Abstract
There is a lack of class I evidence concerning the impact of surgery in the treatment of diffuse low-grade glioma; the early maximal resection with preservation of eloquent brain areas has been accepted as the first therapeutic option. We performed a systematic review of the literature using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and protocol. Inclusion criteria: only case series with at least 100 patients containing supratentorial hemispheric diffuse low-grade glioma (according to any of the WHO classification used in papers published between 2000 to 2019), with pre- and postoperative MRI study were included in the qualitative and quantitative analyses. The extent of resection should be defined based on MRI at least in two categories and correlated with patients’ outcomes (with univariate or multivariate analyses) using overall survival (OS) or malignant progression-free survival (MPFS). A total of 18 series with 4386 patients, published in 20 papers, were included in this systematic review. All the series that evaluates the relation between the extent of resection (EOR) and OS showed a statistically significant improvement of OS at univariate and/or multivariate analyzes with a greater EOR. Six studies showed a statistically significant improvement of MPFS with a greater EOR. We demonstrate that when a more rigorous analysis of EOR is performed, a benefit of a more aggressive resection on OS and MPFS is observed. Our review about EOR in different molecular groups of DLGG also suggests a benefit of maximum safe resection for all different subtypes, even though “radical surgery” may be associated with better OS and MPFS in tumors with a more aggressive signature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Importance of the study
We made a systematic review about the importance of the extent of resection (EOR) in diffuse low-grade glioma. This review brings three new aspects in literature:
-
1.
We selected only papers with a systematic assessment of the extent of resection with MRI
-
2.
We detailed the papers with pre- and postoperative volumetric assessments
-
3.
We reviewed the impact of the extent of resection on different molecular subtypes of LGG
Our study reviews the results of large, modern series that assessed the impact of EOR on the outcome of patients with DLGG. Although there are similar studies, this is the first review to combine all series that performed pre- and postoperatively MRI analysis of the tumor and all series that also performed volumetric analyses. We could observe for the first time that all series with a rigorous analysis of the tumor volume with volumetric assessment were unanimous in demonstrating that the higher the EOR the better the prognosis.
Introduction
Supratentorial diffuse low-grade glioma (DLGG), classified as World Health Organization (WHO) Grade II tumors, are diffuse neuroectodermal primary brain tumors [5, 58]. They are characterized by continuous and relatively slow growth at an average rate of 4 mm/year [41, 58]. Their diffuse infiltrative pattern, tumor invasiveness, and migration along the white matter tracts limit the extent of surgical resection, jeopardizing the efficacy of oncological treatments. DLGG will inevitably become malignant [56, 57] and such malignant transformation (MT) will lead to the functional deficit and ultimately to death [13].
Supratentorial DLGG is a complex and heterogeneous group, with a highly variable median survival, ranging from a few months to more than 15 years, variable according to clinical factors, molecular characteristics, and treatment protocol [5, 18, 24, 62].
Treatment of DLGG includes surgery, radiotherapy, and/or chemotherapy. The role of surgery is still to be investigated in a randomized trial, which is unlikely to happen due to ethical reasons [58]. Although there is a lack of class I evidence concerning the impact of surgery, the early maximal resection with preservation of eloquent brain areas has been accepted as the first therapeutic option for most DLGG [5, 40, 45, 63], once multiple clinical series have demonstrated evidence that extent of resection (EOR) is a significant prognostic factor for malignant progression-free survival (MPFS) and overall survival (OS) among those patients [5, 40, 45, 63].
This systematic review aims to evaluate the impact of the EOR in DLGG patients and also assess the impact of the new WHO gliomas classification, based on the molecular signature, on the surgical treatment of different subtypes of DLGG. The originality of this article consists of our paper selection, including only series with a systematic assessment of the EOR comparing pre- and postoperative MRI, and we detailed the papers with pre- and postoperative volumetric assessment.
Methods
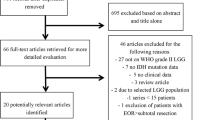
We performed a systematic review of the literature using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and protocol (Fig. 1). A literature search was performed using PubMed, Cochrane Library, EMBASE, MEDLINE, and SCOPUS databases up to October 12, 2019. Search terms included “grade II AND gliomas AND surgery, low-grade AND gliomas AND surgery, and low-grade AND glioma AND surgery.” We selected full-text articles published from January 2000 to September 2019. Screening of titles and abstracts was performed, and further evaluation of full-text publications was used to further exclude studies.
Inclusion criteria
Only case series with at least 100 patients containing supratentorial hemispheric diffuse low-grade glioma (according to any of the WHO classification used in papers published between 2000 to 2019), with pre- and postoperative MRI study were included in the qualitative and quantitative analyses. The extent of resection should be defined based on MRI at least in two categories and correlated with patients’ outcomes (with univariate or multivariate analyses) using OS or malignant progression-free survival (MPFS).
Exclusion criteria
Case series related to non-hemispheric supratentorial diffuse low-grade glioma (e.g.. optic nerve glioma), series in which the EOR was defined based only in the surgeon’s impression or with CT scan, and extension of resection versus outcome assessed using only progression-free survival (PFS) were excluded.
We did not use the PFS as a variable, because we understand that DLGGs present a slow, but continuous growth. Therefore, they would not have a real interval without progression [39]. Mandonnet et al. [29] demonstrated that DLGGs are not stable lesions. They observed that the average slope of the evolution of the mean tumor diameter [MTD = (2 × V)1/3] overtime was corresponding to 4.1 mm/year (95% CI 3.8–4.4 mm/year), and that the MTD of these tumors grows inexorably during the follow-up period in a grossly linear fashion. Pallud et al. [40], in a similar paper with 143 DLGG patients, showed a growth rate in all cases, mean of 4.4 mm/year (ranged from 1 to 35.8 mm/year). The same group published a larger series of DLGG with 407 patients, showing a mean diameter expansion of 5.8 mm/year (median of 3.9 mm/year) [38]. They observed that the spontaneous velocity of diametric expansion modeled as a categorical (< 8 or ≥ 8 mm/year) or as a continuous variable was an independent prognostic factor at multivariate analysis for OS (p < 0.001) and MPFS (p < 0.001). It also has been shown that radiological tumor growth rates remain unchanged after surgical resection [29, 31].
We extracted details on the type of study, which WHO classification was used, patient characteristics including age and sex, initial tumor volume, volumetric assessment, resected percentage, extent of resection per group, OS versus EOR, malignant progression-free survival versus EOR, follow-up years, and number of deaths. Included studies were assessed by two authors (L. J. M. M. F and L. A. F. A.) to ensure that cases were correctly included in the study. Patient data from multiple studies were combined into a table for comparison (Table 1) [1, 5, 6, 8, 12, 17, 20, 22,23,24, 26, 27, 31, 36, 37, 45, 50, 52, 58, 64].
Additional clinical questions
In addition to the systematic review described above, we conducted a second review seeking to answer two questions to assess the role of the molecular profile versus the EOR or RTV in DLGG:
-
1.
Does the EOR or RTV have a positive effect on OS according to different molecular signatures?
-
2.
Does the molecular signature influence the EOR or RTV?
To this review, we included case series containing supratentorial hemispheric diffuse low-grade glioma with pre- and postoperative MRI study. The EOR or RTV should be defined based on MRI and correlated with patients’ outcomes using OS. The EOR or RTV should be correlated with the IDH and/or 1p19q status.
Results
A total of 18 series, published in 20 papers, were included in this systematic review (Table 1). Two series were published twice focusing on different aspects of the same population in the case of Nitta et al. [36, 37], and reviewing the results considering the new WHO classification and with longer follow up in the case of Jakola et al. [21, 23]. Thirteen series were single-center [1, 6, 8, 12, 17, 26, 27, 30, 36, 37, 45, 52, 64], two with two centers [23, 24, 50], and three multicentric [5, 20, 50]. Seventeen series were retrospectives and one had a mixture of retrospective and prospective patients [5]. In all series, the EOR was based in a pre- and postoperative MRI; there were no volumetric assessments in 7 series, volumetric assessment with the ellipse volume in 2 series [5, 10], and volumetric assessment by segmentation in 10 series [8, 17, 22, 26, 36, 37, 45, 50, 52, 58].
A total of 4386 patients were included. The mean age ranged from 34.7 to 45 years old, and the median age ranged from 36 to 44 years old. In all series, men were more predominant (ranging from 54.1 to 76%). The mean initial tumor volume ranged from 38.4 to 75 cm3 and the median initial tumor volume ranged from 28.8 to 69 cc. The mean EOR ranged from 75.5 to 81.4% and the median EOR ranged from 76.1 to 95%.
We found different cutoff thresholds of EOR, as shown in Table 1, and even then, all the series (17/18) that evaluate the relation between EOR and OS showed a statistically significant improvement of OS at univariate and/or multivariate analyzes with a greater EOR. The only series that did not observe such association was the one published by Chaichana et al. [6]. Those authors did not evaluate that parameter—instead, their analysis was focused on recurrence and malignant degeneration after resection of DLGG. Six studies showed a statistically significant improvement of MPFS at univariate and/or multivariate analyzes with a greater EOR [1, 6, 20, 22,23,24, 52].
We observed a heterogeneous follow-up duration, varying from a mean of 3 years to 7.8 years. The median OS varied from 5.8 to 6.7 years at biopsy groups to 14.4–25.1 years in groups that underwent maximal safe resection.
The authors used different WHO classification in their methodology (range from 1993 WHO classification to 2016 WHO classification). It is interesting to observe that with the meticulous analysis of the pre and postoperative tumor volume, a positive correlation between EOR and OS was observed independent of the WHO classification used.
Surgical goal
Regarding OS and MPFS, some authors have demonstrated the benefit of EOR as a continuous variable and others as an ordinal variable [1, 5, 25, 32, 36, 52, 64] (Table 2). However, the threshold of the extent of resection or residual tumor volume (RTV) from which there is a clinical benefit remains a matter of debate. In Table 2, we selected series that correlated in a multivariate fashion the EOR or postoperative residual tumor volume with the overall survival. We observe that for EOR, a positive finding ranges from > 40% of resection to 100% or resection; and for RTV, a positive finding ranges from < 15 to 0 cm3 [1, 5, 18, 22, 25, 26, 32, 36, 37, 44, 51, 52, 56, 62, 64].
Role of molecular signature
With the increasing participation of molecular markers in the management of DLGG, it has been speculated on its real role in clinical practice. We reviewed these markers to answer two main questions related to the main scope of our review (Table 3).
Does the EOR or RTV have a positive effect on OS according to different molecular signatures?
There is no consensus about this data in the literature, as we may see in Table 3. The methodology and focus of these studies are heterogeneous, making it hard to conclude. Some authors observed that a greater resection was beneficial to all the new subgroups of the 2016 WHO classification [24]. Eseonu et al. [17] also believe that all subgroups would benefit from a higher EOR. However, because of a small number of patients with IDHmt without 1p19q codeletion, this subgroup did not reach the statistical significance. On the other hand, Wijnenga et al. [58] only found the benefit of a greater EOR in this specific subgroup with IDHmt without 1p19q codeletion.
Analyzing in isolation the IDHmt, Jungk et al. [25] and Scherer et al. [50] concluded that IDHmt patients would benefit from a higher EOR, which was not seen by Patel et al. [41]. And analyzing in isolation the IDHwt, Patel et al. [41] and Scherer et al. [50] observed a higher OS in patients with greater EOR. Poulen et al. [43] could not correlate positively the EOR with OS; however, all the patients in this series benefited from a large resection as we comment below in the discussion.
When observing the 1p 19q isolated, Scherer et al. [50] observed a greater EOR benefit in these patients.
Does the molecular signature influence the EOR or RTV?
Cordier et al. [10] retrospectively investigated the predictive value of 1p19q, IDH1, p53 expression, and Ki67 index for the EOR in 200 patients with DLGG, trying to discover if a better EOR is correlated to a more favorable genetic profile. There was no significant correlation between IDH1, p53, or Ki67 and the EOR. However, they observed a statistical significance of lower EOR in patients with codeletion 1p19q (p = 0.0463).
Wijnenga et al. showed that the molecular subtype of the tumor did not correlate with postoperative tumor volume [58]. Scherer et al. [50] and Rossi et al. [46] did not find any association between IDH status and postoperative RTV or EOR, respectively.
Discussion
The impact of the EOR on the outcome of DLGG patients is controversial. Different factors likely play a role to justify such heterogeneous results, including tumor biology, case selection, and method for assessment of the extent of resection. The inconsistency of results might be influenced by the fact that EOR was not routinely assessed on postoperative MRI but, instead, it was sometimes based on the neurosurgeon’s estimation of tumor resection or a single computed tomography scan, in some studies, what has been shown to be much less accurate [13, 22].
According to European guidelines [53] and as demonstrated by our review, maximal resection with preservation of eloquent brain areas is an essential part of the treatment of DLGG [53]. The classic strategy of biopsy followed by a “wait and see” approach is associated with inferior outcomes and should be reserved for cases where tumor resection may lead to significant neurological deficit [23, 24, 45]. It may jeopardize the treatment by allowing the continuous progression of the disease and there is the chance of underestimation of the tumor grade [33].
Extent of resection
The largest surgical series comparing EOR and prognosis was made by a French multicenter and multidisciplinary study group (Réseau d’Étude des Gliomes), in which each center applied a different therapeutic approach along the timespan covered by this study [5]. A total of 1097 patients with DLGG were collected retrospectively since January 1985 and prospectively from 1996 up to December 2007. In multivariate analysis, the results of the study demonstrated that EOR, as well as postsurgical residual volume, were independent prognostic factors significantly associated with longer overall survival.
Until now, no randomized controlled trial assessing the different extent of resection rates and clinical outcomes has been conducted in patients with DLGG [45]. However, the current literature presents accumulating evidence to support the benefits of early surgical intervention. Additionally, it is unlikely that such a randomized trial will ever be performed for ethical reasons.
Two near-randomized series compared the OS of patients with DLGG in similar populations, exposed to distinct surgical approaches [23, 24, 45]. Jakola et al. [23] compared the results of parallel cohorts of DLGGs from 2 independent hospitals with different surgical strategies: in one hospital, only biopsy followed by a “wait and scan” approach was the first choice (66 patients—71% biopsy and 29% resection); in the other hospital, the early resections as the main conduct (87 patients—14% biopsy and 86% resection). They observed no significant differences in surgical complications (9% vs. 8%; p = .82) or new deficits (18% vs. 21%; p = .70) between the two institutions. However, the malignant transformation was more common when the biopsy was the initial approach (56% vs. 37%; p = 0.02). There was also a relevant difference in median survival between the centers, at the center favoring biopsy it was 5.8 years and at the center favoring early resection, it was not reached (p < 0.001), estimated 5-year survival was 57% vs. 81% respectively.
Roelz et al. [45] also published another near randomized study in a single institution series with two different departments (Department of Stereotactic Neurosurgery and Department of Neurosurgery) with different and independent approaches to DLGG patients. These patients were referred to either department by a near-randomized process. A total of 126 patients with DLGG entered the study, 77 patients were initially submitted to stereotactic biopsy, and 49 patients underwent an early surgical approach. They observed a significantly better OS for patients managed by early surgical management (p = 0.0018). The 5/10-year OS rate was 82%/67% for early resection versus 54%/38% for initial biopsy. Interestingly, a later surgical intervention as performed in 22 patients initially submitted to only a biopsy (after a mean of 2.8 years) did not have a favorable impact on OS, making clear the high importance of the initial treatment decision. It is important to observe that the survival benefit of patients initially submitted to surgical resection was observed in patients with a residual tumor volume ≤ 15 cm3 (p = 0.034).
Two recently published literature reviews deserve mention. Xia et al. [59] did a meta-analysis to assess the relationship between the extent of resection (EOR) and the prognosis of patients with DLGG. They selected 20 studies that fulfilled their inclusion criteria and observed that, if compared with STR, GTR could significantly increase the 5-year survival of patients with DLGG (OR 3.90; 95% CI 2.79~5.45) and the 10-year survival (OR 7.91; 95% CI 5.12~12.22). Additionally, GTR significantly increased survival at 5-years when compared to biopsy only (OR 5.43; 95% CI 3.57~8.26) and 10-years (OR 10.17; 95% CI 4.02~25.71). Finally, when compared to the survival of patients with DLGG submitted to biopsy versus STR, the last one significantly increased survival at 5-years (OR 2.59; 95% CI 1.81~3.71) and 10-years (OR 2.21; 95% CI 1.16~4.25). Yang et al. [61] performed a systematic review and meta-analysis of papers analyzing the impact of EOR in DLGG. They included 60 reports (55 were retrospective, 5 prospective, and no randomized controlled trials) of adult patients with supratentorial DLGG, with 13,289 patients. They found that the mean OS increased from 3.79 years (95% CI 2.37–5.22) in the biopsy group to 6.68 years (95% CI 4.19–9.16) in the STR group and 10.65 years (95% CI 6.78–14.52) in the GTR group. They showed a benefit in OS associated with GTR versus biopsy (p < 0.001); GTR versus STR (p < 0.001); resection of any extent versus biopsy (p = 0.015); and STR versus biopsy (p = 0.04). The incidence of malignant transformation was 53.7% (95% CI 29.5–76.3) in the biopsy group, 47.5% (95% CI 30.3–65.4) in the STR group, and 15.9% (95% CI 4.2–44.7) in the GTR group. Compared with STR, GTR delayed the occurrence of malignant transformation significantly (p = 0.032). Those reviews show that patients with DLGG are expected to benefit from a greater EOR if no new neurological deficit is associated with the procedure. The greater EOR also improve seizure control and delay malignant transformation [61]. Some of the limitations of those studies include no randomized trials available for analysis, the inclusion of old retrospective series, and poor criteria for the assessment of EOR (almost all of the studies defined EOR based on the impression of the surgeon or CT scan, instead of systematic assessment of pre and post-op MRI). Our review is the first in the literature to focus on a series of DLGG outcome with a meticulous assessment of EOR by MRI, what we believe to provide a better level of evidence.
Besides the improvement in OS and MPFS, a maximal EOR has also been associated with better control of seizure, which is a significant source of morbidity in DLGG patients. The review of Yang et al. [61] mentioned above also showed that the GTR group had an 81% seizure-free rate compared with 54.2% in the STR group and 47.8% in the biopsy group. Xu et al. [60] in a multivariate analysis of a series of 128 DLGG patients observed that the EOR was the only parameter to significantly affected the likelihood of postoperative Engel Class I status (all the subgroups of Class I) (p = 0.002). They observed in a receiver operating characteristic (ROC) curve that the ideal threshold to seizure control was an EOR ≥ 80%, with approximately 94% of Engel Class I beyond this cutoff. Still et al. [54] performed a similar study with 346 DLGG patients, and they also found in a multivariate analysis that a higher percent of tumor resection was independently associated with good postoperative seizure control (p < 0.001). Based on a ROC curve they established an optimal cutoff of ≥ 91.1% of EOR and ≤ 19 cm3 of the residual tumor to obtain an optimal total seizure control (only Engel Class IA).
Surgical goal
There is no consensus about the minimal extent of resection needed to obtain any significant clinical benefits. Smith et al. [52] observed a significant association between EOR and survival (p < 0.001). They found that patients with at least 90% resection had 5- and 8-year OSs of 97% and 91%, respectively, whereas patients with less than 90% resection had 5- and 8-year OS rates of 76% and 60%, respectively. EOR remained a statistically significant predictor of OS even when the set of patients analyzed was limited to those with EOR of at least 80% (p = 0.016).
Ius et al. [22] provided evidence that a more aggressive resection correlates with a significant improvement in OS (p = 0.001), as well as in PFS (p < 0.0001) and MPFS (p < 0.0001), compared with a simple debulking procedure. The EOR, treated as both a continuous and an ordinal variable, was associated with significant improvement of both PFS and MPFS values. Patients with an EOR of less than 70% had a much higher risk of death (19.7 times), disease progression (13.6 times), and malignant transformation (9.77 times) than did patients with an EOR of 90% or more.
The largest investigation addressing the influence of EOR on prognosis suggested a residual tumor volume of fewer than 10 cm3 to be the crucial factor for a favorable prognosis, in particular, due to a delay of anaplastic transformation [5]. A large near-randomized series observed that the survival benefit of patients with initial resection was reserved for patients with a residual tumor volume of fewer than 15 cm3 [45].
In a more recent series, Scherer et al. [50] used volumetric analysis in order to quantitatively evaluate the association of RTV and EOR with survival and to address whether survival associations follow continuous or threshold-based principles. For OS, regression was limited to univariate analysis corroborating a significant impact of continuous volumetric measures RV (p < .001) and EOR (p < .002).
Although there is no consensus to how much of resection is “satisfactory,” and probably there is a linear association of the residual disease with survival, current evidence suggests that an extent of resection threshold of > 80% and a residual volume of < 10 cm3 or at least < 15 cm3 as the surgical goal in DLGG needed to be of therapeutic benefit to individual patients [12, 21, 45, 52]. Although EOR and the final residual volume are independent factors associated with improved outcomes, we believe the final residual volume might be a more reliable factor, since it is not dependent on the initial tumor volume—another important predictor of prognosis [12, 22, 32, 45, 52, 63].
As Scherer et al. [50] stated, to define minimum cutoffs is a reasonable approach to guide decision making preoperatively; however, dichotomization may lead to limited statistical information, to simplification of differences within dichotomized subgroups and, hence, oversimplification of the issue.
Different strategies can be used to try to estimate the percentage of the tumor able to be removed (EOR) or the probable final residual volume. The functional atlas of human white matter developed by Sarubbo et al. [48], based on the concept of “minimal common brain” [21], is a good example of a useful tool for such a goal. In a second publication, Sarubbo et al. [47] published a more complete atlas integrating both cortical hubs and white matter tracts critical for brain functions.
Supratotal resection
The boundaries of DLGG cannot be precisely defined by current neuroimaging technology. Pallud et al. [40] studied 16 patients who underwent serial stereotactic biopsies for the diagnosis of untreated supratentorial DLGG, in whom biopsy samples were taken within and beyond MRI-defined abnormalities. They demonstrated that conventional MRI underestimates the actual spatial extent of DLGG, even when they are well delineated. Biopsies performed at distances of 10 to 20 mm from the limit of MRI-defined abnormalities showed a significantly larger number of tumor cells than biopsies at distances greater than 20 mm from MRI-defined abnormalities [21]. Therefore, the amount of brain containing tumor cells is larger than estimated by routine MR-sequences, which may explain recurrences at the resection margins after gross total surgical removal and the prognostic significance of the EOR [48]. These results support the idea that a functional-based extended resection encompassing a margin beyond MRI-defined abnormalities (ideally with 2 cm of margin) should be pursued whenever feasible, in non-eloquent brain areas. This might improve the outcome of DLGGs, by delaying anaplastic transformation [19, 40, 63]. All these aspects favored the creation of supratotal resection (SpTR) conception
Yordanova et al. [63] published a series of 15 patients with DLGG submitted to SpTR and observed that the margin of the resected brain tissue showed tumoral cells in all but 1 case, strengthening the theory that conventional MR imaging (even in FLAIR) underestimates the real extension of these lesions. They hypothesized that SpTR approach would reduce even more the tumoral burden, and consequently improve the OS and MPFS. Based on intraoperative mapping, they extended the resection up to functional boundaries at the cortical and subcortical levels. They compared these 15 patients submitted to SpTR with a control group of 29 patients of “only” complete resection and observed that malignant transformation was observed in 7 cases in the control group but in no case in the SpTR group (p = 0.037). Furthermore, adjuvant treatment was administered in 10 patients in the control group compared with only one patient in the SpTR group (p = 0.043). The mean duration of postoperative follow-up was 35.7 months (range 6–135 months)[58]. The same group published a series of long-term follow up (mean of 11 years, range, 8–16.5 years) in 16 patients with DLGG submitted to SpTR [49]. No patient was submitted to adjuvant treatment. There was no relapse in eight cases. Eight patients experienced tumor recurrence after an average time of 70.3 months (range, 32–105 months), but without malignant transformation [14].
Although the SpTR is not possible in most cases because of the proximity with eloquent areas, it should be pursued whenever possible, because it might positively change the natural history of DLGG, by decreasing the risk of malignant transformation (MT) for a long period
Functional aspects related to EOR
Despite all evidences presented in favor of a broad EOR of the tumor, maximal EOR or even supratotal resection should only be favored in connection with preservation of neurological and neurocognitive functions. A new permanent neurological deficit may also influence the overall survival, and then must be prevented.
There is an increased amount of evidences showing the benefit of brain mapping in awake surgeries for DLGG, in preserving a good quality of life/return to work in patients submitted to a maximal EOR or even supratotal resection.
Aiming to assess the postoperative cognitive function and ability to work in patients with DLGG, Muto et al. [34] published a series with 39 cases involving eloquent areas and having a functional-based maximal surgical resection under awake procedures. The mean EOR was 91.0% (total or supratotal resection in 46.2% of cases) and the mean residual volume was 4.8 cm3. They observed functional worsening in 89.7% of patients in the early postoperative period (no relation with the EOR, p = 0.216). In long-term cognitive outcomes, none of the patients without preoperative cognitive deficit had a postoperative cognitive deficit; and in the patients with a preoperative cognitive deficit, 44.4% improved, 36.1% remained stable, and 27.8% worsened as compared to the preoperative evaluation. A total of 28/34 patients (82.4%) with preoperative remunerative employment resumed their previous job. The mean time between surgery and return to work was 6.9 months. Subtotal and total resection (p = 0.014 and p = 0.019 respectively) were independently associated with postoperative return to work. They conclude that functional-based surgical resection does not worsen postoperative cognitive evaluations and even may improve postoperative cognitive evaluations in these patients.
Ng et al. [35], in a series of 74 asymptomatic patients with incidental DLGG submitted to maximal safe resection in awake craniotomies, observed that 3 months after surgery, all patients had totally recovered from transient neurological worsening (no permanent deficits). The mean EOR was 95.7% (total or supratotal resection in 58.1% of cases), with a mean residual tumor volume of 1.3 cm3. Among the 68 employed patients preoperatively, 66 (97.1%) resumed their previous employment. The mean time between awake surgery and return to work was 6.8 months. They observed that the time between surgery and return to work did not statistically correlate with the EOR. They argue in favor of a preventive and maximal safe resection of DLGGs.
Rossi et al. [46] reported a large series of SpTR for DLGG aiming to investigate functional outcome based on an analysis of neuropsychological and quality of life profiles when resection was performed according to functional boundaries in awake craniotomies. In a total of 449 patients (79.5% of DLGG), they achieved SpTR in 145 patients (32.3%), total resection in 183 patients (40.8%), and subtotal/partial resection in 121 patients (26.9%). They observed that the rates of new (within the first week of surgery) deficits were quite high (88.9% in all group; 91.7% in partial/subtotal resection group; 88% in total resection group; and 87.6% in supratotal resection group) and independent from the EOR achieved (p = 0.498). Most deficits recovered in a few weeks. The incidence of permanent deficit (persistent at 1 month after surgery) was low (2% in all group; 6.6% in partial/subtotal resection group; 0.55% in total resection group; and 0.69% in supratotal resection group), but was significantly higher in the subtotal/partial resection group versus the total/supratotal group (p < 0.001). They concluded that the SpTR can be safely achieved in a considerable number of patients in the clinical routine when a functional approach is used.
Adjuvant treatment in DLGG
There is no defined approach to adjuvant therapy in DLGG. In young patients, whose tumors have a favorable molecular profile and who have undergone a total or supratotal resection, it is safe to not offer additional therapy initially. However, in older patients with significant residual tumor, additional treatment should be considered. Buckner et al. [3] published a series of 251 DLGG patients with a median follow-up time of the surviving patients of 11.9 years. Patients who were 18 to 39 years of age (with subtotal resection or biopsy), and those who were 40 years of age or older were randomized to receive radiotherapy alone or radiotherapy plus chemotherapy (PCV—procarbazine, CCNU, and vincristine). This phase 3 trial showed a survival benefit among patients with DLGG who were treated with radiation therapy plus chemotherapy, as compared with those who received radiation therapy alone. They also demonstrated that patients with tumoral IDH1 mutations had a significantly longer OS in the group treated with radiotherapy plus chemotherapy. [3] We cannot fail to mention the risks of late cognitive decline related to radiotherapy and the adverse events of chemotherapy. Buckner et al [3] also demonstrated, as expected, that the frequency and severity of toxic effects were greater in the group that received radiation therapy plus chemotherapy than in the group that received radiation therapy alone.
This is the reason why the EORTC group has recently proposed to use MGMT methylation score in order to “identify patients who benefit from first-line treatment with TMZ, to defer RT for long-term preservation of cognitive function and quality of life.” [2]. Indeed, thanks to a maximal safe resection up to functional boundaries, it is possible to postpone adjuvant treatment, especially radiotherapy, to preserve a better cognition—according to the needs of the patient—including in DLGG with foci of malignant transformation, as shown by Darlix et al. [11] in a series with 50 patients with 95% of survival at 5 years. In their series, the median time interval between surgery and the initiation of new treatment was 3.4 years (median time interval of 9.5 years between RT and the first surgery) [11]. In this state of mind, Mandonnet et al. [30] published a recursive algorithm to guide an individualized treatment: although no adjuvant treatment is recommended when a complete resection has been achieved, in case of initial biopsy and limited resection, epilepsy relapse and/or re-evolution of the tumor with a volume above 15 cm3 and/or growth rate acceleration, further treatment can be considered. The first option is re-operation, if it is estimated that a significant degree of resection as mentioned above is possible, without a higher risk of neurological deficit. If further surgery is not feasible, chemotherapy is considered. If the lesion remains radiologically stable with chemotherapy, one can only follow the patient with neuroimaging. If there is a reduction in tumor volume, the possibility of a new surgery may be re-considered. Radiotherapy should be considered in cases where there is no response to chemotherapy and if there is no possibility of a new surgery.
To conclude, in the recent literature, adjuvant treatment after surgery for DLGG is still matter of debate [15].
Role of molecular signature
The WHO 2016 edition has radically changed the classification of gliomas, updated based on molecular markers, which offers more precise prognostication and prediction of treatment response [28]. Gliomas are therefore classified using a layered diagnosis according to histologic as well as molecular criteria into astrocytomas and oligodendrogliomas [4, 28]. The main change in classification of histological DLGG is the presence or not of a mutation at the isocitrate dehydrogenase (IDH 1 or IDH 2; IDHmt henceforth for a mutation in either IDH1 or IDH2) and the presence or not of 1p19q codeletion. That allows classification into three distinct groups of DLGG[21, 27, 42, 47, 55]:
-
1.
IDH mutated with 1p and 19q codeletion—oligodendroglioma. They are associated with favorable outcomes [16, 21]. Median overall survival may be more than 14 years, up to 17.1 years in some series [4, 7].
-
2.
IDH mutated with no 1p and 19q codeletion—IDH mutated astrocytoma. Median overall survival of 9 to 11 years [4, 7, 58].
-
3.
IDH not mutated—IDH wild-type astrocytoma. A more aggressive form of low-grade glioma. Its prognosis may be similar to that of glioblastoma [46]. Median overall survival of 1.7 to 2.1 years [21, 43, 47]. However, it may present a heterogeneous median survival, per mutation, e.g., TERT mutant—1.76 years, TERT wt—10.65 years) [7].
The effect of EOR or RTV in OS at different DLGG groups (WHO 2016)
Jungk et al. [25] evaluated the prognostic impact of EOR in astrocytomas stratified with IDH1mt (38 patients). IDH1 mutation was an independent positive prognosticator for OS in multivariate analysis (p = 0.002). In a subgroup of patients with ≥ 40% EOR (n = 39), initial and residual tumor volumes were prognostic for OS (HR 1.03, p = 0.005 and HR 1.08, p = 0.007, respectively), persistent after adjustment for IDH1 mutation status.
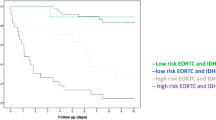
Five years after the first publication of their series, Jakola et al. [24] published new analyses of their cohort now in light of the established molecular markers, dividing patients into 3 groups: (1) the low-risk group being IDH mutated, 1p19q codeleted; (2) the intermediate-risk group being IDH mutated and 1p19q non-codeleted; and (3) the high-risk group being IDH wild-type. They found that the survival benefit of surgical strategy remained after adjustment for molecular groups (p = 0.001), supporting the results of their previous publication. They concluded that surgical resection is effective in all molecular subgroups.
Patel et al. [41] aiming to assess the impact of EOR on MPFS and OS in IDHmt and IDHwt DLGG performed a retrospective review of 74 patients (two groups: 52 IDH1 mutations and 22 IDH wild-type) with WHO grade II gliomas undergoing resection at a single institution. The 3-year OS rate was 95.2% in IDHmt patients’ versus 64.2% in the IDHwt group. Patients with IDHmt had correspondingly longer median MPFS (6.5 years) and median OS (10.9 years) versus IDHwt patients (median MPFS: 3.2 years, median OS: not reached). Greater EOR was the only variable assessed that prolonged MPFS (p = 0.009) and OS (p = 0.03) (150). However, when they performed an age-adjusted Cox regression model, stratified by IDH mutation status revealed that a greater EOR was only associated with prolonged MPFS and OS in IDHwt patients (MPFS—p < 0.001 and OS—p = 0.003), but not for IDHmt patients (MPFS—p = 0.83 and OS—p = 0.48). Therefore, greater EOR seems to be particularly associated with improved survival in a subgroup of DLGG with IDHwt status. Although these are interesting results, they should be carefully interpreted and the limitations of the study need to be considered, such as a small number of patients, retrospective nature of the study, baseline differences between groups, and short follow up.
Wijnenga et al. [58] (Table 1) studied the correlation of molecular profiles and RTV on OS. In a univariate analysis of the whole, the amount of postoperative RTV (as a continuous variable) was significantly associated with OS, with a hazard ratio (HR) of 1.01 per 1 cm3 increase in volume (p < 0.0001). However, there was a different impact of the RTV in astrocytomas (IDHmt, 1p19q retained) versus oligodendrogliomas (IDHmt, 1p19q codeleted). In the astrocytoma group, even with minimal residual volume (0.1–5.0 cm3), OS was impaired compared with no residual tumor (0.0 cm3). In the oligodendroglioma group, a trend toward better OS with more extensive resection was observed, although there was no significant improvement in OS in patients with GTR versus small residual tumor (0.1–5.0 cm3).
Poulen et al. [43] performed a retrospective review of 31 patients who underwent resection for histopathologically confirmed, IDHwt, non–1p19q codeleted gliomas. No patient received immediate postoperative adjuvant treatment, according to the authors due to the high extent of resection (94% on average) and because these grade II astrocytoma patients were managed before the new 2016 WHO classification. The median velocity of diameter expansion (VDE) was 2.45 mm/year (range 0.4–5 mm/year). The median follow-up was 74 months (range 24–157 months). They observed substantial heterogeneity in survival (OS at 5 years was 77.3% for the entire group). However, two groups were identified: subgroup 1 with 5 patients who underwent subtotal resection and died rapidly (median time from radiological diagnosis of 3.5 years); subgroup 2, no death of 21 patients with a long-term follow up over 5 years (2 patients with more than 10 years of follow up). Those results suggest that greater EOR may improve OS outcomes even in the absence of IDH mutation and 1p19q codeletion.
Eseonu et al. [17] assessed the role of EOR on OS based on genetic classification of DLGG: type 1 (IDHmt, 1p19q codeleted), type 2 (IDH1mt, TP53 mutation, ATRX mutation), and type 3 (IDHwt). They found that EOR was significantly associated with OS for type 1 DLGG with an EOR ≥ 70% (p < 0.001). EOR was also significantly associated with OS for type 3 DLGG with an EOR ≥ 79% (p = 0.040). However, they did not contain sufficient type 2 DLGG patients to conduct an EOR survival analysis. They concluded that this different EOR cutoff suggests that the genetic makeup of a DLGG could influence how effective the EOR is on survival.
Scherer et al. [50] found that IDHmt had a significant association with OS (p = 0.005), and that GTR had a beneficial effect on OS regardless of the molecular profile (based on IDH or 1p19q). Possible implications of molecular biomarkers on surgical efficacy and EOR could not be found.
As shown above, greater EOR is related to a better OS when all DLGG are analyzed as a single group (independent of molecular subtypes). EOR has also been proven to significantly affect survival, after the establishment of molecular biomarkers [24, 58], which we believe it is true. However, the question is how beneficial the degree of resection is for each subgroup. Possibly groups with a less favorable prognosis profile benefit more from a wider resection.
The molecular signature influence in the EOR or RTV
The relationship of EOR and glioma molecular markers, such as the 1p19q codeletion or IDH mutation, is not clear [10]. Probably, as seen in Table 3, favorable molecular markers are not related to a greater EOR. Cordier et al. [10] observed a statistical significance of lower EOR in patients with codeletion 1p19q, which reinforces the idea that a greater EOR is an important prognostic factor per se, independently of the molecular pattern.
Limitations of the review
Some of the limitations of our review are (1) the absence of randomized trials since none available in the literature. (2) We established an arbitrary cutoff of 100 patients as an inclusion criterion of our systematic review, which may exclude important smaller series. (3) We did not analyze the PFS reported in the series. Although it is a very used outcome, we believe it is not accurate enough because the DLGG presents continuous growth (so there is not a real-time with progression-free survival) and the definition of this parameter is heterogeneous in literature. (4) Among the papers added in this review, we may observe a wide variety of WHO classification (range from WHO 1993 to WHO 2016).
Conclusion
Our study reviews the results of large, modern series that assessed the impact of EOR on the outcome of patients with DLGG. Although there are similar studies, this is the first review to combine all series that performed pre and postoperatively MRI analysis of the tumor and all series that also performed volumetric analysis. We could observe for the first time that all series with a rigorous analysis of the tumor volume with volumetric assessment were unanimous in demonstrating that the higher the EOR the better the prognosis.
Surgery remains the first treatment option for the management of low-grade gliomas. There is accumulating evidence that supports early surgical resection rather than the classic conservative “wait and see” approach or stereotactic biopsy.
A better understanding of the different molecular signature demonstrates the heterogeneity of DLGG. Current data suggest a benefit of maximum safe resection for all different subtypes; however, “radical surgery” may be associated with better OS and MPFS in tumors with a more aggressive signature (IDHwt tumors; or IDH mutated non-codeleted tumors). Further understanding of the behavior and response to treatment of different DLGG subtypes will likely have an impact on treatment strategies shortly, with the advent of target therapies and new radiotherapy modalities and protocols. The incorporation of those modalities, the development of surgical techniques, and further understanding of the brain connectome may contribute to a significant improvement not only in patients’ survival but also in the quality of life.
References
Ahmadi R, Dictus C, Hartmann C, Zurn O, Edler L, Hartmann M et al (2009) Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir 151(11):1359–1365
Bady P, Kurscheid S, Delorenzi M, Gorlia T, van den Bent MJ, Hoang-Xuan K, Vauléon É, Gijtenbeek A, Enting R, Thiessen B, Chinot O, Dhermain F, Brandes AA, Reijneveld JC, Marosi C, Taphoorn MJB, Wick W, von Deimling A, French P, Stupp R, Baumert BG, Hegi ME (2018) The DNA methylome of DDR genes and benefit from RT or TMZ in IDH mutant low-grade glioma treated in EORTC 22033. Acta Neuropathol 135(4):601–615. https://doi.org/10.1007/s00401-018-1810-6
Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, Coons S, Ricci P, Bullard D, Brown PD, Stelzer K, Brachman D, Suh JH, Schultz CJ, Bahary JP, Fisher BJ, Kim H, Murtha AD, Bell EH, Won M, Mehta MP, Curran WJ Jr (2016) Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med 374(14):1344–1355. https://doi.org/10.1056/NEJMoa1500925
Cancer Genome Atlas Research N, Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372(26):2481–2498
Capelle L, Fontaine D, Mandonnet E, Taillandier L, Golmard JL, Bauchet L, Pallud J, Peruzzi P, Baron MH, Kujas M, Guyotat J, Guillevin R, Frenay M, Taillibert S, Colin P, Rigau V, Vandenbos F, Pinelli C, Duffau H, _ _ Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg 2013;118(6):1157-1168.
Chaichana KL, McGirt MJ, Laterra J, Olivi A, Quiñones-Hinojosa A (2010 Jan) Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J Neurosurg 112(1):10–17. https://doi.org/10.3171/2008.10.JNS08608
Clark VE, Cahill DP (2019) Extent of Resection Versus Molecular Classification: What Matters When? Neurosurg Clin N Am 30(1):95–101
Claus EB, Horlacher A, Hsu L, Schwartz RB, dello-Iacono D, Talos F, Jolesz FA, Black PM (2005) Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 103(6):1227–1233. https://doi.org/10.1002/cncr.20867
Coburger J, Merkel A, Scherer M, Schwartz F, Gessler F, Roder C, Pala A, König R, Bullinger L, Nagel G, Jungk C, Bisdas S, Nabavi A, Ganslandt O, Seifert V, Tatagiba M, Senft C, Mehdorn M, Unterberg AW, Rössler K, Wirtz CR (2016 Jun) Low-grade glioma surgery in intraoperative magnetic resonance imaging: results of a multicenter retrospective assessment of the German study group for intraoperative magnetic resonance imaging. Neurosurgery. 78(6):775–786. https://doi.org/10.1227/NEU.0000000000001081
Cordier D, Goze C, Schadelin S, Rigau V, Mariani L, Duffau H (2015) A better surgical resectability of WHO grade II gliomas is independent of favorable molecular markers. J Neuro-Oncol 121(1):185–193
Darlix A, Rigau V, Fraisse J, Gozé C, Fabbro M, Duffau H (2020) Postoperative follow-up for selected diffuse low-grade gliomas with WHO grade III/IV foci. Neurology 94(8):e830–e841
Duffau H, Lopes M, Arthuis F, Bitar A, Sichez JP, Van Effenterre R et al (2005) Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985-96) and with (1996-2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry 76(6):845–851
Duffau H, Taillandier L (2015) New concepts in the management of diffuse low-grade glioma: proposal of a multistage and individualized therapeutic approach. Neuro-oncology 17(3):332–342
Duffau H (2016) Long-term outcomes after supratotal resection of diffuse low-grade gliomas: a consecutive series with 11-year follow-up. Acta Neurochir 158(1):51–58
Duffau H (2018) Paradoxes of evidence-based medicine in lower-grade glioma: to treat the tumor or the patient? Neurology 91(14):657–662. https://doi.org/10.1212/WNL.0000000000006288
Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, Sarkar G, Caron AA, Kollmeyer TM, Praska CE, Chada AR, Halder C, Hansen HM, McCoy LS, Bracci PM, Marshall R, Zheng S, Reis GF, Pico AR, O’Neill BP, Buckner JC, Giannini C, Huse JT, Perry A, Tihan T, Berger MS, Chang SM, Prados MD, Wiemels J, Wiencke JK, Wrensch MR, Jenkins RB (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372(26):2499–2508
Eseonu CI, Eguia F, ReFaey K, Garcia O, Rodriguez FJ, Chaichana K, Quinones-Hinojosa A (2017) Comparative volumetric analysis of the extent of resection of molecularly and histologically distinct low grade gliomas and its role on survival. J Neuro-Oncol 134(1):65–74. https://doi.org/10.1007/s11060-017-2486-9
Franceschi E, Mura A, De Biase D, Tallini G, Pession A, Foschini MP et al (2018) The role of clinical and molecular factors in low-grade gliomas: what is their impact on survival? Future Oncol 14(16):1559–1567
Gerin C, Pallud J, Deroulers C, Varlet P, Oppenheim C, Roux FX, Chretien F, Thomas SR, Grammaticos B, Badoual M (2013) Quantitative characterization of the imaging limits of diffuse low-grade oligodendrogliomas. Neuro-oncology. 15(10):1379–1388
Gousias K, Schramm J, Simon M (2014) Extent of resection and survival in supratentorial infiltrative low-grade gliomas: analysis of and adjustment for treatment bias. Acta Neurochir 156(2):327–337. https://doi.org/10.1007/s00701-013-1945-0
Hervey-Jumper SL, Berger MS (2019) Evidence for improving outcome through extent of resection. Neurosurg Clin N Am 30(1):85–93
Ius T, Angelini E, Thiebaut de Schotten M, Mandonnet E, Duffau H (2011) Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a "minimal common brain". NeuroImage 56(3):992–1000
Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgard G et al (2012) Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. Jama 308(18):1881–1888
Jakola AS, Skjulsvik AJ, Myrmel KS, Sjavik K, Unsgard G, Torp SH et al (2017) Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol 28(8):1942–1948
Jungk C, Scherer M, Mock A, Capper D, Radbruch A, von Deimling A, Bendszus M, Herold-Mende C, Unterberg A (2016) Prognostic value of the extent of resection in supratentorial WHO grade II astrocytomas stratified for IDH1 mutation status: a single-center volumetric analysis. J Neuro-Oncol 129(2):319–328
Liu S, Wang Y, Fan X, Ma J, Ma W, Wang R, Jiang T (2016) Anatomical involvement of the subventricular zone predicts poor survival outcome in low-grade astrocytomas. PLoS One 11(4)
Louis DN, Perry A, Burger P, Ellison DW, Reifenberger G, von Deimling A, Aldape K, Brat D, Collins VP, Eberhart C, Figarella-Branger D, Fuller GN, Giangaspero F, Giannini C, Hawkins C, Kleihues P, Korshunov A, Kros JM, Beatriz Lopes M, Ng HK, Ohgaki H, Paulus W, Pietsch T, Rosenblum M, Rushing E, Soylemezoglu F, Wiestler O, Wesseling P, International Society Of Neuropathology--Haarlem (2014) International Society Of Neuropathology--Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol 24(5):429–435
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131(6):803–820
Mandonnet E, Delattre JY, Tanguy ML, Swanson KR, Carpentier AF, Duffau H, Cornu P, van Effenterre R, Alvord EC, Capelle L (2003) Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol 53(4):524–528
Mandonnet E, Duffau H (2018) An attempt to conceptualize the individual onco-functional balance: why a standardized treatment is an illusion for diffuse low-grade glioma patients. Crit Rev Oncol Hematol 122:83–91
Mandonnet E, Pallud J, Fontaine D, Taillandier L, Bauchet L, Peruzzi P, Guyotat J, Bernier V, Baron MH, Duffau H, Capelle L (2010) Inter-and intrapatients comparison of WHO grade II glioma kinetics before and after surgical resection. Neurosurg Rev 33(1):91–96
McGirt MJ, Chaichana KL, Attenello FJ, Weingart JD, Than K, Burger PC et al (2008) Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 63(4):700–707 author reply 7-8
Muragaki Y, Chernov M, Maruyama T, Ochiai T, Taira T, Kubo O, Nakamura R, Iseki H, Hori T, Takakura K (2008) Low-grade glioma on stereotactic biopsy: how often is the diagnosis accurate? Minim Invas Neurosur 51(5):275–279
Muto J, Dezamis E, Rigaux-Viode O, Peeters S, Roux A, Zanello M, Mellerio C, Sauvageon X, Varlet P, Oppenheim C, Pallud J (2018) Functional-based resection does not worsen quality of life in patients harboring a diffuse low-grade glioma involving eloquent brain regions: a prospective cohort study. World Neurosurg 113:e200–e212. https://doi.org/10.1016/j.wneu.2018.01.213
Ng S, Herbet G, Moritz-Gasser S, Duffau H. Return towork following surgery for incidental diffuse low-grade glioma: a prospective series with 74 patients. Neurosurgery. 2019 9:nyz513. doi: https://doi.org/10.1093/neuros/nyz513. Online ahead of print.PMID: 31813972
Nitta M, Muragaki Y, Maruyama T, Ikuta S, Komori T, Maebayashi K, Iseki H, Tamura M, Saito T, Okamoto S, Chernov M, Hayashi M, Okada Y (2015) Proposed therapeutic strategy for adult low-grade glioma based on aggressive tumor resection. Neurosurg Focus 38(1):E7
Nitta M, Muragaki Y, Maruyama T, Iseki H, Ikuta S, Konishi Y, Saito T, Tamura M, Chernov M, Watanabe A, Okamoto S, Maebayashi K, Mitsuhashi N, Okada Y (2013) Updated therapeutic strategy for adult low-grade glioma stratified by resection and tumor subtype. Neurol Med Chir (Tokyo) 53(7):447–454
Pallud J, Blonski M, Mandonnet E, Audureau E, Fontaine D, Sanai N, Bauchet L, Peruzzi P, Frenay M, Colin P, Guillevin R (2013) Velocity of tumor spontaneous expansion predicts long-term outcomes for diffuse low-grade gliomas. Neuro-oncology 15(5):595–606
Pallud J, Mandonnet E, Duffau H, Kujas M, Guillevin R, Galanaud D, Taillandier L, Capelle L (2006) Prognostic value of initial magnetic resonance imaging growth rates for World Health Organization grade II gliomas. Ann Neurol 60(3):380–383
Pallud J, Varlet P, Devaux B, Geha S, Badoual M, Deroulers C, Page P, Dezamis E, Daumas-Duport C, Roux FX (2010) Diffuse low-grade oligodendrogliomas extend beyond MRI-defined abnormalities. Neurology 74(21):1724–1731
Patel T, Bander ED, Venn RA, Powell T, Cederquist GY, Schaefer PM et al (2018) The role of extent of resection in IDH1 wild-type or mutant low-grade gliomas. Neurosurgery 82(6):808–814
Pekmezci M, Rice T, Molinaro AM, Walsh KM, Decker PA, Hansen H, Sicotte H, Kollmeyer TM, McCoy LS, Sarkar G, Perry A, Giannini C, Tihan T, Berger MS, Wiemels JL, Bracci PM, Eckel-Passow JE, Lachance DH, Clarke J, Taylor JW, Luks T, Wiencke JK, Jenkins RB, Wrensch MR (2017) Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathol 133(6):1001–1016
Poulen G, Goze C, Rigau V, Duffau H (2018) Huge heterogeneity in survival in a subset of adult patients with resected, wild-type isocitrate dehydrogenase status, WHO grade II astrocytomas. J Neurosurg:1–10
Rezvan A, Christine D, Christian H, Olga Z, Lutz E, Marius H, Stephanie C, Christel HM, Rainer WC, Andreas U (2009) Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir 151(11):1359–1365. https://doi.org/10.1007/s00701-009-0435-x
Roelz R, Strohmaier D, Jabbarli R, Kraeutle R, Egger K, Coenen VA et al (2016) Residual tumor volume as best outcome predictor in low grade glioma—a nine-years near-randomized survey of surgery vs. biopsy. Sci Rep 6:32286
Rossi M, Ambrogi F, Gay L, Gallucci M, Conti Nibali M, Leonetti A, Puglisi G, Sciortino T, Howells H, Riva M, Pessina F, Navarria P, Franzese C, Simonelli M, Rudà R, Bello L (2019) Is supratotal resection achievable in low-grade gliomas? Feasibility, putative factors, safety, and functional outcome. J Neurosurg 17:1–14. https://doi.org/10.3171/2019.2.JNS183408
Sahm F, Schrimpf D, Jones DT, Meyer J, Kratz A, Reuss D et al (2016) Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol 131(6):903–910
Sarubbo S, De Benedictis A, Merler S, Mandonnet E, Balbi S, Granieri E et al (2015) Towards a functional atlas of human white matter. Hum Brain Mapp 36(8):3117–3136
Sarubbo S, Tate M, De Benedictis A et al (2020) Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: an original functional atlas of the human brain. Neuroimage 205:116237
Scherer M, Ahmeti H, Roder C, Gessler F, Jungk C, Pala A, Mayer B, Senft C, Tatagiba M, Synowitz M, Wirtz CR, Unterberg AW, Coburger J (2019) Surgery for Diffuse WHO Grade II Gliomas: Volumetric analysis of a multicenter retrospective cohort from the German study group for intraoperative magnetic resonance imaging. Neurosurgery 1:nyz397. https://doi.org/10.1093/neuros/nyz397
Schomas DA, Laack NN, Rao RD, Meyer FB, Shaw EG, O'Neill BP, Giannini C, Brown PD (2009) Intracranial low-grade gliomas in adults: 30-year experience with long-term follow-up at Mayo Clinic. Neuro-Oncology 11(4):437–445. https://doi.org/10.1215/15228517-2008-102
Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, VandenBerg S, McDermott MW, Berger MS (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26(8):1338–1345
Soffietti R, Baumert BG, Bello L, von Deimling A, Duffau H, Frenay M et al (2010) Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol 17(9):1124–1133
Still ME, Roux A, Huberfeld G, Bauchet L, Baron MH, Fontaine D, Blonski M, Mandonnet E, Guillevin R, Guyotat J, Taillandier L (2019) Extent of resection and residual tumor thresholds for postoperative total seizure freedom in epileptic adult patients harboring a supratentorial diffuse low-grade glioma. Neurosurgery 85(2):E332–E340
Theeler BJ, Yung WK, Fuller GN, De Groot JF (2012) Moving toward molecular classification of diffuse gliomas in adults. Neurology 79(18):1917–1926
van den Bent MJ, Smits M, Kros JM, Chang SM (2017) Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol 35(21):2394–2401
van den Bent MJ (2010) Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician's perspective. Acta Neuropathol 120(3):297–304
Wijnenga MMJ, French PJ, Dubbink HJ, Dinjens WNM, Atmodimedjo PN, Kros JM, Smits M, Gahrmann R, Rutten GJ, Verheul JB, Fleischeuer R, Dirven CMF, Vincent AJPE, van den Bent MJ (2018) The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro-oncology 20(1):103–112
Xia L, Fang C, Chen G, Sun C (2018) Relationship between the extent of resection and the survival of patients with low-grade gliomas: a systematic review and meta-analysis. BMC Cancer 18(1):48
Xu DS, Awad AW, Mehalechko C, Wilson JR, Ashby LS, Coons SW, Sanai N (2018) An extent of resection threshold for seizure freedom in patients with low-grade gliomas. J Neurosurg 128(4):1084–1090
Yang K, Nath S, Koziarz A, Badhiwala JH, Ghayur H, Sourour M, Catana D, Nassiri F, Alotaibi MB, Kameda-Smith M, Manoranjan B, Aref MH, Mansouri A, Singh S, Almenawer SA (2018) Biopsy versus subtotal versus gross total resection in patients with low-grade glioma: a systematic review and meta-analysis. World Neurosurgery 120:e762–ee75
Yeh SA, Ho JT, Lui CC, Huang YJ, Hsiung CY, Huang EY (2005) Treatment outcomes and prognostic factors in patients with supratentorial low-grade gliomas. Br J Radiol 78(927):230–235
Yordanova YN, Moritz-Gasser S, Duffau H (2011) Awake surgery for WHO Grade II gliomas within "noneloquent" areas in the left dominant hemisphere: toward a "supratotal" resection. Clinical article. J Neurosurg 115(2):232–239
Youland RS, Brown PD, Giannini C, Parney IF, Uhm JH, Laack NN (2013) Adult low-grade glioma: 19-year experience at a single institution. Am J Clin Oncol 36(6):612–619
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
LAFA: Conceptualization, methodology, investigation, resources, writing—original draft, writing—review and editing, supervision, project administration. JPA: conceptualization, investigation, resources, writing—original draft, writing—review and editing, supervision. LJMMF: methodology, resources, writing—original draft, writing—review and editing. AFJ: writing—original draft, writing—review and editing, supervision. HD: writing—original draft, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to report. This is an original unpublished article that does not transgress any copyright or intellectual property rights of other people and it is not being evaluated for publication in other journals.
Ethical approval
The research is a literature review. It did not involve human participants, for submission at the Ethical approval.
Informed consent
The research is a literature review. It did involve human participants directly, only from another published series.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
- When systematically measured, the importance of EOR for DLGG is evident in outcome.
- All subgroups of DLGG benefit from a higher EOR.
- Higher EOR may have a greater impact on DLGG with a more aggressive signature.
Rights and permissions
About this article
Cite this article
Albuquerque, L.A.F., Almeida, J.P., de Macêdo Filho, L.J.M. et al. Extent of resection in diffuse low-grade gliomas and the role of tumor molecular signature—a systematic review of the literature. Neurosurg Rev 44, 1371–1389 (2021). https://doi.org/10.1007/s10143-020-01362-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01362-8