Abstract
Neuroprotection has been proposed in neurodegenerative disorders, such as Parkinson’s and Alzheimer’s diseases, to delay or halt disease progression or reverse neuronal deterioration. The inhibitors of type B monoamine oxidase (MAO), rasagiline and (−)deprenyl, prevent neuronal loss in cellular and animal models of neurodegenerative disorders by intervening in the death signal pathway in mitochondria. In addition, rasagiline and (−)deprenyl increase the expression of anti-apoptotic Bcl-2 protein family and neurotrophic factors. Neurotrophic factors, especially glial cell line-derived neurotrophic factor (GDNF) and brain-derived derived neurotrophic factor (BDNF), are required not only for growth and maintenance of developing neurons, but also for function and plasticity of distinct population of adult neurons. GDNF and BDNF have been reported to reduce Parkinson and Alzheimer’s diseases, respectively. GDNF protects the nigra-striatal dopamine neurons in animal models of Parkinson’s disease, and its administration has been tried as a disease-modifying therapy for parkinsonian patients. However, the results of clinical trials have not been fully conclusive and more practical ways to enhance GDNF levels in the targeted neurons are essentially required for future clinical application. Rasagiline and (−)deprenyl induced preferentially GDNF and BDNF in cellular and non-human primate experiments, and (−)deprenyl increased BDNF level in the cerebrospinal fluid of parkinsonian patients. In this paper, we review the induction of GDNF and BDNF by these MAO inhibitors as a strategy of neuroprotective therapy. The induction of prosurvival genes is discussed in relation to a possible disease-modifying therapy with MAO inhibitors in neurodegenerative disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroprotective therapy is proposed to delay disease progression or reverse neuronal loss in Parkinson’s disease (PD), Alzheimer’s disease (AD) and other neurodegenerative disorders. The inhibitors of type B monoamine oxidase (MAO-B), (−)deprenyl (selegiline) and rasagiline, are among the most promising drug candidates for neuroprotection (Ebadi et al. 2006; Youdim et al. 2006; Naoi and Maruyama 2009, 2010; Naoi et al. 2007; Riederer and Laux 2011). MAO-B inhibitors prevent neuronal death in the cellular and animal models through suppression of death signaling and induction of pro-survival, anti-apoptotic Bcl-2 and neurotrophic factor (NTF) genes.
NTFs are associated with the growth and survival of neurons during development, and the synaptic function and plasticity of adult neurons. NTFs are composed of four major groups: neurotrophins [nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3, -4 (NT-3, 4)], glial cell line-derived neurotrophic factor (GDNF) family of ligands (GFLs) [GDNF, neurturin, artemin, persephin], neurotrophic cytokines (neurokines), and a new family of cerebral dopamine (DA) neurotrophic factor and mesencephalic astrocyte-derived neurotrophic factors. NGF and BDNF are required for hippocampal plasticity and their reduction is associated with degeneration of cholinergic neurons and cognition impairment in AD (Conner et al. 2009). On the other hand, GDNF was reported to decrease in surviving neurons of the substantia nigra in the brain of PD patients (Chauhan et al. 2001).
NTFs are proposed as therapeutic agents to protect neurons in PD, AD, Huntington’s disease and amyotrophic lateral sclerosis (Rangasamy et al. 2010; Aron and Klein 2011). GDNF protects neurons in animal and cellular models of PD (Cohen et al. 2011), whereas BDNF protects neurons in AD models (Nagahara et al. 2009). However, clinical trials of NGF in AD failed to prove beneficial effects and had to be abandoned because of adverse events. Therefore, this review deals mainly with GDNF and its role in PD. GDNF cannot be transported into the brain through the blood–brain barrier (BBB), and several delivery methods have been proposed. As an alterative practical system, molecules permeable through the BBB, including MAO-B inhibitors, are proposed to enhance the endogenous synthesis of GFLs and nurotrophins.
This review presents the induction of neuroprotective GDNF and BDNF by rasagiline and (−)deprenyl in cellular models and the molecular mechanism underlying the gene induction. Rasagiline increased GDNF expression in the cerebrospinal fluid (CSF) of non-human primates after systemic administration, whereas (−)deprenyl increased BDNF in the CSF in parkinsonian patients. These results suggest that GDNF or BDNF may be preferentially induced by rasagiline or (−)deprenyl, respectively. The role of MAO inhibitors in the induction of pro-survival genes is discussed as a disease-modifying therapy for neurodegenerative disorders.
Neurotrophic factors in neuro-psychiatric disorders
After the findings on NGF (Levi-Montalcini and Hamburger 1951), NTFs have been expected to protect neurons from degeneration and repair the deteriorated structure and function of specific neurons in the brain. In normal aging and AD, the expression of BDNF and its receptors (TrkB.FL, TrkB.T1, TrkB.T2) was reported to decrease, suggesting their role in cognitive function (Tapia-Arancibia et al. 2008). BDNF supports the survival of cholinergic neurons and also shows a neurotrophic effect on DA neurons in PD models. Postmortem studies of patients with depression showed reduced BDNF levels in the hippocampus and cerebral cortex (Altar 1999). In rat model of depression, BDNF infused in the dentate gyrus of hippocampus produced an anti-depressant effect (Shirayama et al. 2002). These results suggest the association of BDNF with the pathophysiology of depression.
GDNF and neurturin promote survival of DA and other neurons, and are proposed to protect neurons in PD (Hong et al. 2008). In the brain of PD patients, GDNF was reported to decrease (Chauhan et al. 2001) and GDNF administration protected DA neurons in rodent and primate models of PD (Zhang et al. 1997; Grondin et al. 2002; Kells et al. 2010). Treatment of PD patients with GDNF was performed by use of direct infusion into the putamen, BBB-permeable GDNF derivatives, viral vector delivery system with recombinant adeno-associated viruses or lentiviruses, or by transplantation of neural stem cells and genetically modified astrocytes and fibroblasts (Hong et al. 2008; Aron and Klein 2011). The beneficial results of GDNF treatment were reported in the phase I clinical trials (Gill et al. 2003; Slevin et al. 2007), but the phase III trials did not present concrete positive results (Lang et al. 2006). The limited penetration of administered GDNF through the BBB and into target neurons and the rapid clearance from the brain may have caused the disappointing results.
GDNF regulates neuronal survival through activation of cellular signaling, mRNA translation and new protein synthesis. GDNF binds to a selective receptor, GFRα1-α4, and activates GFRα-Ret complex, and its phosphorylation activates the downstream signal pathway. GDNF binds also to another receptor, the neuronal cell adhesion molecules (NCAM), and activates focal adhesion kinase (FK) and Fyn kinase. In addition, GDNF protects neurons by suppressing death receptor–caspase pathway (Yu et al. 2008). GDNF activates pro-survival pathways, including phosphatidyl-3-kinase (PI3K)/Akt, Ras/extracellular-signal-regulated kinase (ERK), Src kinase and phospholipase C-γ (PLC-γ) pathway (Mograbi et al. 2001). GDNF suppresses mitochondria-dependent death pathway by inhibition of caspase-3 activation, endoplasmic reticulum stress and translocation of apoptogenic Bax, and by upregulation of anti-apoptotic Bcl-2 and Bcl-XL (Ghribi et al. 2001; Li et al. 2007). GDNF also suppresses microglia activation and subsequent increase in oxidative stress and inflammatory reaction (Xing et al. 2010).
On the other hand, neurotophins activate one or more of the tropomysin-related kinase (Trk) family of receptor tyrosine kinase (TrkA, TrkB and TrkC) and also p75 neurotrophin receptor (p75NTR), a member of tumor necrosis factor receptor superfamily (Reichardt 2006). Neurotrophins activated signal pathways in the downstream of Trks, Ras, PI3-linase, PLC-γ1 and MAP kinase. Through p75NTR, neurotrophins activate nuclear factor-κB (NF-κB) and Jun kinase, and promote NF-κB-dependent neuronal survival.
Neuroprotection by rasagiline and (−)deprenyl and induction of GDNF and BDNF
Rasagiline and (−)deprenyl protect neurons from cell death through preventing oxidative stress and production of neurotoxins from protoxicants, such as MPP+ from MPTP. In intrinsic apoptosis, rasagiline and (−)deprenyl directly suppress the activation of mitochondrial death pathway (Naoi and Maruyama, 2009, 2010). (−)Deprenyl, rasagiline and structurally related propargylamine derivatives of MAO inhibitors protect neurons in animal models of PD (Kupsch et al. 2001), multiple system atrophy (Stefanova et al. 2008) and amyotrophic lateral sclerosis (Kupershmidt et al. 2009).
These MAO-B inhibitors induce neuroprotective genes, including anti-apoptotic Bcl-2 (Akao et al. 2002) and GDNF (Maruyama et al. 2004), and anti-oxidant enzymes and redox protein (Andoh et al. 2005; Nakaso et al. 2006).
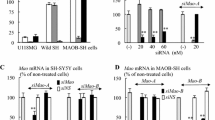
Rasagiline and (−)deprenyl preferentially increase the expression of mRNA and protein of GFLs or neurotrophins, respectively, in human dopaminergic neuroblastoma SH-SY5Y cells (Maruyama et al., in preparation). The amounts of GDNF and neurotrophins were quantified as reported previously using the enzyme immunoassay (EIA) (Maruyama et al. 2004). Figure 1 shows that rasagiline increased GDNF protein in the cells more markedly than (−)deprenyl and the increase was the highest at 10−7 M. (−)Deprenyl induced BDNF expression and the increase was marked at 10−9–10−8 M. Ladaostigil [TV3326, (N-propargyl)-(3R)-aminoindan-5-yl]-ethyl methyl carbamate], a cholinesterase and MAO inhibitor, increased BDNF levels to 10−5–10−4 M, but did not affect GDNF. The (S)-enantiomers of rasagiline and deprenyl, desmethyldeprenyl and aminoindan, a rasagiline metabolite, did not affect the GDNF levels.
Effects of (−)deprenyl, rasagiline and ladostigil on NTF expression in SH-SY5Y cells. SH-SY5Y cells were incubated with the MAO-B inhibitors for 24 h, in Hanks’ minimum essential medium (MEM). GDNF (a) and BDNF (b) were quantified by the EIA. Experiments were repeated at least three to four times in triplicate or quadruplicate measurements, and the column and bar represent the mean and SD. Differences were statistically evaluated by analysis of variance (ANOVA) followed by Scheffe’s F test. * and ** significantly different from control, p < 0.05 and 0.01
NTF induction by rasagiline has been confirmed in animal experiments. Rasagiline and (–)deprenyl increased mRNA levels of GDNF, NT-3 and NGF, and rasagiline increased BDNF protein levels in rat midbrain (Weinreb et al. 2009). We also proved NTF induction by rasagiline in non-human primates. Rasagiline (0.1, 0.25 and 2 mg/day) was systematically administered in Japanese monkeys (n = 4) for 4 weeks by daily subcutaneous injection and the cerebrospinal fluid (CSF) was taken once a week (Maruyama et al., in preparation). The levels of GDNF and neurotrophins (NGF, BDNF, NT-3) in the CSF were quantified by the EIA assay. As shown in Fig. 2, rasagiline at 0.25 mg/day significantly increased GDNF levels. Rasagiline at 0.25 and 0.1 mg/day induced also NGF, BDNF and NT-3, but at 1 mg/day did not increase these four NTF levels in the CSF. Increased levels of NTF, caused by rasagiline, returned to the basal values after 1 week’s washout.
Effects of systematic administration of rasagiline on NFT levels in the CSF from Japanese monkeys. Rasagiline (2, 0.25 and 0.1 mg/day) and saline as control were subcutaneously administered in Japanese monkeys (n = 4 for each groups), and the lumbar CSF was taken from anesthetized monkeys four times, once in every week. White and black boxes, black and white circles represent the NFT levels in monkey group treated with saline or 0.1, 0.25 and 2 mg/day rasagiline, respectively. a, b, c, and d: GDNF, BDNF, NGF and NT-3 levels. NTF levels were quantified by the EIA and the point and bar represent the mean of triplicate measurements of four samples. *, significantly different from the basal levels, p < 0.05, using Student’s two-tailed t test. [The Ethical Committee of Kyoto University Primate Research Institute, Inuyama, Aichi, Japan, approved the research project and the experiment protocol]
NTF induction by (−)deprenyl was investigated in parkinsonian patients; (−)deprenyl increased BDNF levels in the CSF (Maruyama et al., in preparation). Eight patients, who were diagnosed as having PD on the basis of neurological examinations and neuroimaging in Kushiro Rosai Hospital (Kushiro, Hokkaido, Japan), were subjected to this experiment. Before and after treatment with (−)deprenyl (5 mg/day, for 2–8 weeks orally), the lumbar CSF was taken and NTF contents were quantified by EIA assay. In five patients, BDNF levels increased markedly (Fig. 3). The mean and SD of the BDNF levels in the CSF before and after the treatment were 1.93 ± 1.06 and 7.17 ± 4.98 pg/ml and the difference was significant, p < 0.025, using Student’s two-tailed t test. GDNF was not increased by (−)deprenyl in a majority of the patients, but increased markedly in three patients with the basal levels lower than 4 pg/ml. The average GDNF values were not changed by (−)deprenyl treatment: 3.56 ± 2.59 versus 3.63 ± 3.43 pg/ml.
Effects of (−)deprenyl on BDNF and GDNF levels in the CSF from parkinsonian patients. The CSF was taken before and after (−)deprenyl treatment. BDNF (a) and GDNF (b) were quantified by EIA, and each connected point represents the sample from the same patients (n = 8). *Significantly different from the basal levels, p < 0.05, using Student’s two-tailed t test. [The patients were fully informed about the aims and the potential risks of CSF sampling and the analysis of BDNF and GDNF. The Ethical Committee of Kushiro Hospital, Kushiro, Hokkaido, Japan, approved the protocol of this study]
The role of MAO in the induction of pro-survival genes by MAO-B inhibitors
The role of MAO in the induction of pro-survival genes, including NTFs and anti-apoptotic Bcl-2, by rasagiline and (−)deprenyl has not been clarified. These MAO-B inhibitors increase neuroprotective genes at concentrations quite lower than those required for the inhibition of enzymatic activity, suggesting that gene induction does not depend on inhibition. Rasagiline and (−)deprenyl increase pro-survival genes in cells expressing only MAO-A, but not MAO-B, such as SH-SY5Y and PC12 cells. Only the (R)-isomer of rasagiline and deprenyl can increase Bcl-2 and NTFs, suggesting the presence of the binding site that initiates signal pathway to induce pro-survival genes. We found that MAO-A is required for the induction of pro-survival Bcl-2 (Inaba-Hasegawa et al. 2012). The reduction of MAO-A expression with short-interfering (si)RNA suppressed the Bcl-2 induction by rasagiline, (−)deprenyl and befloxatone, a reversible MAO-A inhibitor. The involvement of MAO-A was also indicated by Bcl-2 induction of moclobemide, another reversible MAO-A inhibitor (Chiou et al. 2006). These results suggest that compounds with affinity to MAO-A also might induce neuroprotective genes.
The intracellular signal pathway to induce pro-survival genes may be common for Bcl-2 and NTFs. The ERK 1/2-NF-κB pathway mediates the induction of GDNF and Bcl-2 (Maruyama et al. 2004; Inaba-Hasegawa et al. 2012). The GDNF gene has multiple transcription factor binding sites at the promoter sequence, such as camp response element (CRE) binding protein (CREB), which may function as a transcription factor in regulating GDNF expression. CREB phosphorylation is induced by activation of distinct signal systems: protein kinase C (PKC), calmodulin kinase II (CaMKII) and ERK 1/2 and serine/threonine kinase Akt. Rasagiline activates these signal pathways and upregulates the endogenous NTF synthesis (Weinreb et al. 2007).
We found that the type of MAO may be associated with the preferential induction of GDNF or BDNF by rasagiline and (−)deprenyl (Inaba-Hasegawa et al., in preparation). Rasagiline and (−)deprenyl induced GDNF preferentially in neuroblastoma MAO-A-expressing SH-SY5Y cells and neurotrophin human glioblastoma MAO-B-expressing U118MG cells. These results suggest that the two classes of NTFs may be induced in the targeted cell types, depending on the presence of MAO-A or MAO-B. MAO-A and MAO-B are distributed in distinct neuron populations and brain regions. MAO-A is expressed in the catecholaminergic and dopaminergic cell groups, whereas MAO-B is present in the serotonergic and histaminergic neurons, as well as astrocytes (Bortolato et al. 2008). The compounds which can selectively induce GFLs and neurotrophins may be applied as neuroprotective therapy targeted at distinct neuronal or glial population in the brain.
MAO inhibitors may bind to MAO at the site without inhibiting the activity, or to other amine oxidase, D1, D2 receptors or other protein in neuronal cells (see references in the review, Naoi and Maruyama 2009). In addition to MAO inhibitors, NTFs are induced by compounds increasing DA transmission (DA agonists, L-DOPA), anti-depressants, anti-psychotics, anti-dementia drugs, immunophilin ligands and neurotrophic hormones (melatonin, estrogen, vitamin D3) (Saavedra et al. 2008). DA, noradrenaline and adrenaline increase the mRNA and protein levels of NT-3 in primary cultured astrocytes (Mele et al. 2010). The studies on the target protein and binding site of these compounds may bring us clues to develop novel neuroprotective agents.
The criteria for considering neuroprotective drug candidates in PD have been proposed: scientific rationale; penetration of the BBB; safety and tolerability and efficiency in animal PD models; an indication of benefit in clinical studies for parkinsonian patients (Ravina et al. 2003). The effects on disease progression have been presently determined symptomatically using the United PD Rating Scale (UPDRS) or PD Quality of Life (PDQUAL) score. Using imaging with position emission tomography (PET) and single photon emission tomography (SPECT), the integrity of the DA system in the nigra striatum may be monitored (Booij and Berendsee 2011). However, these methods are not sensitive or specific to quantify the effects on progressive neuronal loss in neurodegenerative disorders. As a surrogate marker, NTF levels may indicate the potency of agents to delay, halt or repair neuronal loss in neurodegenerative disorders.
Abbreviations
- AD:
-
Alzheimer’s disease
- BBB:
-
Blood–brain barrier
- BDNF:
-
Brain-derive neurotrophic factor
- DA:
-
Dopamine
- GDNF:
-
Glial cell line-derived neurotrophic factor
- GFLs:
-
GDNF family of ligands
- NGF:
-
Nerve growth factor
- NT-3:
-
Neurotrophic factor-3
- NTF:
-
Neurotrophic factor
- PD:
-
Parkinson’s disease
References
Akao Y, Maruyama W, Yi H, Shamoto-Nagai M, Youdim MB, Naoi M (2002) An anti-Parkinson’s disease drug, N-propargyl-1(R)-aminoindan (rasagiline) enhances expression of antiapoptotic Bcl-2 in human dopaminergic SH-SY5Y cells. Neurosci Lett 326:105–108
Altar CA (1999) Neurotrophins and depression. Trends Pharmacol Sci 20:59–61
Andoh T, Chock B, Murphy DL, Chiueh CC (2005) Role of the redox protein thioredoxin in cytoprotective mechanism evoked by (−)-deprenyl. Mol Pharmacol 68:1408–1414
Aron L, Klein R (2011) Repairing the parkinsonian brain with neurotrophic factors. Trends Neurosci 34:88–100
Booij J, Berendsee HW (2011) Monitoring therapeutic effects in Parkinson’s disease by serial imaging of the nigrostriatal dopaminergic pathway. J Neurol Sci 310:40–43
Bortolato M, Chen K, Shih JC (2008) Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev 60:1527–1533
Chauhan NB, Siegel GJ, Lee JM (2001) Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson’s disease brain. J Chem Neuroanat 21:277–288
Chiou SH, Ku HH, Tsai TH et al (2006) Moclobemide upregulated Bcl-2 expression and induce neural stem cell differentiation into serotoninergic neurons via extracellular-regulated kinase pathway. Br J Pharmacol 148:587–598
Cohen AD, Zigmond MJ, Smith AD (2011) Effects of intrastriatal GDNF on the response of dopamine neurons to 6-hydroxydopamine: time course of protection and neurorestoration. Brain Res 1370:8–88
Conner JM, Franks KM, Titterness AK, Russell K, Christie BR, Sejnowski TJ, Tuszynski MH (2009) NGF is essential for hippocampal plasticity and learning. J Neurosci 29:10883–10889
Ebadi M, Brown-Borg H, Ren J, Sharma S, Shavali S, ReFacy HE, Carlson EC (2006) Therapeutic efficacy of selegiline in neurodegenerative disorders and neurological diseases. Curr Drug Targets 7:1513–1529
Ghribi O, Herman MM, Forbes MS, DeWitt DA, Savory J (2001) GDNF protects against aluminum-induced apoptosis in rabbits by upregulating Bcl-2 and Bcl-XL and inhibiting mitochondrial Bax translocation. Neurobiol Dis 8:764–773
Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P (2003) Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9:589–595
Grondin R, Zhang Z, Yi A (2002) Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain 125:2191–2301
Hong M, Mulhida K, Mendez I (2008) GDNF therapy for Parkinson’s disease. Expert Rev Neurother 8:1125–1139
Inaba-Hasegawa K, Akao Y, Maruyama W, Naoi M (2012) Type A monoamine oxidase is associated with induction of neuroprotective Bcl-2 by rasagiline, an inhibitor of type B monoamine oxidase. J Neural Transm 119:405–414
Kells AP, Eberling J, Su X et al (2010) Regeneration of the MPTP-lesioned dopaminergic system after convention-enhanced delivery of AAV2-GDNF. J Neurosci 30:9567–9577
Kupershmidt L, Weinreb O, Amit T, Mandel S, Carri MT, Youdim MBH (2009) Neuroprotective and neuritogenic activities of novel multimodal iron-chelating drugs in motor-neuron-like NSC-34 cells and transgenic mouse model of amyotrophic lateral sclerosis. FASEB J 23:3766–3779
Kupsch A, Sautter J, Götz ME et al (2001) Monoamine oxidase-inhibition and MPTP-induced neurotoxicity in the non-human primate: comparison of rasagiline (TVP1012) with selegiline. J Neural Transm 108:985–1009
Lang AE, Gill S, Patel NK et al (2006) Randomized controlled trial of intraputamental glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59:459–466
Levi-Montalcini R, Hamburger V (1951) Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool 116:321–362
Li X, Peng C, Li L, Ming M, Yang D, Le W (2007) Glial cell-derived neurotrophic factors protects against proteasome inhibition-induced dopamine neuron degeneration by suppression of endoplasmic reticulum stress and caspase-3 activation. J Gerontol 62A:943–950
Maruyama W, Nitta A, Shamoto-Nagai M, Hirata Y, Akao Y, Youdim M, Furukawa S, Nabeshima T, Naoi M (2004) N-Propargyl-1-(R)-aminoindan, rasagiline, increases glial cell line-derived neurotrophic factor (GDNF) in neuroblastoma SH-SY5Y cells through activation of NF-κB transcription factor. Neurochem Int 44:293–400
Mele T, Carman-Krzan M, Juric DM (2010) Regulatory role of monoamine neurotransmitters in astrocytic NT-3 synthesis. Int J Dev Neurosci 28:13–19
Mograbi B, Bocciardi R, Bourget I, Busca R, Rochet N, Farahi-Far D, Juhel T, Rossi B (2001) Glial cell line-derive neurotrophic factor-stimulated phosphatidylinositol 3-kinase and Akt activities exert opposing effects on the ERK pathway. J Biol Chem 276:45307–45319
Nagahara AH, Merrill DA, Coppola G et al (2009) Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med 15:331–337
Nakaso K, Nakamura C, Sato H, Imamura K, Takeshima T, Nakashima K (2006) Novel cytoprotective mechanism of anti-parkinsonian drug deprenyl: pI3K and Nrf2-derived induction of antioxidative proteins. Biochem Biophys Res Commun 339:915–922
Naoi M, Maruyama W (2009) Functional mechanism of neuroprotection by inhibitors of type B monoamine oxidase in Parkinson’s disease. Expert Rev Neurother 9:1233–1250
Naoi M, Maruyama W (2010) Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr Pharmaceut Design 16:2799–2817
Naoi M, Maruyama W, Yi H, Akao Y, Yamaoka Y, Shamoto-Nagai M (2007) Neuroprotection by propargylamines in Parkinson’s disease: intracellular mechanism underlying the anti-apoptotic function and search for clinical markers. J Neural Transm Suppl 72:121–131
Rangasamy AB, Soderstrom K, Bakay RAE, Kordower JH (2010) Neurotrophic factor therapy for Parkinson’s disease. Progress Brain Res 134:237–264
Ravina BM, Fagan SC, Hart RG et al (2003) Neuroprotective agents for clinical trials in Parkinson’s disease: a systematic assessment. Neurology 60:1234–1240
Reichardt LF (2006) Neurotrophin-regulated signaling pathways. Phil Trans R Soc B 361:1545–1564
Riederer P, Laux G (2011) MAO-inhibitors in Parkinson’s disease. Experiment Neurobiol 20:1–17
Saavedra A, Baltazar G, Duarte E (2008) Driving GDNF expression: the green and the red traffic lights. Progress Neurobiol 86:186–215
Shirayama Y, Chen ACH, Nakagawa S, Russel DS, Duman RS (2002) Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22:3251–3261
Slevin JT, Gash DM, Smith CD et al (2007) Unilateral intraputaminal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J Neurosurg 106:614–620
Stefanova N, Poewe W, Wenning GK (2008) Rasagiline is neuroprotective in a transgenic model of multiple system atrophy. Exp Neurol 210:421–427
Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S (2008) New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev 59:201–220
Weinreb O, Amit T, Bar-Am O, Youdim MBH (2007) Induction of neurotrophic factors GDNF and BDNF associated with the mechanisms of neurorescue action of rasagiline and ladostigil: new insights and implications for therapy. Ann NY Acad Sci 1122:155–168
Weinreb O, Amit T, Sagi Y, Drigues N, Youdim MBH (2009) Genomic and proteomic study to survey the mechanism of action of the anti-Parkinson’s disease drug, rasagiline compared with selegiline, in the rat brain. J Neural Transm 116:1456–1472
Xing B, Xin T, Zhao L, Hunter RL, Chen Y, Bing G (2010) Glial cell line-derived neurotrophic factor protects midbrain dopaminergic neurons against lipopolysaccharide neurotoxicity. J Neuroimmunol 225:43–51
Youdim MBH, Edmondson D, Tipton KF (2006) The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 7:295–309
Yu L, Saarma M, Arumae U (2008) Death receptors and caspases but not mitochondria are activated in the GDNF- or BDNF-derived dopaminergic neurons. J Neurosci 28:7467–7475
Zhang Z, Miyoshi Y, Lapchak PA et al (1997) Dose response to intraventricular glial cell line-derived neurotrophic factor administration in parkinsonian monkeys. J Pharm Exper Thera 282:1396–1401
Acknowledgments
This work was supported by the Research Grant for Longevity Sciences (21A-13, 23A-2) from the Ministry of Health, Labour and Welfare, Japan (W. M., M. N).
Author information
Authors and Affiliations
Corresponding author
Additional information
70th Birthday Professor Riederer.
Rights and permissions
About this article
Cite this article
Maruyama, W., Naoi, M. ‘‘70th Birthday Professor Riederer’’ Induction of glial cell line-derived and brain-derived neurotrophic factors by rasagiline and (−)deprenyl: a way to a disease-modifying therapy?. J Neural Transm 120, 83–89 (2013). https://doi.org/10.1007/s00702-012-0876-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-012-0876-x