO-A in SH-SY5Y cells, in which only MAO-A protein and enzymatic activity are expressed. To examine the role of MAO-A and MAO-B, MAO-A expression was silenced with siRNA, and MAO-B was overexpressed by enforced transfection of MAO-B DNA in SH-SY5Y cells. The molecular mechanism behind MAO-A induction by rasagiline and selegiline was investigated in relation to R1, a transcriptional repressor of MAO-A (Chen et al. 2005) and the following transcriptional processes. The results are discussed in concern to the role of MAO-A in regulation of neuronal death and survival in neurodegenerative disorders.
Materials and methods
Materials
Rasagiline, selegiline and clorgyline were kindly donated from TEVA (Netanya, Israel), Dr. Knoll (Semmelweis University, Budapest, Hungary) and May and Baker (Dagenham, UK), respectively. Kynuramine and mithramycin-A were purchased from Sigma (St. Louis, MO, USA), an inhibitor of NF-κB, sc-3060, and its inactive control, sc-3061, from Santa Cruz Biotechnology (Santa Cruz, CA, USA), actinomycin-D, Dulbecco’s modified Eagle’s medium (DMEM) and other chemicals from Wako (Osaka, Japan).
Cell culture and MAO-B transfection
SH-SY5Y cells were cultured in Cosmedium-001 tissue culture medium (CosmoBio, Tokyo, Japan) supplemented by 5 % fetal calf serum in 95 % air–5 % CO2. Stable clone expressing MAO-B was established in SH-SY5Y cells by DNA transfection of MAO-B gene (MAO-B SH cells) (Yi et al. 2006). Using pIRES1neo eukaryotic expression vector (Invitrogen, San Diego, CA, USA), pIRES1neo-MAO-B was constructed and transfected in SH-SY5Y cells using cationic liposomes (Lipofectamine 2000), according to the manufacturer’s Lipofection protocol (Invitrogen). Selection was started 2 days after the transfection using the culture medium containing 0.7 mg/ml geneticin (Sigma).
Quantitative assay for protein levels of MAO-A, R1 and NF-κB p65 by Western blot analysis
MAO-A protein levels were quantified by Western blot analysis with densitometry, as reported previously (Inaba-Hasegawa et al. 2012). SH-SY5Y cells (3 × 105 cells/well) were cultured for 24 h in a 6-well poly-l-lysine coated culture flask (Iwaki, Asahi Glass, Tokyo, Japan), then treated with 10−5–10−12 M MAO inhibitors for further 24 h. The cells were gathered, washed with phosphate-buffered saline (PBS) and suspended in RIPA buffer (10 mM Tris–HCl buffer, pH 7.5, containing 1 % NP-40, 0.1 % sodium deoxycholate, 0.1 % sodium dodecyl sulfate, 150 mM NaCl and 1 mM EDTA 2Na), containing a protease inhibitor cocktail (Roche, Mannheim, Germany). Lyzed protein was separated by SDS-PAGE using 10–20 % gradient polyacrylamide gel (Bio-Rad Lab., Hercules, VA, USA). Resolved proteins were electroblotted onto PVDF membranes (Amersham Hybond-P, GE Healthcare, Buchinghamshire, UK). After blocked with 5 % fat-free milk, MAO-A was detected with the antibody recognizing MAO-A (SC-20156 ×500 dilution, Santa Cruz Biotechnology), and β-actin as control with anti-β-actin antibody (×5,000 dilution, Sigma) on the same membrane. R1 (JPO2/RAM2) protein was stained with the antibody against R1 (JPO2/RAM2) (×500 dilution, Bethyl, Montgomery, TX, USA) and NF-κB p65 with anti-p65 antibody (×2,000 dilution, Code No. 100-4165, Rockland Immunochemicals, Gilbertsville PA, USA). Then, the membrane was treated with the second antibody at the room temperature. The second antibody for MAO-A, R1 and NF-κB/p65 was anti-rabbit IgG horseradish peroxidase-linked species-specific whole antibody (GE Healthcare), and for β-actin anti-mouse IgG (GE Healthcare), respectively. The immunoreactive bands were visualized using Amersham ECL plus Western blotting detection reagents (GE Healthcare) or Immunostar LD (Wako). Protein amounts were quantitatively determined using a Fujifilm LAS-4000 luminescent image analyzer (Tokyo, Japan). The amounts were quantified by densitometry, and the values were expressed as the relative density intensity against background, normalized with corresponding β-actin, and calculated as the ratio to control. To determine MAO-A, R1 and β-actin on the same membrane, MAO-A was stained with anti-MAO-A antibody, quantified, and the antibody was washed out, then R1 and β-actin were visualized with the respective antibody and quantitatively assayed.
Quantitative determination of MAO-A mRNA levels by real-time RT-PCR
MAO mRNA levels were quantitatively measured by real-time reverse transcription-polymerase chain reaction (RT-PCR) method (Inaba-Hasegawa et al. 2012). SH-SY5Y cells (3 × 105 cells/well) cultured in a six-well culture flask were treated with MAO inhibitors (10−5–10−12 M) for 6 or 24 h. The cells were gathered and washed with PBS, and the total RNA was extracted using Trizol plus RNA purification kit according to the manufacture’s protocol (Invitrogen). The cDNA was generated by reverse transcription of the total RNA (100 ng) and the cDNA fragments were amplified. β-actin mRNA was quantified with HA067803-F/-R as a PCR primer and used as an internal standard. The relative concentrations of PCR products were determined from the standard curve prepared by real-time PCR of serial dilutions of template cDNAs. MAO-A mRNA levels were quantitatively determined from cycle threshold (C t) of the PCR reaction analyzed by Real time system software (TaKaRa Bio) and the standard curve, and expressed as the relative values against control after normalized with corresponding β-actin levels.
Assay for MAO activity
The effects of rasagiline on MAO activity were examined in SH-SY5Y cells cultured in a 6-well poly-l-lysine coated culture flask for 24 h, and treated with rasagiline (10−7–10−11 M) for further 24 h. The cells were gathered, washed with PBS and mitochondrial faction was prepared, according to Desagher et al. (1999). MAO activity was quantitatively determined by the fluorometric assay using kynuramine as a substrate, according to Kraml (1965). Protein concentration was quantified according to Bradford (1976).
Silencing MAO-A expression with siRNA and the effects of inhibitors of transcription and NF-κB
Silencing MAO-A expression was carried out in SH-SY5Y cells using siRNA targeting MAO-A mRNA (sc-35847, Santa Cruz Biotechnology) (Yi et al. 2006). The cells (1.5 × 105 cells/well) were cultured in a six-well culture flask for 24 h, and treated with siRNA for 48 h. The siRNA (40 nM) was transfected into the cells using Lipofectamine™ RNAiMAX (Invitrogen), according to the manufacturer’s Lipofection protocol. The transfection efficiency was evaluated by the Western blot analysis of MAO-A protein. The siRNA-treated cells were treated with rasagiline or selegiline for further 24 h and MAO-A protein levels were quantitatively measured.
In experiments using mithramycin-A, an inhibitor of Sp1 binding (0.1 or 1 μM), or actinomycin-D, a transcriptional inhibitor (1 or 5 μg/ml), SH-SY5Y cells (9 × 105 cells/well) were cultured in a six-well culture flask for 24 h, then treated with mithramycin-A or actinomycin-D for 1 h, and with rasagiline (10−6 or 10−9 M) for further 24 h. The levels of MAO-A mRNA were quantified as described above.
To investigate nuclear translocation of NF-κB by rasagiline, SH-SY5Y cells were treated with sc-3060 (30 or 40 μg/ml) or sc-3061 (40 μg/ml) as control for 1 h, then with rasagiline (10−6 or 10−9 M) for 24 h. The cells were gathered and the nuclear fraction was prepared with Nuclear/Cytosol fraction kit (MBL, Nagoya, Japan) according to the manufacture’s protocol. After SDS-PAGE, NF-κB p65 and MAO-A protein were quantitatively determined and the values were expressed as the relative ratio to those in cells treated with sc-3060 in the absence of rasagiline.
Statistics
Experiments were repeated at least 3–4 times in triplicate or quadruplicate measurements, and the results were expressed as the mean and SD. Differences were statistically evaluated by analysis of variance (ANOVA) followed by Scheffe’s F test. A p value less than 0.05 was considered to be statistically significant.
Results
Rasagiline and selegiline increased the protein, mRNA and enzymatic activity of MAO-A.
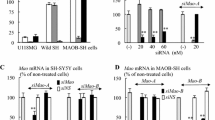
The effects of rasagiline and selegiline on MAO-A protein levels were examined in SH-SY5Y cells after treatment at 10−5–10−12 M for 24 h. These MAO-B inhibitors increased MAO-A protein significantly, as shown by Western blot analysis (Fig. 1a). By staining with anti-MAO-A antibody, only one protein band with molecular mass of about 60 kDa was detected. MAO-A protein was quantitatively determined by densitometry, and rasagiline at 10−5–10−8 M and selegiline at 10−6–10−12 M increased MAO-A significantly (p < 0.05, from control) (Fig. 1b). The increase of MAO-A by rasagiline was marked at the higher concentrations, whereas selegiline upregulated MAO-A at the lower concentrations. On the other hand, clorgyline did not affect the MAO-A levels. Serotonin (5-hydroxytryptamine), a MAO-A substrate, and β-phenylethylamine, a MAO-B substrate, did not affect MAO-A levels (data not shown).
Effects of rasagiline and selegiline on MAO-A protein levels in SH-SY5Y cells. The cells were treated with the MAO-B inhibitors (10−5–10−12 M), and MAO-A levels were determined. a Western blot analyses for MAO-A. β-Actin was used as control. b The quantitative determination of MAO-A levels. The values were presented as the relative ratio to control treated without MAO inhibitors [(−)]. Black, white and gray columns show the values in cells treated with rasagiline, selegiline and clorgyline, respectively. The column and bar represent the mean and SD of four experiments. Significantly different from control and between rasagiline- and selegiline-treated cells, respectively, *,§ p < 0.05
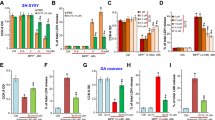
Rasagiline and selegiline increased also MAO-A mRNA, as quantified by real-time RT-PCR method. Figure 2a, b shows the increased mRNA levels after 6 and 24 h treatment with these MAO-B inhibitors. Rasagiline increased MAO-A mRNA at 10−7–10−9 M and 10−10–10−12 M after 6 and 24 h treatment, respectively. Selegiline enhanced MAO-A mRNA levels at 10−8–10−12 M except 10−9, and 10−8–10−12 M after treatment for 6 and 24 h, respectively. The increase by selegiline was more marked than rasagiline at the lower concentrations.
Effects of rasagiline and selegiline on MAO-A mRNA levels and of rasagiline on MAO activity. MAO-A mRNA levels in SH-SY5Y cells after treated with rasagiline (a) or selegiline (b) at 10−5–10−12 M for 6 h (black column) or 24 h (white column). MAO-A mRNA was quantified by real time RT-PCR, and the column and bar represent the mean and SD of four independent experiments. c MAO activity in mitochondrial fraction prepared from cells treated with rasagiline (10−7–10−11 M) for 24 h. The column and bar represent the mean and SD of the quadruplicate measurements of three experiments. Statistically significant from control, *p < 0.05
The effects of rasagiline on MAO-A enzymatic activity was studied in mitochondrial fraction prepared from SH-SY5Y cells treated with rasagiline at 10−7–10−11 M for 24 h. The enzymatic activity was significantly increased after incubation with rasagiline at 10−9 and 10−10 M: 72.6 ± 5.8 and 64.2 ± 3.6 pmol/min/mg protein from control, 56.5 ± 2.3 pmol/min/mg protein (p < 0.05) (Fig. 2c). Rasagiline at 10−7 M inhibited MAO activity significantly, indicating that rasagiline, a MAO-B inhibitor, could inhibit MAO-A activity at the higher concentration. The effects of rasagiline and selegiline varied on the mRNA, protein and catalytic activity of MAO-A, suggesting that the post-transcriptional modification and the direct inhibition of the activity might determine their distinct expression levels.
MAO-A silencing suppressed rasagiline-induced MAO-A expression
The effects of siRNA treatment were investigated on the rasagiline-dependent MAO-A induction. MAO-A-targeted siRNA (MAO-A-siRNA) (40 nM) reduced MAO-A protein markedly to 42.9 ± 6.1 % of control, and the catalytic activity to 46.7 ± 9.9 % of control (Inaba-Hasegawa et al. 2012). In MAO-A-silenced cells, rasagiline did not affect MAO-A protein levels, but it upregulated MAO-A in cells treated with non-specific (NS)-siRNA (Fig. 3a, b). These results suggest that MAO-A itself might mediate MAO-A induction by rasagiline.
Effects of silencing MAO-A expression with siRNA on MAO-A protein. a Western blot analysis of MAO-A protein in SH-SY5Y cells treated with MAO-A-specific [MAO-A-siRNA] or non-specific (NS)-siRNA [NS-siRNA]. Cells treated with 40 nM siRNA for 48 h, and further 24 h without [(−)] or with rasagiline (10−6–10−12 M). b Quantitative analyses of MAO-A levels in cells treated with specific- (black column) or NS-siRNA (white column). The protein levels were expressed as the relative values to those in cells treated without rasagiline. The column and bar represent the mean and SD. Significantly different from control, and between the specific- and NS-siRNA-treated cells, respectively. *,§ p < 0.05
Rasagiline reduced R1 a transcription repressor and Sp1 transcription factor was associated with MAO-A induction, but not in the case with selegiline.
To confirm the intracellular mechanism behind increased MAO-A expression, the effects of rasagiline and selegiline on protein levels of R1, a MAO-A specific repressor, were investigated. Western blot analyses show that rasagiline decreased R1 and increased MAO-A (Fig. 4a). R1 protein with molecular mass of about 56 kDa was detected with anti-R1 antibody recognizing a region between residue 400 and the C terminus (residue 454) of human R1 (JPO2, RAM2). Rasagiline (10−6 and 10−9 M) significantly down-regulated R1 levels to 70 and 86 % of control (p < 0.01 and p < 0.05, respectively), and it upregulated MAO-A expression significantly (p < 0.01) (Fig. 4b). On the other hand, selegiline did not reduce, but rather increased R1 protein at 10−6 M and did not affect R1 level at 10−9 M.
Effects of rasagiline and selegiline on R1 levels and the inhibitors of Sp1 transcription on MAO-A mRNA. a Western blot analyses of R1 and MAO-A in the cells treated with rasagiline and selegiline (10−6 and 10−9 M) for 24 h. b Quantitative analyses of R1 and MAO-A levels. The values of R1 (black column) and MAO-A (white column) were presented as the relative values against those in control cells treated without MAO inhibitors. Column and bar represent the mean and SD of three independent experiments. Significantly different from control, *p < 0.05. c The effects of mithramycin-A (0.1 and 1 μM) and actinomycin-D (1 and 5 μg/ml) on MAO-A mRNA levels. SH-SY5Y cells treated with these inhibitors and further with rasagiline. I Control, II and III cells treated with rasagiline at 10−6 and 10−9 M. Column and bar represent the mean and SD. All the results after treatment with mithramycin-A and actinomycin-D were significantly different from corresponding control cells treated without and with rasagiline, *p < 0.01. Significantly different from cells treated without rasagiline or with 10−6 M rasagiline in the absence of mithramycin-A or actinomycin-D
In addition, Sp1 transcription factor, a controlling factor of MAO-A, was associated with the increase of MAO-A mRNA by rasagiline. Mithramycin-A, a specific inhibitor of Sp1 binding to GC-rich regions of gene promoters (Sp1 response elements) significantly inhibited MAO-A mRNA expression by rasagiline (Fig. 4c). Mithramycin-A (0.1 and 1 μM) reduced the basal values of MAO-A mRNA to 34 and 23 % of control, and inhibited the rasagiline-dependent upregulation to 25–39 % of control. The reduction was statistically significant from control and cells treated with rasagiline in the absence of mithramycin-A (p < 0.01). Actinomycin-D (1 and 5 μg/ml), a transcriptional inhibitor, also suppressed rasagiline-induced MAO-A mRNA expression, and significantly reduced the basal mRNA value to less than 50 % of control (p < 0.01 from control) (Fig. 4c). The reduction in the basal MAO-A mRNA levels by mithramycin-A and actinomycin-D may be due to elevated basal expression of NF-κB-dependent genes in the absence of activated nuclear NF-κB (Hirano et al. 1998).
Rasagiline increased MAO-A expression through activating NF-κB
Rasagiline was found to induce neuroprotective GDNF and Bcl-2 through extracellular-regulated kinase 1/2 (ERK 1/2)-nuclear factor-κB (NF-κB) pathway (Maruyama et al. 2004; Inaba-Hasegawa et al. 2012). The role of NF-κB in rasagiline-induced MAO-A expression was investigated using sc-3060, a selective inhibitor of nuclear translocation of the NF-κB active complex (Fig. 5a). Nuclear translocation of NF-κB p65 was determined in the nuclear fraction prepared from SH-SY5Y cells treated with sc-3060 or sc-3061, and then with rasagiline. By staining with antibody against NF-κB p65 subunit, only one protein band with about 65 kDa corresponding NF-κB p65 was detected in lysate of the nuclear fraction. Rasagiline (10−6 and 10−9 M) increased NF-κB in the nuclear fraction, and sc-3060 (30 and 40 μg/ml) inhibited the nuclear translocation, but sc-3061 a negative control (40 μg/ml) did not. Treatment with sc-3060 (40 μg/ml) suppressed rasagiline-dependent MAO-A induction, whereas sc-3061 at the same concentration did not affect MAO-A levels, as shown by Western blot analyses (Fig. 5b). Figure 5c shows the quantitative determination of MAO-A protein and sc-3060 markedly suppressed MAO-A increase by rasagiline, but sc-3061 did not. The difference of the affects of sc-3060 was statistically significant from control and the cells treated with sc-3061 (p < 0.05).
Effects of a NF-κB inhibitor, sc-3060, on rasagiline-induced nuclear translocation of NF-κB and expression of MAO-A in SH-SY5Y cells. The cells were cultured in a six-well tissue culture flask, treated with sc-3060 or sc-3061 for 1 h, and then cultured without or with rasagiline (10−6 or 10−9 M) for further 24 h. a Western blot analyses of NF-κB p65 in the nuclear fraction. I, II, III and IV Control, treated with sc-3060 at 30 and 40 μg/ml, or sc-3061 as control at 40 μg/ml. b Western blot analyses of MAO-A protein after treated with rasagiline in sc-3060-pretreated cells. I, II and III Control and cells treated with 40 μg/ml sc-3060 or sc-3061. c Quantitative determination of MAO-A detected by Western blot analyses. The values were expressed as the ratio to those in control cells treated without sc-3060 and rasagiline. The white, black and gray columns represent MAO-A in the cells pretreated without and with sc-3060 or sc-3061. The column and the bar represent the mean and SD of four independent experiments. Significantly different from the cells treated without rasagiline, or with rasagiline after treated with sc-3061, *p < 0.05
Role of MAO-B in MAO-A induction by rasagiline and selegiline in MAO-B expressed SH-SY5Y cells
In SH-SY5Y cells, neither MAO-B protein nor the enzymatic activity was detected, even though the RT-PCR analysis detected MAO-B mRNA. Therefore, the role of MAO-B in MAO-A induction by rasagiline and selegiline was examined in MAO-B overexpressed SH-SY5Y (MAO-B SH) cells. In wild SH-SY5Y cells, only MAO-A with 59.7 kDa was detected by Western blot analysis (Inaba-Hasegawa et al. 2012). MAO-B transfection markedly expressed MAO-B protein with 58.0 kDa, but MAO-A protein was still detected. In MAO-B SH cells, MAO activity was increased significantly to 985.7 from 93.7 pmol/min/mg protein in control (p < 0.01). MAO-B activity contributed about 92 % of the total MAO activity in MAO-B SH cells, whereas MAO-A activity remained same as in wild SH-SY6Y cells (82.9 pmol/min/mg protein). Transfection-enforced MAO-B overexpression did not affect the protein and enzymatic activity of MAO-A.
In MAO-B SH cells, rasagiline and selegiline at 10−5–10−11 M did not increase MAO-A protein, as shown by Western blot analyses (Fig. 6a, b). These MAO-B inhibitors significantly suppressed MAO-B activity in MAO-B SH cells in dose-dependent way (Inaba-Hasegawa et al. 2012), suggesting that MAO-B might not be associated with MAO-A induction by these MAO-B inhibitors.
Effects of MAO-B overexpression on MAO-A induction by rasagiline and selegiline. a Western blot analyses of MAO-A protein in MAO-B SH cells after treated with the MAO inhibitors (10−6–10−12 M). b The quantitative measurement of MAO-A protein in MAO-B SH cells after treated with rasagiline (black columns) or selegiline (white columns). The values were expressed as the relative ratio against control treated in the absence of the MAO-B inhibitors. The column and bar represent the mean and SD of four independent experiments. The difference of all the results was not statistically significant from control
Discussion
Rasagiline and selegiline increased the mRNA, protein and catalytic activity of MAO-A in SH-SY5Y cells, whereas clorgyline did not affect MAO-A protein levels and furthermore caused apoptosis at the high concentration. However, increased MAO-A expression by rasagiline and selegiline has never been reported in the brain of experimental animals. After chronic treatment, rasagiline and selegiline reduced MAO-A activity (15 and 40 % inhibition, respectively) in the rat striatum, whereas they inhibited MAO-B activity by 90 % (Lamensdorf et al. 1996). On the other hand, several compounds increase MAO-A expression in cellular and animal models. Glucocorticoid and androgen increase MAO-A and MAO-B promoter and enzymatic activities (Ou et al. 2006b). Dopamine and bromocriptine, a D2-receptor agonist, up-regulated the mRNA, protein and catalytic activity of MAO-A in rat mesangial cells (Pizzinat et al. 2003). But, we could not confirm MAO-A induction by serotonin or β-phenylethylamine. Calcium increased MAO-A activity in the monkey brain (Egashira et al. 2003) and the mRNA and activity in human cerebellar and rat glial C6 cell extracts by activation of 38(MAPK) signal pathways, whereas MAO-B activity remained unaffected (Cao et al. 2009). Valproic acid, a mood stabilizer and anticonvulsant, induced MAO-A expression through activation of Akt/FoxO1 signaling pathway (Wu and Shih 2011). These results suggest that various stimuli might increase the expression and activity of MAO-A and perturb monoamine neurotransmitter systems, as being observed in some classes of psychiatric and behavioral disorders (Shih et al. 1999; Youdim and Bakhle 2006). However, it remains elusive if MAO-A itself is associated with MAO-A induction by these stimuli.
R1-Sp1 pathway was found to mediate MAO-A induction by rasagiline, whereas selegiline might increase MAO-A though a different signal pathway. MAO-A expression is suppressed by R1 and activated by a transcription factor Sp1 (Shih et al. 2011), which are downstream of p38 mitogen activated protein kinase (MAPK) functions (De Zutter and Davis 2001; Ou et al. 2006a). On the other hand, Sp1 and Sp4 activate MAO-B promotor activity, which is downregulated by a transcription repressor Sp3 (Shih et al. 2011). MAO-B promotor activity is increased by phorbol 12-myristate through activating MAPK pathway, including protein kinase C, MAP kinase 1, ERK 1/2, and c-Jun NH2-terminal kinase (Wong et al. 2002). Ethanol induced nuclear translocation of GAPDH and increased MAO-B activity, leading to cell death in U118MG and SH-SY5Y cells (Ou et al. 2009).
Rasagiline and selegiline protect neurons against cell death in cellular and animal models of neurodegenerative disorders, but they show different potencies to protect neuronal cells (Tazik et al. 2009). In clinical trials, rasagiline is more potent as an anti-Parkinson drug than selegiline (Parkinson Study Group 2002). Rasagiline is metabolized to aminoindan with a neuroprotective function (Bar Am et al. 2004, 2010), whereas metabolites of selegiline are neurotoxic l-methamphetamine and l-amphetamine (Abu-Raya et al. 2002). These metabolites may account for different functions of these MAO-B inhibitors (Zhu et al. 2008). In addition, rasagiline increases GDNF more markedly than neurotrophins [BDNF, NGF and neurotropin-3 (NT-3)], and vice versa selegiline preferentially increases neurotrophins (Maruyama and Naoi 2012). At present, it remains to be clarified whether the different molecular mechanism to induce MAO-A might be associated with the distinct neuroprotective functions of rasagiline and selegiline.
Rasagiline and selegiline are selective inhibitors of MAO-B, but they bind to MAO-A, as shown by the inhibition of MAO-A activity at the high concentrations or longer treatment. Rasagiline adducts with the FAD moiety of both MAO types, even the affinity to MAO-A is less than 1/100th of that to MAO-B (Hubalek et al. 2004; Edmondson et al. 2007). Our presented results present that the binding of MAO-B inhibitors to MAO-A, not to MAO-B, may initiate MAO-A induction, which does not depend on inhibition of the catalytic activity of either MAO types. The different responses of MAO-A and MAO-B to these inhibitors might be due to the difference in the substrate-binding site, the occurrence of MAO-A as a monomer, whereas MAO-B as a dimer (Edmondson et al. 2007), or to the different localization on mitochondrial outer membrane (Wang and Edmondson 2011). MAO-A occurs on the cytosolic face and MAO-B on the surface facing the intermembrane space. The binding to the FAD cofactor of MAO alters the environment of aromatic residues in the substrate-binding site, “aromatic cage” and causes the conformational changes in MAO-A (Hynson et al. 2004). Or, a novel binding of rasagiline and selegiline may occur in MAO-A with quite high affinity, but the imidazoline I2-binding site in MAO was not associated with gene induction by these MAO-B inhibitors (Naoi et al. 2011).
It remains to be clarified whether MAO-A induction by these MAO-B inhibitors may regulate signal pathway, either in a way to accelerate cell death by activating death signaling, or to protect neurons by inducing pro-survival and anti-apoptotic genes. In addition, we should be cautious to apply our results observed in neuroblastoma SH-SY5Y cells to neurodegenerative process in the human brain, and we should reconfirm our results at least in animal subclinical models. Nevertheless, understanding of the regulation mechanism mediated by MAO-A to induce pro-survival genes and finding of new compounds with affinity to MAO-A will bring us a new way to develop novel classes of neuroprotective agents for PD and related neurodegenerative disorders.
Abbreviations
- MAO:
-
Monoamine oxidase
- MAO-A and MAO-B:
-
Type A and B MAO
- PD:
-
Parkinson’s disease
- ROS:
-
Reactive oxygen species
- SiRNA:
-
Small interfering RNA
References
Abu-Raya S, Tabakman R, Blaugrund E, Trembovler V, Lazarovici P (2002) Neuroprotective and neurotoxic effects of monoamine oxidase-B inhibitors and derived metabolites under ischemia in PC12 cells. Eur J Pharmacol 434:109–116
Akao Y, Maruyama W, Shimizu S, Yi H, Shamoto-Nagai M, Youdim MB, Tsujimoto Y, Naoi M (2002) Mitochondrial permeability transition mediates apoptosis induced by N-methyl(R)salsolinol, an endogenous neurotoxin, and its inhibited by Bcl-2 and rasagiline, N-propargyl-1(R)-aminoindan. J Neurochem 82:913–923
Bar Am O, Amit T, Youdim MBH (2004) Contracting neuroprotective and neurotoxic action of respective metabolites of anti-Parkinson drugs rasagiline and selegiline. Neurosci Lett 355:169–172
Bar-Am O, Weinreb O, Amit T, Youdim MBH (2010) The neuroprotective mechanism of 1-(R)-aminoindan, the major metabolite of anti-parkinsonian drug rasagiline. J Neurochem 112:1131–1137
Bortolato M, Chen K, Shih JC (2008) Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Delivery Rev 60:1527–1533
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Anal Biochem 72:248–254
Cao X, Li X-M, Mousseau DD (2009) Calcium alters monoamine oxidase-A parameters in human cerebellar and rat glial C6 cell extracts: possible influence by distinct signal pathways. Life Sci 85:262–268
Chen K, Ou X-M, Chen G, Choi SH, Shih JC (2005) R1, a novel repressor of the human monoamine oxidase A. J Biol Chem 280:11552–11559
De Girolamo LA, Hargreaves AJ, Billett EE (2001) Protection from MPTP-induced neurotoxicity in differentiating N2a neuroblastoma cells. J Neurochem 76:650–660
De Zutter GS, Davis RJ (2001) Pro-apoptotic gene expression mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Proc Natl Acad Sci USA 98:6168–6173
Desagher S, Osen-Sand A, Nichols A et al (1999) Bid-induced conformational changes of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol 144:891–901
Ebadi M, Brown-Borg H, Ren J, Sharma S, Shavali S, ReFaey HE, Carlson EC (2006) Therapeutic efficiency of selegiline in neurodegenerative disorders and neurological diseases. Curr Drug Targets 7:1513–1529
Edmondson DE, Binda C, Mattevi A (2007) Structural insights into the mechanism of amine oxidation by monoamine oxidases A and B. Arch Biochem Biophys 464:269–276
Egashira T, Sakai K, Sakurai M, Takayama F (2003) Calcium disodium edetate enhances type A monoamine oxidase activity in monkey brain. Biol Trace Elem Res 94:203–211
Finberg JPM, Youdim MBH (2002) Pharmacological properties of the anti-Parkinson drug rasagiline; modification of endogenous brain amines, reserpine reversal, serotonergic and dopaminergic behaviours. Neuropharmacology 43:1110–1118
Fitzgerald JC, Ufer C, De Girolamo LA, Kuhn H, Billet EE (2007) Monoamine oxidase-A modulates apoptotic cell death induced by staurosporine in human neuroblastoma cells. J Neurochem 103:2189–2199
Heikkila RE, Manzino L, Cabbat FS, Duvoisin RN (1985) Studies on the oxidation of the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by monoamine oxidase B. J Neurochem 45:1049–1054
Hirano F, Tanaka H, Hirano Y, Hiramoto M, Handa H, Makino I, Scheidereit C (1998) Functional interference of Sp1 and NF-κB through the same DNA binding site. Mol Cell Biol 18:1266–1274
Hubalek F, Binda C, Ki M, Herzig Y, Sterling J, Youdim MBH, Mattevi A, Edmondson DE (2004) Inactivation of purified human recombinant monoamine oxidase A and B by rasagiline and its analogues. J Med Chem 47:1760–1768
Hynson RMG, Kelly SM, Price NC, Ramsay RR (2004) Conformational changes in monoamine oxidase A in response to ligand binding or reduction. Biochim Biophys Acta 1672:60–66
Inaba-Hasegawa K, Akao Y, Maruyama W, Naoi M (2012) Type A monoamine oxidase is associated with induction of neuroprotective Bcl-2 by rasagiline, an inhibitor of type B monoamine oxidase. J Neural Transm 119:405–414
Konradi C, Riederer P, Youdim MB (1986) Hydrogen peroxide enhances the activity of monoamine oxidase type B but not of type-A: a pilot study. J Neural Transm Suppl 22:61–63
Kraml M (1965) A rapid microfluorometric determination of monoamine oxidase. Biochem Pharmacol 14:1684–1686
Lamensdorf I, Youdim MBH, Finberg JPM (1996) Effect of long-term treatment with selective monoamine oxidase A and B inhibitors on dopamine release from rat striatum in vivo. J Neurochem 67:1532–1539
Maruyama W, Naoi M (2012) Induction of glial cell line-derived and brain-derived neurotrophic factors by rasagiline and (−)deprenyl: a way to disease-modifying therapy? J Neural Transm. doi:10.1007/s00702-0876-x
Maruyama W, Nitta A, Shamoto-Nagai M, Hirata Y, Akao Y, Youdim M, Furukawa S, Nabeshima T, Naoi M (2004) N-Propargyl-1-(R)-aminoindan, rasagiline, increases glial cell line-derived neurotrophic factor (GDNF) in neuroblastoma SH-SY5Y cells through activation of NF-κB transcription factor. Neurochem Int 44:293–400
Naoi M, Maruyama W (2010) Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr Pharmaceut Des 16:2799–2817
Naoi M, Maruyama W, Yi H, Akao Y, Yamaoka Y, Shamoto-Nagai M (2007) Neuroprotection by propargylamines in Parkinson’s disease: intracellular mechanism underlying the anti-apoptotic function and search for clinical markers. J Neural Transm Suppl 72:121–131
Naoi M, Maruyama W, Inaba-Hasegawa K, Akao Y (2011) Type A monoamine oxidase regulates life and death of neurons in neurodegeneration and neuroprotection. Inter Rev Neurobiol 100:85–106
Ou X-M, Chen K, Shih JC (2006a) Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc Natl Acad Sci USA 103:10923–10928
Ou X-M, Chen K, Shih JC (2006b) Glucocorticoid and androgen activation of monoamine oxidase A is regulated differently by R1 and Sp1. J Biol Chem 281:21512–21525
Ou X-M, Lu D, Johnson C, Chen K, Youdim MBH, Rajkowska G, Shih JC (2009) Glyceraldehyde-3-phosphate dehydrogenase-monoamine oxidase B-mediated cell death-induced by ethanol is prevented by rasagiline and 1-R-aminoindan. Neurotox Res 16:148–159
Parkinson Study Group (2002) A controlled trial of rasagiline in early Parkinson disease: the TEMPO study. Arch Neurol 59:1937–1943
Pizzinat N, Marchal-Victorion S, Maurel A, Ordener C, Bompart G, Parini A (2003) Substrate-dependent regulation of MAO-A in rat mesangial cells: involvement of dopamine D2-like receptors. Am J Physiol Renal Physiol 284:F167–F174
Riederer P, Lachenmayer L, Laux G (2004) Clinical applications of MAO-inhibitors. Curr Med Chem 11:2033–2043
Shih JC, Chen K, Ridd MJ (1999) Monoamine oxidase: from genes to behavior. Ann Rev Neurosci 22:197–217
Shih JC, Boyang J, Chen K (2011) Transcriptional regulation and multiple functions of MAO genes. J Neural Transm 118:979–986
Tazik S, Johnson S, Lu D, Johnson C, Youdim MBH, Stockmeier OuX-M (2009) Comparative neuroprotective effects of rasagiline and aminoindan with selegiline on dexamethasone-induced brain cell apoptosis. Neurosci Res 15:284–290
Wang J, Edmondson DE (2011) Topological probes of monoamine oxidases A and B in rat liver mitochondria: inhibition by TEMPO-substituted pargyline analogues and inactivation by proteolysis. Biochemistry 50:2499–2505
Wong WK, Ou XM, Chen K, Shih JC (2002) Activation of human monoamine oxidase B gene expression by a protein kinase C MAPK signal transduction pathway involves c-Jun and Egr-1. J Biol Chem 277:22222–22230
Wu JB, Shih JC (2011) Valproic acid induces monoamine oxidase A via Akt/Forkhead Box O1 activation. Mol Pharmacol 80:714–723
Yi H, Akao Y, Maruyama W, Chen K, Shih J, Naoi M (2006) Type A monoamine oxidase is the target of an endogenous dopaminergic neurotoxin, N-methyl(R)salsolinol, leading to apoptosis in SH-SY5Y cells. J Neurochem 96:541–549
Youdim MBH, Bakhle YS (2006) Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. Br J Pharmacol 147:S287–S296
Youdim MBH, Edmondson D, Tipton KF (2006) The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 7:295–309
Zhu W, Xie W, Pan T, Jankovic J, Youdim MBH, Le W (2008) Comparison of neuroprotective and neurorestorative capacities of rasagiline and selegiline against lactacystin-induced nigrostriatal dopaminergic degeneration. J Neurochem 105:1970–1978
Acknowledgments
This work was supported by the Research Grant for Longevity Sciences (21A-13, 23A-2) from the Ministry of Health, Labour and Welfare (W. M., M. N).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inaba-Hasegawa, K., Akao, Y., Maruyama, W. et al. Rasagiline and selegiline, inhibitors of type B monoamine oxidase, induce type A monoamine oxidase in human SH-SY5Y cells. J Neural Transm 120, 435–444 (2013). https://doi.org/10.1007/s00702-012-0899-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-012-0899-3