Abstract
Objective
Decompressive hinge craniotomy (DHC) is an alternative treatment option to decompressive craniectomy (DC) for elevated intracranial pressure (ICP). The aim of this study was to characterize the difference in pressure–volume relationship between DHC and DC.
Methods
We compared the intracranial pressure–volume relationship in a human cadaver model following either DHC, DC, or fixing of the bone plate by titanium clamps. We inserted an intracranial expandable device in two human cadaver specimens, performed either DHC, DC, or bone plate fixation, and gradually increased the intracranial volume while measuring ICP. Following DHC, we also performed CT-scans at pre-defined intervals.
Results
Before ICP exceeded a threshold of 20 mmHg, a fixed bone plate tolerated an increase of 130 ml of intracranial volume, while DHC and DC allowed an increase of 190 ml and 290 ml, respectively. CT-derived calculations following DHC determined that the increase in intracranial volume at ICP 22 mmHg was 65 ml, the maximal increase of intracranial volume was 84 ml, the maximal bone displacement was 21 mm, and the bone plate volume to be 82 ml. Manual stress test of the hinged bone plate did not allow misalignment or intracranial displacement of the bone plate.
Conclusion
DHC increases the intracranial volume by up to 84 ml and allows for approximately 60 ml increase of intracranial volume before ICP exceeds 20 mmHg. This indicates, when comparing with results from previous studies of herniation volumes, that DHC will be sufficient in many patients with head injury or cerebral infarction with treatment refractory intracranial hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Decompressive hinge craniotomy (DHC) is a promising surgical technique to supplement the surgical decompressive treatment options for medically intractable elevated intracranial pressure and impending cerebral herniation following traumatic brain injury (TBI) or middle cerebral artery infarction. Specifically, DHC eliminates the need for a subsequent cranioplasty, a procedure with a high complication rate (up to 31% [18]). Other advantages of the technique is the reduction of frequent complications related to conventional decompressive craniectomy (DC), such as hygroma formation, sunken skin flap, hydrocephalus, and syndrome of the trephined [10]. DHC is likely to reduce axonal strain, a process suggested to lower functional outcome in patients treated with DC [11].
DHC allows an expanding cerebral mass lesion to displace a hinged bone plate outwards, thereby increasing the intracranial volume. The procedure, like DC, interrupts the normal pressure–volume relationship of the closed skull following an expanding mass lesion. DHC is a proposed alternative to DC in some cases; however, the pressure–volume relationship of this procedure has yet to be described or compared to DC.
The aim of this study was to investigate the volume of decompression and the intracranial pressure–volume relationship following DC and DHC, respectively. We used a human cadaver model with an implanted expandable sizer to simulate a patient with a gradually increasing mass lesion and measured ICP during expansion of the sizer after DC, DHC, and when fixing the bone plate to the cranium.
Methods
Surgical procedure
DHC
Several surgical techniques have been described, some involving surgical hinging by plates or sutures [5], others have used “hinging” to the temporalis muscle [2], and in some cases a “floating” unhinged cranial plate has been utilized [4].
In our clinical practice, the bone plate is fixed by a 5 to 7 cm long flexible titanium mesh (1.3 screen plate, Dupuy Synthes) anchoring the bone plate to the skull, approximately 1–2 cm parasagittal to the midline (see Fig. 1). The titanium mesh was tested for fatigue during 2.5 million micromovements exerted by pulsation of the brain in a construct simulating DHC (Synthes GMBH, Material and Testing, Oberdorf, Switzerland, supplementary material). The skin incision and bone removal are the same as in a standard decompressive craniectomy—allowing the surgeon to choose between hinging the bone plate and removing the bone during the procedure. The hinging of the bone plate at the parasagittal aspect causes the maximal displacement of the bone plate to be located over the temporal lobe, thereby reducing the risk of uncal herniation. The convex shape of the bone plate in conjunction with the rigid hinge prevents the bone plate from being intracranially displaced. No cranioplasty or other surgical reconstruction is therefore needed.
Simple hinge craniotomy procedure. A Parasagittal hinge approximately 7 cm placed between bone plate and skull. B Anterior view of DHC potential bone plate displacement following brain edema. C Inferior view of DHC bone plate displacement allowing its maximal displacement over the temporal lobe thereby reducing uncal herniation. D The convex structure of the bone plate in conjunction with the hinge function does not allow the bone plate to be displaced intracranially when brain edema subsides. No cranioplasty is therefore needed
Human cadaver procedures
In order to evaluate the decompressive hinge craniotomy efficacy and clinical applicability, we performed the hinge craniotomy on two separate intact human cadaver head specimens, one frozen freshly thawed specimen and one alcohol preserved specimen. In both specimens, we expected the compliance of the galea and soft tissue layers in the myocutaneous flap to be the limiting factor for decompression.
In both specimens, we performed three separate procedures: first a standard DC, second a DHC, and third, we fixed the bone plate with three titanium clamps (CranioFix, B. Braun). A large hemicraniectomy involving the fronto-, parietal-, and temporal bones was performed approximately 1.5 cm from the sagittal suture and to the floor of the middle fossa, measuring 17 by 13.5 cm and 16 by 12 cm, respectively. In all cases, the dura was opened in a stellate fashion and left in situ on top of the sizer. Each procedure was performed in succession unilaterally on each cadaver using the same skin opening, bone plate, and skin suture technique.
ICP measurement
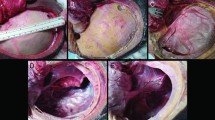
We implanted an ICP-measuring sensor (Codman Microsensor) in each cadaver ipsilateral to the surgical procedure with the sensor tip placed approximately 2 cm inside the brain parenchyma. To simulate a real-life cerebral swelling, we implanted an expandable sizer (used in plastic surgery to estimate breast implant size), which was fitted on top of the cerebral cortex after the dural opening, and a tube was led to an external saline solution. The bone flap was either removed (DC), attached with a hinge (DHC), or fixed by titanium clamps (Craniofix, B. Braun) (see Fig. 2). The skin (but not the galea) was closed with running ethilon nylon sutures. After performing each procedure, the sizer was gradually injected with 10 ml saline solution and an ICP value was recorded simultaneously. Volume expansion was continued until ICP reached 50 mmHg or until maximum capacity of the sizer (see Fig. 2).
Cadaver procedures. A Lateral view of hemicraniectomy. The expansion device (implant sizer) is placed on top of the right hemisphere. B Superior view of DHC. Parasagittally placed hinge. Also visible is the ICP sensor and the external line to the expansion device. C Postero-superior view of the fixed bone plate. D Postero-superior view following skin sutures
In our lab, we first performed all three procedures on both cadaver heads. At a separate occasion, we repeated measurements following all three procedures two additional times, in random order, in our alcohol preserved specimen. Due to the rapid decay of unfixed brain tissue, our freshly thawed human cadaver was unavailable for further laboratory work. We conducted the procedures again and in random order to avoid bias from changing tissue compliance or dehydration.
CT derived volume calculations
To investigate the volume expansion that was attainable with DHC, we performed the volume expansion while performing CT scans at intervals.
CT scans were performed on a Siemens Somatom Definition (Scanning settings 120 kV, 075 slice thickness, pitch factor 0.6 mm, reconstruction algorithm H60f (sharp), pixel size 0.57 mm) and were then analyzed using the Mimics software (Materialise). Intracranial volume was calculated for each scan at intervals by 3D segmenting the brain and the bone plate. At 3D segmentation, the maximal distance from the temporal cranial bone in the middle fossa to the most displaced part of the temporal edge of the bone plate was also measured (see Fig. 3).
Stress test of DHC bone plate
A concern regarding the DHC technique is whether adhering the bone to the skull only at the parasagittal aspect, and removing further temporal bone over the middle cranial fossa, might allow the bone plate to be displaced intracranially, when brain edema subsides and outside forces are applied. We tested if the bone plate might displace, or the edges of the bone plate might misalign to the skull edges, following lateral displacement of the bone plate. We tested this by first displacing the plate laterally and afterwards allowing the plate to spontaneously return to its position. We then manually exerted force on the bone plate by more than 40 kg (392 Newton) in the anterior, middle, and posterior areas, respectively, of the bone plate. The stress was exerted by applying force with three fingers, previously recorded to exert force by more than 40 kg or 392 Newton. We then scrutinized the bone plate for placement, hinge plate integrity and for screw pull-out (Fig. 2).
Results
Pressure–volume results
Measurements from both cadaver specimens indicated significant decompressive effect of the hinge craniotomy compared to rigid fixation of the cranial plate. There were no difference in intracranial pressure/volume relationship development between the freshly thawed or alcohol-preserved specimen. In the DHC model, ICP increased slowly, until reaching a threshold, where minor volume expansion resulted in severe ICP elevation (see Fig. 4). The DHC model tolerated a mean volume expansion to 190 ml before ICP increased over 20 mmHg, whereas the fixed cranial plate tolerated only 130 ml. In comparison, the DC model tolerated 290 ml before ICP increased to more than 20 mmHg (see Table 1).
CT derived volume results of DHC
3D models from CT scans of the DHC model showed that increasing the intracranial volume displaced the bone plate outwards (see Fig. 2). The most displaced point of each bone plate was 16 mm and 21 mm, respectively (see Table 2). By calculating the intracranial volume in each scan as the temporal bone was displaced, we concluded that DHC allows for an increase in intracranial volume of maximum 77.1 ml and 84.1 ml, respectively. Notably, the calculated increased intracranial volume at ICP 22 mmHg was 65 ml. The calculated bone plate volume was 82.3 and 75.9 ml, respectively.
DHC stress test results
When displacing the DHC bone plate laterally and allowing it to remigrate spontaneously, the plate did not displace. The bone edges of the skull defect are continuous and more than 5 mm thick for about 70 to 80% of the circumference of the bone plate. Only temporal, over the floor of the middle cranial fossa, does the bone edge become very thin, and the anterior temporal bone of the circumference is removed, to decompress the temporal lobe. Subsequently, the bone plate, when hinged, rests on the bony edges of the circumference. Much like a pot’s lid, it is not capable of displacing inwards. When applying direct manual pressure exceeding 392 N to the anterior, middle, and posterior part of the bone plate, it did not cause the bone plate to displace or misalign. We did not register any signs of titanium plate failure or screw pull out.
Discussion
In this study, we found the volume gain after DC to be larger than after DHC. This suggests that DHC may provide insufficient decompression, when treating raised ICP. However, several studies have shown similar effects of DHC and DC in lowering ICP post-operatively [2, 5,6,7]. Most notably, Kenning et al. retrospectively reviewed 20 patients treated with DHC to 30 patients treated with DC and found no difference in post-operative ICP control or early clinical outcomes [7].
Horsfall et al. in their scoping review of many minor retrospective studies of different types of dynamic or hinged craniotomies, found them sufficient in handling most cases of elevated ICP [10].
Our results show that DHC allows for approximately 60 ml of additional intracranial volume compared to a fixed bone plate while maintaining an ICP threshold below 20 mmHg. CT-derived calculations found DHC to increase intracranial volume by 65 ml at ICP 22 mmHg. At the same threshold (20 mmHg), however, a DC allows for 160 ml of volume expansion compared to a fixed bone plate. The reduced decompressive volume of DHC compared to DC is partly explained by the volume of the bone plate of approximately 80 ml. It is also of importance that a volume expansion of 200 ml in the DHC experiment only increased the ICP to 25 mmHg (compared to ICP of 43 mmHg with a fixed bone plate).
Abode-lyamah et al. found an average herniation volume of 11.1 ml in their study of cerebral herniation following hemicraniectomy of patients suffering from TBI, when comparing pre- and post-operative CT scans. Of the study’s 56 patients, only 2 patients had a herniation volume exceeding 50 ml [1].
In a similar study, Stoner et al. found that cerebral herniation following ischemic infarction and hemicraniectomy resulted in an average herniation volume of 39.5 ml and only 2 out of 20 patients had an average herniation volume exceeding 65 ml [16].
These studies indicate that the cerebral herniation volume following cerebral infarction or TBI is within the capacity of volume expansion achieved from DHC.
To our knowledge, the present study is the first to characterize the differences in the pressure–volume relationship between a fixed bone plate, DHC, and DC and the first to simulate the decompressive effect by measuring the pressure–volume relationship in a complete human cadaver model including brain parenchyma, cranium, galea, muscle, subcutaneous tissue, and skin.
Khanna et al. [8, 9] conducted cadaver studies testing pressure–volume relationship for cadaver skulls following decompressive hinge craniotomy and a novel dynamic bone plate fixation device. Their studies found significant decompressive effect using both techniques. Their study was performed on cadaver skulls without extracranial soft tissue, which we consider the major limiting factors for bone plate displacement.
Following DHC, the major limiting factor for volume expansion is the extracranial soft tissue. The skin, galea, and muscle are able to expand, when exposed to mechanical stress, a trait also known as mechanical creep [17]. This trait is often exploited by inserting expanders for reconstructive surgery [13]. However, mechanical creep does not seem to be in effect in human cadavers [14], implying that our short term study might underestimate the in vivo decompressive efficacy of DHC, especially if a moderate ICP is allowed to exert mechanical stress to the skin flap.
Cadaver studies have obvious limitations: the physiological responses to an elevated ICP of cerebral blood perfusion, venous drainage, and CSF displacement, which account for the initial accommodation of an increasing intracerebral mass lesion are unaccounted for. However, these responses are often depleted at the time when decompressive surgery is performed.
Our study also omits the physiologic function of pulsative ICP, another aspect of interest following decompressive surgery. The effect of DC on ICP amplitude and waveform is well described from in vivo studies [3, 12] and is difficult to mimic in human cadaver studies. Perhaps, pulsatile ICP movement might also increase displacement of the bone plate following DHC. Decompressive surgery changes a vast aspect of the cerebral dynamics including ICP compliance, oxygenation, and interstitial fluid diversion, and it is not certain than an extrapolated ICP threshold level of 20 or 22 mmHg following decompression is of clinical significance. Sauvigny et al. in their retrospective study of patients treated with DC found a time-dependent threshold level between 10 and 17 mmHg in their group with a favorable outcomes [15].
Our study is limited by the limited sample size (n = 2), providing no basis for advanced statistical analyses, including corrections for anatomical variations of cranium and brain. This study seeks to give insight into the relative difference in volume gain and intracranial pressure/volume relationship from different techniques of decompression of the same cranium and brain specimen. To demonstrate the reproducibility of our findings, we performed the same procedures in different orders at another time, in our alcohol-preserved specimen.
Conclusions
DHC increases the intracranial volume by up to 84 ml by a gradual displacement of the bone plate when ICP rises. Compared to rigid bone plate fixation, DHC allows for an increase of up to 60 ml, in intracranial mass lesion volume, before ICP rises to threshold levels. DHC therefore appears to be a viable surgical treatment option, as an alternative to DC, in cases where extreme brain edema is not obvious at the time of surgery, or imminent.
References
Abode-Iyamah KO, Stoner KE, Close LN, DeVries Watson NA, Flouty OE, Grosland NM, Howard MA 3rd (2018) Volume of brain herniation after decompressive craniectomy in patients with traumatic brain injury. World Neurosurg 118:e414–e421
Adeleye AO (2016) Clinical and radiologic outcome of a less invasive, low-cost surgical technique of osteoplastic decompressive craniectomy. J Neurol Surgery, Part A Cent Eur Neurosurg. https://doi.org/10.1055/s-0035-1566115
Brasil S, Solla DJF, Nogueira R de C, Jacobsen Teixeira M, Malbouisson LMS, Paiva WS (2021) Intracranial compliance assessed by intracranial pressure pulse waveform. Brain Sci. https://doi.org/10.3390/brainsci11080971
Gutman MJ, How E, Withers T (2017) The floating anchored craniotomy. Surg Neurol Int. https://doi.org/10.4103/sni.sni_460_16
Kano T, Kurosaki S, Wada H (2012) Retrospective analysis of hinge technique for head trauma or stroke. Neurol Med Chir (Tokyo). https://doi.org/10.2176/nmc.52.816
Kenning TJ, Gandhi RH, German JW (2009) A comparison of hinge craniotomy and decompressive craniectomy for the treatment of malignant intracranial hypertension: early clinical and radiographic analysis. Neurosurg Focus. https://doi.org/10.3171/2009.4.FOCUS0960
Kenning TJ, Gooch MR, Gandhi RH, Shaikh MP, Boulos AS, German JW (2012) Cranial decompression for the treatment of malignant intracranial hypertension after ischemic cerebral infarction: decompressive craniectomy and hinge craniotomy. J Neurosurg 116(6):1289–1298
Khanna R (2017) Dynamic decompressive craniotomy with a novel reversibly expandable plate. J Neurol Surgery, Part A Cent Eur Neurosurg. https://doi.org/10.1055/s-0036-1594013
Khanna R, Ferrara L, Khanna S (2019) Biomechanics of a novel reversibly expandable dynamic craniotomy bone flap fixation plate. J Neurosurg. https://doi.org/10.3171/2018.8.JNS172614
Layard Horsfall H, Mohan M, Devi BI et al (2019) Hinge/floating craniotomy as an alternative technique for cerebral decompression: a scoping review. Neurosurg Rev. https://doi.org/10.1007/s10143-019-01180-7
Li X, Von Holst H, Kleiven S (2013) Decompressive craniectomy causes a significant strain increase in axonal fiber tracts. J Clin Neurosci. https://doi.org/10.1016/j.jocn.2012.04.019
Lilja-Cyron A, Andresen M, Kelsen J, Andreasen TH, Fugleholm K, Juhler M (2019) Long-term effect of decompressive craniectomy on intracranial pressure and possible implications for intracranial fluid movements. Neurosurgery. https://doi.org/10.1093/neuros/nyz049
Raposio E (2018) Scalp expansion: surgical considerations and possible future directions. Indian J Plast Surg Off Publ Assoc Plast Surg India 51(1):84–88
Raposio E, Cella A, Barabino P, Santi P (1999) Ineffectiveness of acute scalp expansion. Plast Reconstr Surg 103(6):1645–1649
Sauvigny T, Göttsche J, Czorlich P, Vettorazzi E, Westphal M, Regelsberger J (2018) Intracranial pressure in patients undergoing decompressive craniectomy: new perspective on thresholds. J Neurosurg 128(3):819–827
Stoner KE, Abode-Iyamah KO, Grosland NM, Howard MA (2016) Volume of brain herniation in patients with ischemic stroke after decompressive craniectomy. World Neurosurg. https://doi.org/10.1016/j.wneu.2016.08.095
Wilhelmi BJ, Blackwell SJ, Mancoll JS, Phillips LG (1998) Creep vs. stretch: a review of the viscoelastic properties of skin. Ann Plast Surg 41(2):215–219
Zanaty M, Chalouhi N, Starke RM et al (2015) Complications following cranioplasty: incidence and predictors in 348 cases. J Neurosurg. https://doi.org/10.3171/2014.9.JNS14405
Acknowledgements
We thank professor of clinical anatomy Jørgen Tranum-Jensen and laboratory chief technician Johnny Grandt, Institute of Cellular and Molecular Medicine, for invaluable help with cadaver specimens and technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was conducted with human cadaver specimens donated from consenting Danish adults with their intention of participating in medical science following their natural death. All patients and data are anonymous and the study is exempted from approval from the Danish Ethics Committee.
Competing interests
The authors declare no conflict of interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurosurgical intensive care
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Søndergaard, C.B., Villa, C., Jacobsen, C. et al. The intracranial pressure–volume relationship following decompressive hinge craniotomy compared to decompressive craniectomy—a human cadaver study. Acta Neurochir 165, 271–277 (2023). https://doi.org/10.1007/s00701-022-05409-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05409-1