Abstract
Background
Multicentric malignant gliomas are well-separated tumours in different lobes or hemispheres, without anatomical continuity between lesions. The purpose of this study was to explore the clinical features, the pathology and the outcome according to the management strategies in a consecutive series of patients treated at a single institution. In addition, an analysis of the existing literature is presented.
Methods
For the institutional analysis, a retrospective review of all patients who underwent treatment for multicentric gliomas in the last 7 years was performed. For the analysis of the literature, a MEDLINE search with no date limitations was accomplished for surgical treatment of multicentric malignant gliomas.
Results
Two hundred and thirty-nine patients with glioma were treated in our department. Eighteen patients (7.5 %) with a mean age of 64 years (age range, 37–78 years) presented multicentric malignant gliomas. Thirteen patients (72 %) underwent surgical resection of at least one lesion that was followed by adjuvant treatment in all but one case. Five patients (28 %) underwent stereotactic biopsy and thereafter received chemotherapy. A survival advantage was associated with resection of at least one lesion followed by adjuvant treatment (median overall survival 12 months) compared with 4 months for stereotactic biopsy followed by chemotherapy. Similar results were obtained from the review of the literature.
Conclusions
Resection of at least one lesion seems to play a significant role in the management of selected patients with multicentric malignant gliomas. Multi-institutional studies on larger series are warranted to define how aggressively the patients with malignant multicentric gliomas should be treated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multicentric gliomas are well-separated lesions, localised in different lobes or hemispheres, without evidence of dissemination through a known anatomical route for spread between lesions (commissural pathways, cerebrospinal fluid, blood or local extension) [1]. This condition is rare, accounting for 2–16.2 % of cases and its management remains controversial [1–6]. We sought to review our results for the management of high-grade multicentric gliomas and to review the pertinent literature in order to investigate the outcome of these lesions.
Clinical material and methods
Institutional data
A retrospective chart review was performed on patients treated for pathologically proved multicentric glioblastoma multiforme (GBM) at the Neurosurgical Department of the University of Pisa between January 2006 and December 2012. Patients with neurofibromatosis or multiple sclerosis were excluded [7]. In addition, according to Batzdorf and Malamud’s criteria [1], patients with multifocal GBM, i.e. multiple lesions resulting from dissemination or growth by an established route of spread, were excluded. The patient’s age, sex and Karnofsky Performance Scale (KPS) scores at the time of presentation were noted. All patients underwent a preoperative gadolinium (Gd)-enhanced magnetic resonance imaging (MRI) scan of the whole brain on 1.5-tesla Magneton (Siemens, Stuttgart, Germany). Multicentricity was defined as more than one lesion within the brain without connecting signal alteration in FLAIR sequences. A functional MRI (fMRI) was performed when the tumour localisation was presumed close to language, motor and visual areas. A neuronavigation system was used in all operations. In addition, intra-operative neurophysiology was performed in all cases of presumed motor tumour localisation and included localisation of the central sulcus with the help of median nerve phase-reversal technique. Tumour location with respect to the proximity to eloquent brain was characterised by functional grade as reported by Sawaya et al. [8] and modified by Lacroix et al. [9] (Table 1). Postoperatively, all patients underwent early computed tomography (CT) scans to exclude the occurrence of haematoma and Gd-enhanced MRI to evaluate the extent of tumour resection. Surgical complications were defined as those occurring within 30 days of the operation. The postoperative KPS scores were assessed 4 weeks after surgery and compared with the preoperative KPS scores. Gd-enhanced MRI was obtained every 2 months during the follow-up until death or the end of the study. Kaplan-Meier estimates of overall survival (OS) were obtained for patients receiving surgery followed by adjuvant treatment and patients receiving only radiotherapy and/or chemotherapy after stereotactic biopsy.
Literature review
A MEDLINE search was performed for the key words “multicentric glioma”, “glioblastoma”, “multiple cerebral lesions” and “glioma”. No date limitations were imposed in the search criteria. Articles referenced in other articles were also included. Solely studies reporting detailed information on demographics, histology, treatments and follow-up were considered. Exclusion criteria included studies reporting multifocal gliomas, paediatric and low-grade multicentric gliomas. Data collected included the number of patients, average age, number of lesions, type of treatment and OS.
Results
Institutional analysis
Between January 2006 and December 2012, 239 consecutive adult patients with glioma diagnosis were referred for surgery to our neurosurgical department. Of these patients, 18 (7.5 %) had multicentric glioma and complete follow-up information available for review. The clinical characteristics of patients are summarised in Table 2. There were ten female and eight male patients, ranging in age from 37 to 78 years (mean age, 64 years). The preoperative KPS scores ranged from 40 to 90 (median 60). A motor deficit was observed in five patients (33.3 %) and speech disturbances were evident in four patients (22 %). Fifteen patients presented synchronous lesions and three patients with metachronous lesions. The preoperative MRI disclosed two lesions in ten patients (56 %), three lesions in four patients (22 %), four or more lesions in the remaining four patients (22 %). In 50 % of cases the multicentric gliomas were in the same hemisphere, and 55 % of them were located on the left side. Seventy percent of patients had one or more focus located in eloquent brain regions according to Sawaya and co-workers [8, 9] (functional grade III). Surgical treatment was offered to 13 patients (72 %) and consisted of resection of one lesion in 10 patients and two lesions in the remaining 3 (Fig. 1). In four cases surgical resection was performed after stereotactic biopsy. Two (15 %) patients experienced surgical complications. One patient had a new mild hemiparesis, which improved to functional independence after 2 months. The other patient had superficial wound infection successfully treated with antibiotic medications. Resection was followed by temozolomide chemotherapy in all patients but one and by radiotherapy in seven cases. The postoperative KPS scores ranged from 50 to 100 (median 80). According to the findings of postoperative MRI, ten lesions underwent gross total resection and six lesions underwent near total resection. Five patients (28 %) with KPS of 50 or lower or presenting with three or more lesions underwent stereotactic biopsy and thereafter received temozolomide chemotherapy (Fig. 2). The perioperative morbidity from stereotactic biopsy was nil. Histological examination was available for 21 tumours and demonstrated GBM in 17 cases and anaplastic astrocytoma (AA) in four cases. Three patients had histological examination of two lesions with diagnosis of the same grade of malignancy (GBM). In our series, glioblastoma presented as multicentric in 6 % of cases and AA in 10.5 %.
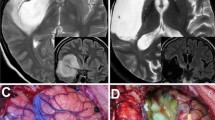
Patient 1. Preoperative axial, T1-weighted contrast-enhanced (a) and coronal FLAIR MR images (b) demonstrating three multicentric lesions located in the right parieto-occipital, frontal and insular regions. Stereotactic biopsy of the parieto-occipital lesion was diagnostic for glioblastoma and the patient underwent surgical resection of the lesion followed by chemotherapy and radiotherapy. Postoperative axial T1-weighted contrast-enhanced MR image obtained 3 months after surgery demonstrated total removal of the parieto-occipital glioblastoma (c)
Patient 18. Axial (a, b) and coronal (c) contrast-enhanced T1-weighted MR images disclosing a left pontine lesion and a left frontal paraventricular ring-enhancing lesion. The coronal FLAIR image (d) shows no extensions between the lesions through the white matter. Stereotactic biopsy of the frontal lesion was diagnostic for glioblastoma and the patient underwent chemotherapy
The median OS time for the 18 patients included in this study was 11 ± 1.55 months. With respect to the treatment, the median OS was 12 ± 1.63 months after surgical treatment and adjuvant therapy (12 cases) and 4 ± 1.34 months after stereotactic biopsy and adjuvant treatment (five cases) (Fig. 3). One patient refused the adjuvant treatment after surgery and is not included in this analysis. Median PFS was 8.5 ± 1.33 months after surgical treatment (12 cases). With respect to PFS, no differences were found between patients who underwent single or multiple surgical resections. The tumour’s histology conditioned average OS after surgical treatment (GBM = 10.7 ± 1.04 months; AA = 21.5 ± 3.9 months) and stereotactic biopsy (GBM = 5 ± 0.9 months; AA = 7.5 ± 3.46 months).
Analysis of literature review
The results of our analysis are summarised in Table 3. According to our search criteria, 56 cases [5, 10–18] of multicentric gliomas have been reported in the literature, including the 18 cases described in this article. Patient age at first presentation ranged from 32 to 78 years (average age 52.5 years). Eighty-seven percent of patients (49 cases) harboured two documented lesions, with the remaining patients harbouring three or more lesions. Seventy-five percent of patients (42 cases) underwent surgical resection followed by adjuvant therapy in 39 cases. Surgical treatment consisted of resection of two lesions in 31 cases and one lesion in 11 cases. Twenty-five percent of patients (14 cases) received stereotactic biopsy (SB) and adjuvant treatment. Histological examination was available for 105 tumours and demonstrated GBM in 72 cases, AA in 22 cases and low-grade astrocytoma (LGA) in two cases. In eight cases, including both LGAs, a different histology between two lesions was evidenced. The median OS for patients receiving surgery and adjuvant treatment was 12 ± 1.2 months. The median OS for patients receiving only radiotherapy and/or chemotherapy was 4 ± 1.7 months (Fig. 4).
Discussion
Since the original description of multiple gliomatous foci by Bradley in 1880 [19], few clinical series investigated this phenomenon and the management of this entity is still a matter of controversy. In 1963, Batzdorf and Malamud [1] provided pathological and radiological criteria to differentiate multiple gliomatous lesions into multicentric and multifocal gliomas. They stated that multifocal gliomas result from growth along an established route, including white matter tracts, cerebrospinal fluid channels or local extension by satellite formation. In contrast, multicentric gliomas were defined as widely separated lesions without macroscopic and microscopic continuity [1]. This distinction seems of clinical value since patients with newly diagnosed multifocal glioblastomas show a trend towards shorter survival time after surgical treatment [18]. In recent clinical studies, the rate of multicentricity in gliomas has been described as ranging from 2 to 16.2 % [3–5]. Interestingly, continuous advances in MRI coil technology produced an increased identification rate of multiple gliomas. Recently, Thomas et al. [20] reviewed their experience with glioblastoma and found an overall incidence of multiple lesions at the time of diagnosis of 35 %. Despite significant advances in understanding the biology and oncology of glioma cells, the pathophysiology of multicentricity remains largely elusive. It has been suggested that multicentricity results from de novo growth of multiple foci of disease from separate areas of the brain. Although this theory is corroborated by the cancer stem cell hypothesis, a growing body of evidence from studies using high field MRI suggests that multicentricity follows the commitment of glioma cells to migrate and invade adjacent and distant normal brain [4, 20, 21]. It has been reported that changes in T2-weighted images and FLAIR sequences outside the contrast-enhanced areas of the brain can reflect modification in extracellular matrix induced by invading glioma cells [4, 20, 22]. Accordingly, even lesions that seem to be solitary on radiological investigations can demonstrate tumour cells spread diffusely through the brain on autopsy [2]. Our results agree with the previous reports concerning the demographics of patients with multicentric gliomas including the presence of different histological lesions with a greater incidence of glioblastoma [5, 12, 21, 23–26] and the frequency of multicentricity, which in our series is of 7.5 %. Interestingly, this value coincides with the incidence of multicentric gliomas detected in large autoptic series. In fact, Barnard and Geddes [2] found 18 cases (7.5 %) of multicentric gliomas in an unselected series of 241 gliomas, after excluding cases with neurofibromatosis, tuberose sclerosis and multiple sclerosis. These authors reported that celloidin-embedded whole brain sections in conjunction with routine paraffin wax blocks proved invaluable for establishing whether two tumours are microscopically distinct and for detection of unsuspected diffuse spread.
The management of patients with multicentric gliomas is still under discussion and ranges from aggressive cytoreductive resection followed by adjuvant therapy to stereotactic biopsy followed by chemotherapy and/or radiation treatment [5, 18, 20, 21]. In the neurosurgical oncology literature, several studies demonstrated an association between extent of resection and overall survival in patients with glioblastoma [9, 27, 28]. Lacroix et al. [9] demonstrated that resection of 98 % or more of the tumour is an independent variable associated with longer survival times in patients with glioblastoma (median survival 13 months). In multicentric gliomas, cytoreduction should be carefully balanced with the risk of neurological morbidity. In fact, retrospective studies demonstrated an association between new postoperative deficits and decreased overall survival or worsened functional outcome [29, 30]. However, a provocative recent report by Hassaneen et al. [18] suggests that aggressive resection of all lesions in selected patients with multifocal and multicentric glioblastomas optimises patients’ outcomes, resulting in a survival duration comparable with that of patients undergoing surgery for a single lesion without an increase in postoperative complications. Some limitations, including the absence of a comparative group of patients with multifocal or multicentric glioblastomas treated with biopsy or subtotal resection and variations in postoperative treatment protocols, limit the strength of their conclusions. However, the results of the study of Hassaneen and co-workers stimulated the debate on the role of surgical treatment for multiple glioblastomas [31]. In the present series of 18 consecutive patients with multicentric gliomas, the management was tailored depending on preoperative KPS, the number and location of lesions. According to this policy, 72 % of patients underwent resection through a single craniotomy of at least one lesion, which was followed by chemotherapy and/or radiation treatment in all but one patient. Our results showed that the median OS in patients with multicentric glioblastomas treated with surgical resection and adjuvant therapy was 12 ± 1.63 months, whereas it was 4 ± 1.34 months after stereotactic biopsy and chemotherapy. Interestingly, the results of literature analysis are quite consistent with the results of our series disclosing a median OS for patients receiving surgery and adjuvant treatment of 12 ± 1.2 months and a median OS for patients receiving only radiotherapy and/or chemotherapy 4 ± 1.7 months.
The advantage of our study was that it included a non-selected consecutive series of patients with multicentric glioblastomas, which were treated over a relatively limited period of 7 years. However, the results presented in this study are retrospective and from a single investigational centre by design, and therefore entail several drawbacks and only allow reduced statistical inference. In addition, the clinical and radiological differences between patients with multicentric gliomas treated by resection or biopsy are a limitation of this study, because interfere with comparability of two groups.
Conclusions
Surgical resection of at least one lesion seems to have a beneficial effect on survival of selected patients with multicentric gliomas. Although in contrast to conventional wisdom, this finding was confirmed by our analysis of the literature on this challenging clinical population. Further studies on larger series, mainly focusing on patient selection and postoperative survival, are warranted to define the effectiveness of such an approach.
References
Batzdorf U, Malamud N (1963) The problem of multicentric gliomas. J Neurosurg 20:122–136
Barnard RO, Geddes JF (1987) The incidence of multifocal cerebral gliomas. A histologic study of large hemisphere sections. Cancer 60:1519–1531
Djalilian HR, Shah MV, Hall WA (1999) Radiographic incidence of multicentric malignant gliomas. Surg Neurol 51:554–558
Hefti M, von Campe G, Schneider C, Roelcke U, Landolt H (2010) Multicentric tumor manifestations of high grade gliomas: independent proliferation or hallmark of extensive disease? Cen Eur Neurosurg 71(1):20–25
Salvati M, Caroli E, Orlando ER, Frati A, Artizzu S, Ferranti L (2003) Multicentric glioma: our experience in 25 patients and critical review of the literature. Neurosurg Rev 26:275–279
Terakawa Y, Yordanova YN, Tate MC, Duffau H (2013) Surgical management of multicentric diffuse low-grade gliomas: functional and oncological outcomes: clinical article. J Neurosurg 118:1169–1175
Reagan TJ, Frieman IS (1973) Multiple cerebral gliomas in multiple sclerosis. J Neurol Neurosurg Psychiatry 36:523–528
Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, Wildrrick DM (1998) Neurosurgical ouctomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42:1044–1055
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95(2):190–198
Chadduck WM, Roycroft D, Brown MW (1983) Multicentric glioma as a cause of multiple cerebral lesions. Neurosurgery 13:170–175
Kato T, Aida T, Abe H, Ogata A, Nakamura N, Nagashima K, Kitaoka K (1990) Clinicopathological study of multiple gliomas—report of three cases. Neurol Med Chir (Tokyo) 30:604–609
Franco CM, Malheiros SM, Nogueira RG, Batista MA, Santos AJ, Abdala N, Stavale JN, Ferraz FA, Gabbai AA (2000) Multiple gliomas. Illustrative cases of 4 different presentations. [Article in Portuguese] Arq Neuropsiquiatr 58(1):150–156
Synowitz M, von Eckardstein K, Brauer C, Hoch HH, Kiwit JC (2002) Case history: multicentric glioma with involvement of the optic chiasm. Clin Neurol Neurosurg 105:66–68
Jawahar A, Weilbaecher C, Shorter C, Stout N, Nanda A (2003) Multicentric glioblastoma multiforme determined by PET: a case report. Clin Neuro Neurosurg 106:38–40
Iza B, Mateo-Sierra O, Ruiz-Juretszke F, Garbizu J, Guzmán de Villoria J, Carrillo R (2006) Familiar glioblastoma presenting as a true multicentric tumor: etiopathogenic and prognostic features [Article in Spanish]. Neurocirugía (Astur) 17:340–347
Ampil F, Burton GV, Gonzalez-Toledo E, Nanda A (2007) Do we need whole brain irradiation in multifocal or multicentric high-grade glioma? Review of cases and literature. J Neuorooncol 85:353–355
Colavolpe C, Guedj E, Metellus P, Barrie M, Figarella-Branger D, Mundler O, Chinot O (2008) FDG-PET to predict different patterns of progression in multicentric glioblastoma: a case report. J Neuroncol 90:47–51
Hassaneen W, Levine NB, Suki D, Salaskar AL, de Moura LA, McCutcheon IE, Prabhu SS, Lang FF, DeMonte F, Rao G, Weinberg JS, Wildrick DM, Aldape KD, Sawaya R (2011) Multiple craniotomies in the management of multifocal and multicentric glioblastoma. Clinical article. J Neurosurg 114:576–584
Bradley WL (1880) Case of gliosarcomatous tumors of the brain. Proc Conn Med Soc 2:39–41
Thomas RP, Xu LW, Lober RM, Li G, Nagpal S (2013) The incidence and significance of multiple lesions in glioblastoma. J Neurooncol 112(1):91–97
Patil CG, Eboli P, Hu J (2012) Management of multifocal and multicentric gliomas. Neurosurg Clin N Am 23:343–350
Stuckey SL, Wijedeera R (2008) Multicentric/multifocal cerebral lesions: can fluid-attenuated inversion recovery aid the differentiation between glioma and metastases? J Med Imaging Radiat Oncol 52(2):134–139
Budka H, Podreka I, Reisner T, Zeiler K (1980) Diagnostic and pathomorphological aspects of glioma multiplicity. Neurosurg Rev 3:230–241
Courville CB (1936) Multiple primary tumors of the brain. Review of the literature and report of twenty-one cases. Am J Cancer 26:703–731
Nakhl F, Chang EM, Shiau JS, Alastra A, Wrzolek M, Odaimi M, Raden M, Juliano JE (2010) A patient with multiple synchronous gliomas of distinctly different grades and correlative radiographic findings. Surg Neurol Int 16(1):48
Turola MC, Schivalocchi R, Ramponi V, De Vito A, Nanni MG, Frivoli GF (2009) A rare case of multicentric synchronous bi-frontal glioma in a young female. Diagnostic and therapeutic problems. Cases J 2(1):81
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62:753–764
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115(1):3–8
Gulati S, Jakola AS, Nerland US, Weber C, Solheim O (2011) The risk of getting worse: surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg 76(6):572–579
McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, Weingart JD, Brem H, Quiñones-Hinojosa AR (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110:156–162
Sarkar A, Chiocca EA (2011) Multiple craniotomies. J Neurosurg 114(3):574–575, discussion 575
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
di Russo, P., Perrini, P., Pasqualetti, F. et al. Management and outcome of high-grade multicentric gliomas: a contemporary single-institution series and review of the literature. Acta Neurochir 155, 2245–2251 (2013). https://doi.org/10.1007/s00701-013-1892-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1892-9