Abstract
Introduction
Decompressive craniectomy (DC) is the most common surgical procedure to manage increased intracranial pressure (ICP). Hinge craniotomy (HC), which consists of fixing the bone operculum with a pivot, is an alternative method conceived to avoid some DC-related complications; nonetheless, it is debated whether it can provide enough volume expansion. In this study, we aimed to analyze the volume and ICP obtained with HC using an experimental cadaver-based preclinical model and compare the results to baseline and DC.
Methods
Baseline conditions, HC, and DC were compared on both sides of five anatomical specimens. Volume and ICP values were measured with a custom-made system. Local polynomial regression was used to investigate volume differences.
Results
The area of the bone opercula resulting from measurements was 115.55 cm2; the mean supratentorial volume was 955 mL. HC led to intermediate results compared to baseline and DC. At an ICP of 50 mmHg, HC offers 130 mL extra space but 172 mL less than a DC. Based on local polynomial regression, the mean volume difference between HC and the standard craniotomy was 10%; 14% between DC and HC; both are higher than the volume of brain herniation reported in the literature in the clinical setting. The volume leading to an ICP of 50 mmHg at baseline was less than the volume needed to reach an ICP of 20 mmHg after HC (10.05% and 14.95% from baseline, respectively).
Conclusions
These data confirm the efficacy of HC in providing sufficient volume expansion. HC is a valid intermediate alternative in case of potentially evolutionary lesions and non-massive edema, especially in developing countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the acute phase, the multidisciplinary management of severe TBI aims to prevent and treat secondary damage and stabilize the patient’s general and neurological condition. The goal of this treatment, medical and possibly surgical, is also the control of brain swelling and intracranial pressure (ICP) [1, 8, 10, 15, 16, 40]. ICP monitoring is essential for decision-making and medical and surgical treatment modulation [6, 37]. The decompressive craniectomy (DC) is considered the last step in the context of a “staircase approach” in treating traumatic intracranial hypertension [24, 46]. However, it has two main problems: the lack of clear indications for treatment and a shared ICP threshold. Multiple factors also seem to affect the outcome: for example, higher mortality and unfavorable outcomes have been reported in patients over 65 years of age [13]. The sum of all these uncertainties has led to the realization of two large randomized trials, the DECRA and RESCUEicp studies [9,10,11, 19, 22, 21, 48], which confirmed the effectiveness of DC in reducing ICP and as a life-saving procedure. However, reducing ICP does not automatically translate into improving the outcome [9, 11, 22, 21]. According to the latest update of guidelines, DC is recommended in case of a late increase in ICP that is refractory to therapy to improve mortality and outcome [19, 22]. Despite being a very effective tool in the control of ICP, DC is potentially burdened by non-negligible complications, together with cranioplasty and its related complications (10–40% in some series) [11, 22, 20, 31, 42, 43].
Another indication for DC is the malignant middle cerebral artery infarction or space-occupying hemispheric infarction. According to the European Stroke Organisation guidelines, in adult patients aged 60 years or younger, who can be treated within 48 h of stroke onset, DC is indicated to reduce the risks of death or a poor outcome [53]. Nevertheless, surgery should only be done after a shared decision process, including a careful discussion with the patient or their representatives about the risk of survival with substantial disability. Furthermore, the involvement of the internal carotid artery, or anterior or posterior cerebral arteries territories, the side of the stroke, the age cutoff, and how to behave after 48 h are still debated [53]. Additionally, only expert consensus statements exist regarding the role of ICP measurements in brain infarction. For these reasons, we mainly focused on the TBI in this study.
Although the DC is regarded as the standard procedure [19], the hinge craniotomy (HC) technique has been recently introduced [41]. HC allows for overcoming some DC complications and could play a crucial role in managing severely traumatized patients, especially in areas where resources are limited. A factor limiting its widespread use is fear that the technique does not allow a sufficient volume for brain expansion. HC consists in repositioning the bone operculum and fixing it with titanium plates that maintain a hinged margin, thus allowing a subtotal opening [41]. Clinical series (for a total of almost 300 patients) have shown that HC is an effective technique in controlling ICP of moderate-grade cerebral edema [32]. It allows maintenance of brain protection, reduces post-surgical complications, avoids cranioplasty, and is associated with an excellent esthetic result [32]. In recent years, several clinical studies have been conducted on cadavers and 3D models to demonstrate the feasibility and clinical effectiveness of HC. Nevertheless, achieving an adequate volume of brain expansion and determining if and how many patients may need a subsequent standard DC have yet to be determined [32, 44]. To our knowledge, only another recent study focused on measuring the difference in the pressure-volume relationship between HC and DC in a preclinical setting [45].
Therefore, this study aimed to analyze, with a novel experimental cadaver-based preclinical model, the available volume obtained with an HC and its ICP trend and to compare the results with those of a baseline condition (craniotomy fixed with plates) following a DC.
Materials and methods
This work was conducted in accordance with the institutional ethical committee guidelines and was performed according to the ethical standards of our institutional review board. Five fresh anatomical specimen heads, provided by MedCure (MedCure, Inc. Portland, USA), were used for the current study. All procedures were carried out in the human anatomy laboratory at the University of Brescia. The arterial system was injected with a silicone resin (Xiameter® RTV, Dow Corning, Midland, MI, USA) and stained with red dye (Pintasol®, Mixol Red E-L3mix, Kirchheim unter Teck, Germany) to highlight the course of the arterial network. The preparations were defrosted 24 h before and placed at 4 °C until when used. On each specimen, a DC and an HC were performed on each side (n = 10 for each technique).
Surgical technique
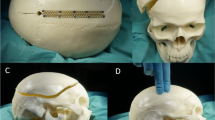
The procedures were carried out after fixing the specimen to the operating table with a Mayfield skull clamp and rotating it contralaterally. Following a large frontal-temporal-parietal “trauma flap” incision, the temporal muscle was incised, dissected, and retracted antero-inferiorly to allow adequate exposure of the pole and base of the temporal lobe. Two burr holes were performed, using a perforator (Bien-Air Surgery® SA; Le Noirmont, CH), at the pterional key hole and the posteroinferior margin of the craniotomy, respectively. The margins of the craniotomy were drawn with a marker, and the measurements were made with respect to the minimum dimensions recommended by guidelines (Fig. 1) [19]. A frontal-temporal-parietal craniectomy was performed using a craniotome (Bien-Air Surgery SA; Le Noirmont, CH). Then, the largest diameters of the bone volet were recorded. The craniectomy was completed at the temporal base and pole level until adequate exposure was obtained. Subsequently, the dura mater was incised and opened (Fig. 1), and the brain parenchyma, up to the level of the tentorial hiatus and the falx along its entire length, was removed. This last step created a large chamber to allow the measuring system to best adapt to the cranial cavity. To avoid a transtentorial herniation with possible alteration of the volumetric and pressure data, a duroplasty, reinforced with a titanium mesh, was performed at the level of the tentorial hiatus (Fig. 1). An enlarged burr hole was made posteriorly, close to the midline, for the insertion of the plastic casing connected to the washing and pressure measurement system,
Dissection steps. A Bone flap measurements after dissecting epicranial tissues; B craniotomy as wide as reported in guidelines on DC; C dural opening to prepare the experimental model; D removal of the falx cerebri to create a unique supratentorial space; and E duroplasty at the level of the tentorial notch
Intracranial pressure measurement system
The ICP measurement system (Fig. 2) consisted of a plastic bag with a volume of 3 L, whose extremity was connected to a washing catheter containing an ICP probe (IntegraTM Camino®, Integra Lifescience, Princeton, NJ, USA). The end of the catheter was connected to a syringe system for injecting and aspirating saline solution from a graduated can through a three-way connector. Saline solution was then infused until a mean physiological value of 10 mmHg ICP was reached [35]. The corresponding volume was defined as the baseline value for that specific specimen.
Subsequently, ICP values were progressively documented every 5/10 mL of saline infusion steps until achieving 50 mmHg ICP. Although not relevant in clinical practice, this value was chosen to acquire more values and improve the study’s validity. The exact process was repeated on each side in the following conditions: baseline (craniotomy fixed with plates), HC, and DC.
Baseline craniotomy
Once the system was inserted, the bone operculum was repositioned and fixed with plates. Skin and subcutaneous tissues were sutured. Measurements were then taken as described previously.
Hinge craniotomy
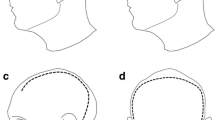
The sutures were removed, and the bone flap was fixed only along the frontal and parietal margin at the level of the midline with the use of two plates (Fig. 3). This system, functioning as a hinge or pin along the medial edge, allowed the bone volet to rise along the basal, anterior, and posterior margins. The sinking of the bone operculum at the resolution of the raised ICP state can be avoided by placing plates along the free margins but fixing them only to the volet. Our technique is comparable to those employed by Søndergaard et al., who used a parasagittal titanium mesh (whose rigidity prevents the bone from sinking when intracranial pressure decreases) [45].
At this point, skin and subcutaneous tissue were sutured again. Subsequently, the initial saline solution volume was restored, and seriate measurements were repeated.
Decompressive craniectomy
As a third step, the stitches and the bone operculum were removed. The skin and subcutaneous tissues were then sutured again. As before, the baseline volume was restored, and serial measurements were repeated.
After completing measurements on one side, the system was emptied, and the bone flap was repositioned and fixed with screws. The same steps were then repeated contralaterally.
Data processing
The volumes and corresponding pressure values were recorded on a spreadsheet. The values were recorded for each specimen, divided into the right and left sides, and then in the three steps (basal, HC, DC). Next, the percentage increase in volume compared to baseline (corresponding to 10 mmHg value) was calculated up to 50 mmHg. Measurements were compared between the different techniques to highlight the differences in volume obtained with each specimen. Subsequently, a statistical approximation was performed using the kernel-weighted local polynomial regression of two variables, which analyzes the distribution of the different methods using SPSS® statistic software (IBM®, Armonk, NY, USA).
Results
The average diameter of the bone opercula was 14 ± 0.9 × 10.6 ± 0.73 cm for a total mean area of 115.55 cm2. The mean volume of the supratentorial compartment was 955 mL (Table 1).
Figure 4 shows the pressure values obtained by progressive volume changes, expressed in mmHg, on the y-axis for each specimen. In all ten sides, a homogeneity of distribution of values within each type of craniotomy was observed. The values of fluid volume, expressed in terms of volume difference from baseline and percentage at different ICP measurements, are reported in Table 1. The HC appeared to be approximately placed in an intermediate position with respect to the other two procedures; at 20 mmHg, indeed, the HC allows for a 143 mL further expansion compared to the baseline, but 137 mL less than DC. Assuming that a pressure of 50 mmHg represents the maximum expansion of the system, HC offers approximately 130 mL of extra space, compared to baseline, and about 172 mL less than a DC.
Relationship between the volume changes in mL (x-axis) and the intracranial pressure (ICP) in mmHg (y-axis). Each graph corresponds to one side of the five specimens (1–5) and shows the pressure values obtained with the three procedures. DC, decompressive craniectomy; HC, hinge craniotomy; ICP, intracranial pressure; L, left side; R, right side
Figure 5 shows the distribution of the three different procedures as a function of ICP (x-axis) and percentage of volume variations (y-axis). Using ordinary least squares, we estimated the best polynomial fit and, from this, the expected mean value of ICP associated with each volume. Comparing the values obtained with each procedure, the mean difference in volume between HC and the standard craniotomy was 10% of the baseline volume, whereas between DC and HC was 14%.
Representation of the “kernel-weighted” local polynomial regression between ICP in mmHg (x-axis) and percentage of volume increase (y-axis). The points correspond to single measurements in each specimen. The lines show each procedure’s best-fit values (with a 95% confidence interval). HC, hinge craniotomy; DC, decompressive craniectomy
It should be noted that after the simulation and reading of the pressures of the HC, there was no noticeable damage to the system, breakage of plates, or loosening of screws.
Discussion
Traumatic brain injury (TBI) contributes to disabilities and deaths worldwide much more than any other traumatic pathology, especially in low- and middle-income countries [14, 39, 51]. Head trauma initiates a cascade of pathophysiological mechanisms that may result in a severe patient prognosis or a fatal outcome [14]. Primary damage is determined directly by the mechanical action of traumatic forces. However, secondary damage, which may develop after the trauma due to circulatory disorders, hypoxia, hypercapnia, or hypoglycemia, can be even more dangerous than primary traumatic force [8, 6]. Therefore, it is essential to prevent its effects by applying measures, eventually including surgery, according to the values of ICP [10, 6]. DC, despite being a very effective tool in the control of ICP, is potentially burdened by non-negligible complications [20, 31, 42, 43], which HC could overcome [41, 30, 32]. Indeed, this appears even more relevant in developing countries. Not only are these countries the most affected by TBI, but their healthcare systems are often non-existent or inaccessible [14].
Surgical technique for HC
The techniques described in the literature differ to some extent, but they all follow two main principles: have a bone margin hinged to the skull to allow free movement along its axis; have support systems that avoid the sinking of the operculum once the ICP decreases. Described for the first time by Ko et al. and Schmidt et al. [29, 41], there were later various publications from different world regions, based on small case series, in the following years. In most studies, in the initial phase, the same steps of DC are followed: unilateral incision of the skin and subcutaneous tissue, removal of the bone operculum (at least 12 cm wide) [41], dural opening, and possible evacuation of the post-traumatic lesion. As with DC, performing a sizeable dural plastic is advisable, following the technique and materials used in individual institutions. The fixing of the bone operculum is where the most significant variability between the published studies is observed. The cardinal principle consists in obtaining a margin of the bone operculum “hinged” to the cranial theca, leaving the other margins free to move but not to sink into the intracranial space, thanks to the support of plaques. A Y-plate is placed along the medial margin in such a way as to allow the bone flap to rise in case of cerebral edema. Along the anterior, posterior, and possibly basal margins, plates are fixed only to the mobile operculum. The temporal muscle is repositioned but not fixed tightly; then, the skin and subcutaneous layer suturing are done. After a few weeks, the bone operculum gradually returns to its original anatomical position [41]. In one study, the use of temporal muscle was proposed as the anchor point of the mobile bone operculum, partially secured with suture [3]; in other cases, sutures were used on the mobile bone operculum [18]; in one case, a resin mold of the removed bone operculum was implanted [4]. Recently, several clinical studies have been carried out on cadavers and 3D models to demonstrate the technique’s feasibility and clinical effectiveness.
Previous clinical studies
A recent meta-analysis, including the major studies published on HC, identified 283 patients [32]. Approximately 80% were victims of severe head trauma; the remaining suffered brain edema from a stroke. The most commonly encountered tomographic anomalies were midline shifts greater than 5 mm and obliteration of basal cisterns and convexity sulci [3,4,5, 17, 18, 25, 27, 29, 34, 36, 38, 41, 50, 52]. The effectiveness of HC in ICP control emerged from the same studies’ clinical experience. No significant difference between HC and DC was observed in terms of postoperative ICP (HC 12.1 ± 2.6 mmHg and DC 15.0 ± 6.3 mmHg) [26]; Gutman et al., starting from a mean pre-intervention ICP of 32.7 ± 8.1 mmHg, obtained a postoperative ICP of 16.0 ± 12.1 mmHg [18]. In the study by Valença et al., ICP decreased from 15–35 mmHg to 6–12 mmHg postoperatively [52]. Some authors comparing DC and HC showed that the two methods’ control of ICP in the postoperative period was equivalent [27]. Furthermore, a regression of the midline shift with both procedures has been reported [26, 38].
The data regarding outcomes are poor and heterogeneous, mainly described as comparable to those of DC patients or even better [50]. The observed survival rate following HC was 74.6% [32], with a better long-term outcome than DC [27]. A second DC surgery may be necessary in 3.2% of cases due to uncontrolled intracranial hypertension or failure of the previous surgery [32]. One case required a second surgery to secure the bone operculum (too mobile) [26]; one case needed cranioplasty for esthetic reasons (bone resorption) [12], but in most cases the esthetic result is satisfactory [4].
Previous cadaver studies
Studies on cadavers have also confirmed the efficacy in reducing ICP. One study measured ICP after a posterior circular hinge craniotomy (CPHC), demonstrating a significant reduction in ICP and a 10% increase in intracranial volume obtained with a frontal elevation of the cranial vault between 9 and 21 mm [49]. Three different surgical techniques were compared in another study: standard craniotomy (baseline), HC, and “dynamic telescopic craniotomy” (DTC), developed by the authors. Both techniques controlled ICP effectively: DTC and HC were superior in reducing ICP compared to standard craniotomy, allowing reasonable pressure control up to a volume of 120 mL greater than baseline. Above this volume, the telescopic seemed more effective [28]. Søndergaard et al. employed a preclinical cadaver model similar to the one described in our paper. They found that before ICP exceeded a threshold of 20 mmHg, HC and DC allowed an increase of 190 mL and 290 mL, respectively. They also reported computed tomography–derived calculations following HC: increased intracranial volume at ICP 20 mmHg equal to 60 mL, maximal increase of intracranial volume of 84 mL, and bone plate volume around 80 mL [45].
Results of the current study and comparison with other experimental cadaver studies
One of the primary limits of previous studies was using a skull without considering the effect of the presence of skin, subcutaneous tissue, and muscle. In addition, the geometry of convexity craniotomies may not reflect the dynamics observed in temporal and pterional areas. Furthermore, materials designed and built specifically for the study (i.e., telescope plates) were sometimes employed; these could hardly find a diffuse application in other contexts. Søndergaard et al. used cadavers with intact soft tissues and left brains inside the intracranial cavity. However, one of them (used for repeated measures) was alcohol fixed, therefore potentially altered in elastic properties [33, 45]. In our study, we used anatomical preparations that recreate in vivo dynamics as faithfully as possible to overcome these limitations. A standard surgical technique (wide frontal-temporal-parietal craniectomy) was performed; surgical materials commonly found in almost all operating rooms (plates and standard fixing screws) were employed; and the presence of skin, subcutaneous tissue, and muscle increased the fidelity of the experimental model. All three procedures were performed on both sides to better compare the results of the different surgical techniques (craniotomy, HC, and DC) on the same specimen. In detail, HC provided an extra volume for brain expansion of 188 mL before the ICP reached 30 mmHg and potentially up to 226 mL before 50 mmHg, compared to standard craniotomy. A DC offered 334 mL and 398 mL of extra volume, respectively. In the work by Khanna et al., surprisingly, the infusion of 240 mL did not increase ICP above 14 mmHg [28]. These data differ from our experience was probably because the craniotomy simulated in their experiment was a circular craniotomy at the level of the vertex, and there was no epicranial tissue to oppose resistance. In the editorial commentary on the work by Søndergaard et al., the small number of specimens was highlighted as a limitation [33], and we agree with that. Nevertheless, despite the differences between our model (brain and falx removal, exclusion of the posterior fossa with suture/mesh plates) and the model by Søndergaard et al., we achieved similar results, especially regarding the additional volume achieved with HC and DC from baseline, confirming the results of both preclinical models.
Comparing these results to clinical studies with volumetric analysis of brain herniation after a DC, Stoner et al. found a mean herniated volume of 30.48 ± 23.56 mL [47], significantly less than the space provided by the HC. Similar results have been found by Jasielski et al., who reported a mean volume of extra space filled by the swollen brain equal to 42.2 mL ± 40.7 [23]. One clear result from our measurement is that the percentage of volume increase that leads to an ICP of 50 mmHg at baseline is less than the percentage increase that produces an ICP of 20 mmHg when an HC is performed (10.05% and 14.95%, respectively; Table 1). Søndergaard et al. also measured the volume of bone flaps, which could partially explain the additional volume difference between DC and HC [45]. We did not perform such a measurement, as we feel that skull size, bone thickness, and bone flap width may influence the results too heavily. However, our data and those from their paper prove the volume gain, regardless of bone volume, but indirectly including the effect of removing it. Furthermore, it is well known now that a dural opening is required to achieve adequate ICP reduction [7]. Therefore, other factors play a significant role despite bone removal itself.
We estimated the best predictor of volumetric variation for a given ICP value by analyzing the regression curves and using ordinary least squares. An HC produces a mean volume gain of 10% from baseline. DC leads to an additional 14% increase. This data shows that, at the level of volumetric expansion, HC represents a valid intermediate choice between a standard craniotomy and DC. Indeed, in the study by Abdullah et al., the percentage of brain volume increase after a DC is approximately 9.6% [2]. From a clinical point of view, the effectiveness of the technique has been documented in several series published in the literature, and it is likely that, in most cases, volumes achieved with an HC are sufficient to ensure the adequate expansion of the cerebral parenchyma, also confirming previously reported results [45]. Based on the combined data from the two studies, the HC can be a valid alternative in case of potentially evolutionary lesions when the clinical and radiological picture does not undoubtedly indicate that DC is needed. This could have significant implications in treating patients requiring refractory ICP, potentially reserving DC as a second step after HC if the ICP still increases.
The next step would be to increase the significance of preclinical measurements and apply the HC in clinical practice, hypothetically starting with patients undergoing craniotomy for hematoma evacuation rather than secondary craniectomy for refractory ICP.
Study limitations
The first limitation is related to the HC itself, as it is difficult to predict the degree of swelling in individual patients. Thus, from a clinical point of view, the surgeon will have difficulties deciding if the patient needs a DC or if the HC could be sufficient.
The experimental model used has two main limitations. The elasticity and thickness of soft tissues, such as skin and muscle of an anatomical preparation, do not faithfully reflect the characteristics of the same tissues in vivo; furthermore, possible subgaleal blood collection or edema of the temporal muscle could decrease the available volume provided by HC in the clinical scenario. It should also be emphasized that the ICP values observed in the current study obviously do not reflect the complex pathophysiological mechanisms that regulate ICP in case of head trauma or stroke.
Furthermore, the small sample size prevents us from doing advanced statistical analysis [33]. However, our results confirmed previously reported data, increasing evidence regarding the HC procedure.
Conclusion
The experimental model confirmed the effectiveness of HC in providing sufficient volume for brain expansion. The recorded volumetric variations were half of those obtained through a DC. For this reason, we believe that the HC can be a valid alternative in case of potentially evolutionary lesions and non-massive edema. This fact could have important implications in treating patients requiring DC, especially in developing countries where the treatment of head trauma remains a significant problem. Using materials in our model commonly found in many operating rooms could facilitate this process. Further clinical studies are needed to confirm this promising surgical technique’s utility and specific indications.
References
Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM (2006) Outcome following decompressive craniectomy for malignant swelling due to severe head injury. JNS 104(4):469–479
Abdullah JY, Rajion ZA, Martin AG, Jaafar A, Ghani ARI, Abdullah JM (2019) Shape-based interpolation method in measuring intracranial volume for pre- and post-operative decompressive craniectomy using open source software. Neurocirugía 30(3):115–123
Adeleye A (2016) Clinical and radiologic outcome of a less invasive, low-cost surgical technique of osteoplastic decompressive craniectomy. J Neurol Surg A Cent Eur Neurosurg 77(02):167–175
Ahn D-H, Kim D-W, Kang S-D (2009) In situ floating resin cranioplasty for cerebral decompression. J Korean Neurosurg Soc 46(4):417
Ao A, Al A (2011) Decompressive craniectomy bone flap hinged on the temporalis muscle: a new inexpensive use for an old neurosurgical technique. Surg Neurol Int 2(1):150
Bratton SL, Chestnut RM, Ghajar J et al (2007) VII. Intracranial pressure monitoring technology. J Neurotrauma 24(supplement 1):S45–S54
Burger R, Duncker D, Uzma N, Rohde V (2008) Decompressive craniotomy: durotomy instead of duroplasty to reduce prolonged ICP elevation. In: Steiger H-J (ed) Acta Neurochirurgica Supplements. Springer Vienna, Vienna, pp 93–97
Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA (1993) The role of secondary brain injury in determining outcome from severe head injury. J Trauma 34(2):216–222
Chi JH (2011) Craniectomy for traumatic brain injury: results from the DECRA trial. Neurosurgery 68(6):N19–N20
Cooper DJ, Rosenfeld JV, Murray L et al (2011) Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 364(16):1493–1502
Cooper DJ, Rosenfeld JV, Murray L et al (2020) Patient outcomes at twelve months after early decompressive craniectomy for diffuse traumatic brain injury in the randomized DECRA clinical trial. J Neurotrauma 37(5):810–816
Cruz-Flores S, Berge E, Whittle IR (2012) Surgical decompression for cerebral oedema in acute ischaemic stroke. Cochrane Database Syst Rev 1:CD003435. https://doi.org/10.1002/14651858.CD003435.pub2
De Bonis P, Pompucci A, Mangiola A, Paternoster G, Festa R, Nucci CG, Maviglia R, Antonelli M, Anile C (2011) Decompressive craniectomy for elderly patients with traumatic brain injury: it’s probably not worth the while. J Neurotrauma 28(10):2043–2048
Dewan MC, Rattani A, Gupta S et al (2019) Estimating the global incidence of traumatic brain injury. J Neurosurg 130(4):1080–1097
Flint AC, Manley GT, Gean AD, Hemphill JC, Rosenthal G (2008) Post-operative expansion of hemorrhagic contusions after unilateral decompressive hemicraniectomy in severe traumatic brain injury. J Neurotrauma 25(5):503–512
Glushakova OY, Glushakov AV, Yang L, Hayes RL, Valadka AB (2020) Intracranial pressure monitoring in experimental traumatic brain injury: implications for clinical management. J Neurotrauma 37(22):2401–2413
Goettler CE, Tucci KA (2007) Decreasing the morbidity of decompressive craniectomy: the Tucci flap. J Trauma 62(3):777–778
Gutman M, How E, Withers T (2017) The floating anchored craniotomy. Surg Neurol Int 8(1):130
Hawryluk GWJ, Rubiano AM, Totten AM et al (2020) Guidelines for the management of severe traumatic brain injury: 2020 update of the decompressive craniectomy recommendations. Neurosurgery 87(3):427–434
Honeybul S, Ho KM (2012) Incidence and risk factors for post-traumatic hydrocephalus following decompressive craniectomy for intractable intracranial hypertension and evacuation of mass lesions. J Neurotrauma 29(10):1872–1878
Hutchinson PJ, Kolias AG, Timofeev IS et al (2016) Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med 375(12):1119–1130
Hutchinson PJ, Kolias AG, Tajsic T et al (2019) Consensus statement from the International Consensus Meeting on the Role of Decompressive Craniectomy in the Management of Traumatic Brain Injury: Consensus statement. Acta Neurochir 161(7):1261–1274
Jasielski P, Czernicki Z, Dąbrowski P, Koszewski W, Rojkowski R (2019) How does early decompressive craniectomy influence the intracranial volume relationship in traumatic brain injury (TBI) patients? Neurol Neurochir Pol 53:47–54
Jiang J-Y, Xu W, Li W-P, Xu W-H, Zhang J, Bao Y-H, Ying Y-H, Luo Q-Z (2005) Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled study. J Neurotrauma 22(6):623–628
Kano T, Kurosaki S, Wada H (2012) Retrospective analysis of hinge technique for head trauma or stroke. Neurol Med Chir (Tokyo) 52(11):816–821
Kenning TJ, Gandhi RH, German JW (2009) A comparison of hinge craniotomy and decompressive craniectomy for the treatment of malignant intracranial hypertension: early clinical and radiographic analysis. FOC 26(6):E6
Kenning TJ, Gooch MR, Gandhi RH, Shaikh MP, Boulos AS, German JW (2012) Cranial decompression for the treatment of malignant intracranial hypertension after ischemic cerebral infarction: decompressive craniectomy and hinge craniotomy: Clinical article. JNS 116(6):1289–1298
Khanna R, Ferrara L (2016) Dynamic telescopic craniotomy: a cadaveric study of a novel device and technique. JNS 125(3):674–682
Ko K, Segan S (2007) In situ hinge craniectomy. Operative. Neurosurgery 60(4):255–259
Kolias AG, Viaroli E, Rubiano AM et al (2018) The current status of decompressive craniectomy in traumatic brain injury. Curr Trauma Rep 4(4):326–332
Kung W-M, Lin F-H, Hsiao S-H, Chiu W-T, Chyau C-C, Lu S-H, Hwang B, Lee J-H, Lin M-S (2012) New reconstructive technologies after decompressive craniectomy in traumatic brain injury: the role of three-dimensional titanium mesh. J Neurotrauma 29(11):2030–2037
Layard Horsfall H, Mohan M, Devi BI et al (2020) Hinge/floating craniotomy as an alternative technique for cerebral decompression: a scoping review. Neurosurg Rev 43(6):1493–1507
Maas AIR (2022) The resilience of a dead brain: commentary to “The intracranial pressure–volume relationship following decompressive hinge craniotomy compared to decompressive craniectomy—a human cadaver study”. Acta Neurochir 165(2):279–279
Mezue W, Ndubuisi C, Ohaegbulam S, Chikani M, Erechukwu U (2013) Cranial bony decompressions in the management of head injuries: Decompressive craniotomy or craniectomy? Niger J Clin Pract 16(3):343
Miller K, Eljamel S (2016) Does size and site matter in therapeutic decompressive craniectomy? A laboratory-based experimental study. World Neurosurg 95:441–446
Mracek J, Choc M, Mork J, Vacek P, Mracek Z (2011) Osteoplastic decompressive craniotomy—an alternative to decompressive craniectomy. Acta Neurochir 153(11):2259–2263
Olivecrona M, Rodling-Wahlström M, Naredi S, Koskinen L-OD (2007) Effective ICP reduction by decompressive craniectomy in patients with severe traumatic brain injury treated by an ICP-targeted therapy. J Neurotrauma 24(6):927–935
Peethambaran A, Gopal V, Valsalamony J (2015) Four-quadrant osteoplastic decompressive craniotomy: a novel technique for refractory intracranial hypertension - A pilot study. Neurol India 63(6):895
Rusnak M (2013) Giving voice to a silent epidemic. Nat Rev Neurol 9(4):186–187
Sahuquillo J, Dennis JA (2019) Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst Rev 12:CD003983. https://doi.org/10.1002/14651858.CD003983.pub3
Schmidt JH, Reyes BJ, Fischer R, Flaherty SK (2007) Use of hinge craniotomy for cerebral decompression: Technical note. JNS 107(3):678–682
Schuss P, Vatter H, Marquardt G, Imöhl L, Ulrich CT, Seifert V, Güresir E (2012) Cranioplasty after decompressive craniectomy: the effect of timing on postoperative complications. J Neurotrauma 29(6):1090–1095
Schuss P, Vatter H, Oszvald Á, Marquardt G, Imöhl L, Seifert V, Güresir E (2013) Bone flap resorption: risk factors for the development of a long-term complication following cranioplasty after decompressive craniectomy. J Neurotrauma 30(2):91–95
Smith DH, Kochanek PM, Rosi S, Meyer R, Ferland-Beckham C, Prager EM, Ahlers ST, Crawford F (2021) Roadmap for advancing pre-clinical science in traumatic brain injury. J Neurotrauma 38(23):3204–3221
Søndergaard CB, Villa C, Jacobsen C, Lilja-Cyron A, Fugleholm K (2022) The intracranial pressure–volume relationship following decompressive hinge craniotomy compared to decompressive craniectomy—a human cadaver study. Acta Neurochir 165(2):271–277
Stocchetti N, Maas AIR (2014) Traumatic intracranial hypertension. N Engl J Med 370(22):2121–2130
Stoner KE, Abode-Iyamah KO, Grosland NM, Howard MA (2016) Volume of brain herniation in patients with ischemic stroke after decompressive craniectomy. World Neurosurg 96:101–106
Thompson HJ, McCormick WC, Kagan SH (2006) Traumatic brain injury in older adults: epidemiology, outcomes, and future implications: traumatic brain injury and older adults. J Am Geriatr Soc 54(10):1590–1595
Traxler H, Ender HG, Weber G, Surd R, Redl H, Firbas W (2002) Applying circular posterior-hinged craniotomy to malignant cerebral edemas. Clin Anat 15(3):173–181
Tsermoulas G, Shah O, Wijesinghe HE, Silva AHD, Ramalingam SK, Belli A (2016) Surgery for acute subdural hematoma: replace or remove the bone flap? World Neurosurg 88:569–575
Vaishnavi S, Rao V, Fann JR (2009) Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics 50(3):198–205
Valença MM, Martins C, da Silva JC (2010) “In-window” craniotomy and “bridgelike” duraplasty: an alternative to decompressive hemicraniectomy: technical note. JNS 113(5):982–989
Van Der Worp HB, Hofmeijer J, Jüttler E, Lal A, Michel P, Santalucia P, Schönenberger S, Steiner T, Thomalla G (2021) European Stroke Organisation (ESO) guidelines on the management of space-occupying brain infarction. Eur Stroke J 6(2):XC–CX
Author information
Authors and Affiliations
Contributions
Antonio Biroli: methodology, validation, formal analysis, investigation, resources, writing—review and editing, and project administration. Valentina Bignotti: methodology, investigation, and writing—original draft. Pietro Biroli: formal analysis and data curation management. Barbara Buffoli: investigation and resources. Francesco A. Rasulo: methodology, validation, and resources. Francesco Doglietto: validation, resources, and writing—review and editing. Rita Rezzani: validation, resources, and supervision. Alessandro Fiorindi: validation, writing—review and editing, and supervision. Marco M. Fontanella: validation, resources, writing—review and editing, and supervision. Francesco Belotti: methodology, validation, investigation, resources, writing—original draft, writing—review and editing, visualization, and project administration.
Corresponding author
Ethics declarations
Ethical approval
This work was conducted in accordance with the institutional ethical committee guidelines and was performed according to the ethical standards of our institutional review board.
Consent to participate
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Biroli, A., Bignotti, V., Biroli, P. et al. Hinge craniotomy versus standard decompressive hemicraniectomy: an experimental preclinical comparative study. Acta Neurochir 165, 2365–2375 (2023). https://doi.org/10.1007/s00701-023-05715-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05715-2