Abstract
Multiple high-grade gliomas (M-HGGs) are well--separated tumors, differentiated as multifocal (MF) and multicentric (MC) by their MRI features. The authors performed a systematic review and meta-analysis of literature examining epidemiology, clinical and radiological characteristics, management, and the overall survival from M-HGGs. According to PRISMA guidelines, a comprehensive review of studies published between January 1990 and January 2017 was carried out. The authors identified studies that examined the prevalence rate, clinical and radiological characteristics, treatment, and overall survival from M-HGGs in patients with HGG. Data were analyzed using a random-effects meta-analysis model. Finally, we systematically reviewed demographic characteristics, lesion location, and surgical and adjuvant treatments. Twenty-three studies were included in this systematic review. The M-HGGs prevalence rate was 19% (95% CI 13–26%) and the hazard ratio of death from M-HGGs in the HGGs population was 1.71 (95% CI 1.49–1.95, p < 0.0001). The MC prevalence rate was 6% (CI 95% 4–10%), whereas MF prevalence rate was 11% (CI 95% 6–20%) (p < 0.0001). There were no statistically significant differences between MF and MC HGGs in gender, lesion location, histological type, and surgical treatment. Survival analysis of MC tumors showed that surgical resection (gross total resection or subtotal resection) is an independent predictor of improved outcome (HR 7.61 for biopsy subgroup, 95% CI 1.94–29.78, p = 0.004). The prevalence of M-HGGs is approximately 20% of HGGs. The clinical relevance of separating M-HGGs in MF and MC tumors remains questionable and its prognostic significance is unclear. When patient status and lesion characteristics make it safe and feasible, cytoreduction should be attempted in patients with M-HGGs because it improves overall survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple high-grade gliomas (M-HGGs) pose diagnostic and therapeutic challenges and their incidence at the time of diagnosis is reported to be between 0.5 and 35% [5, 9, 15, 42, 47, 51]. In 1963, Batzdorf and Malamud proposed pathological criteria to differentiate multifocal (MF) and multicentric (MC) tumors [5]. Expanding some pathological concepts highlighted by Russell and Rubistein [41], Batzdorf and colleagues proposed that MF gliomas are the result of tumor dissemination or growth by an established route, including white matter tracts (association, projection, and commissural fibers); cerebrospinal fluid channels; or local extension through satellite formation. In contrast, MC gliomas are widely separated lesions occurring in different lobes or hemispheres without a demonstrable diffusion along the pathways previously described. Gradually, the term MC glioma has broadened to include tumors not only separated by location but also by time. Accordingly, synchronous gliomas are already present as multiple lesions at the time of initial radiological investigation, whereas metachronous gliomas occur months after diagnosis. Although the association of the extent of resection and the overall survival in patients with glioblastoma has been clearly reported in literature [27], the role of surgical treatment in patients with multiple gliomatous foci remains controversial. Surgical management paradigms are changing, however, due to the mounting evidence of the role of cytoreduction on patients’ survival when associated with adjuvant treatment, in patients with both single and multiple lesions [42]. Radiation therapy is also coming under renewed scrutiny. While previous studies advised in favor of whole-brain radiation therapy in the management of MF and MC gliobastomas, limited-field irradiation is now increasingly recommended [37, 47]. The primary objectives of the study were (1) to determine the prevalence of M-HGGs, MC, and MF gliomas in HGGs population and (2) to determine the overall hazard ratio of death during follow-up for M-HGGs among HGGs population. Secondary objectives were: (1) to examine M-HGGs treatment modalities and complications and (2) to examine the impact of surgical resection on overall survival (OS) in M-HHGs.
Methods
Literature search
For this study, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [33]. A comprehensive search of three databases (PubMed, Scopus, Ovid EMBASE) was conducted by an experienced librarian. The search key words “glioblastoma,” “high-grade glioma,” “malignant glioma,” “anaplastic astrocytoma,” “multicentric,” “multifocal,” and “multiple cerebral lesions” were used in both “AND” and “OR” combinations. The search strategy is reported in Online Resource 1. The search was limited to articles in English published between January 1990 and January 2017. All studies found to be reporting series of M-HGGs were selected based on the following inclusion criteria: (1) series reporting prevalence of multiple lesions among HGGs population, (2) series reporting prevalence of MF and/or MC lesions among HGGs population, (3) series reporting characteristics and outcomes of patient with MF and/or MC HGGs. Exclusion criteria were the following: (1) studies with less than three patients, (2) case reports, (3) review articles, (4) studies published in languages other than English with no available English translations, and (5) studies with overlapping patient population. In cases of overlapping patient populations, only the series with the largest number of patients or most detailed data were included. Two reviewers with experience in neuro-oncological surgery independently selected the included studies. When discrepancies arose, papers were re-examined by the third author.
Quality scoring
The studies were dichotomized into two groups: case-control and case series studies. The Newcastle-Ottawa Scale [53] was used to assess the quality of the included studies (Table 1). This was done by assessing the patient selection criteria, comparability of the study groups, as well as the outcome assessment. The quality of the case-control group was assessed by answering the following questions: were the definition and the representativeness of M-HGGs, MF, and MC tumors adequate? Were the selection and the definition of HGGs adequate? Were outcomes well ascertained? Was the length of clinical follow-up adequate to assess the outcomes of interest? For cases series studies, we evaluated the quality of the study based on the following questions: were the definition of M-HGGs, MF, and MC tumors adequate? Was the number of treated patients sufficient to estimate outcomes? Was the treatment description reliable? Was the assessment of outcome accurate? “High-quality” studies were defined based on the following criteria: (1) presence of a predefined study protocol, (2) well-defined inclusion and exclusion criteria, (3) adequate distinction between MF and MC tumors, (4) adequate information about treatment and complications post-treatment, (5) adequate length of follow-up, and (6) enough information about overall survival. A star rating of 0 to 9 was allocated to each study based on these parameters. The quality assessment was performed by two authors independently. When discrepancies arose, papers were re-examined by the third author. Studies receiving six or more stars are considered as high-quality.
Data abstraction
From each article, the following baseline data was collected: study design, study time period, imaging modality used to screen for M-HGGs, radiological definition of MC and MF lesions, number of HGG patients, number of HGG patients with multiple cerebral lesions, and distinction between MF and MC tumors, sex, mean age, age range, lesions location, number of lesions per patient, number of synchronous or metachronous lesions, histological type of HGG, mean clinical follow-up, pre-operative clinical features, treatment characteristics, post-operative complications, and overall survival. Multicentricity was defined as multiple gadolinium-enhancing lesions within the brain without a connecting signal alteration in T2-weighted and/or FLAIR sequences (“T2 /FLAIR connection” criterion) [9, 10, 15, 16, 29, 37, 42], or widely separated lesions in one or both cerebral hemispheres (“foci location” criterion) [32, 47]. Pre-operative clinical symptoms were summarized as follows: (1) headache, (2) focal deficit, (3) intracranial hypertension signs, (4) altered mental status, (5) seizure, and (6) other. Pre-operative clinical status was classified using the Eastern Cooperative Oncology Group (ECOG) scale [34], and when reported using the Karnofsky Performance Status (KPS) [19] scale, we duly transformed it in ECOG scale [52]. Surgical treatments were categorized as follows: gross total resection (GTR), subtotal resection (STR), and biopsy. Subsequently, data obtained from abstraction was sub-grouped based on GTR definitions as follows: (1) resection of all contrast-enhanced areas at post-operative MRI [54] and (2) other definitions. Furthermore, surgical resection was defined as “multiple lesions resection” if more than one lesion was surgically removed; otherwise, surgery was classified as “single lesion resection.” GTRs were considered as multiple lesions resection. Adjuvant treatments were categorized as follows: concurrent radio-chemotherapy (RT + CH), radiotherapy or chemotherapy alone, and no adjuvant treatment.
Outcomes
For M-HGGs prevalence meta-analysis, only studies with ascertained prevalence of multiple cerebral lesions were included. When there was no available information about MF or MC population, the study was not included in the meta-analysis. For M-HGGs survival meta-analysis, only studies reporting hazard ratio of death for M-HGGs among HGGs population, were included. Post-operative complications were divided into surgical and medical complications. Surgical complications included post-operative hemorrhage, neurological deficits, seizure, and wound infections. Medical complications included complications not directly related to surgery.
Statistical analysis
For the purposes of statistical analysis, we calculated the overall frequency (percentage) and 95% confidence interval (CI) for all results. The Wald method was used to calculate confidence intervals for event rates. A two-tailed t test was used for continuous data and the chi-square test for categorical variables.
Survival analysis
Survival curves were calculated using the Kaplan-Meier method and the log-rank test was used to evaluate the difference between curves. The OS was defined as the time from first surgery to the final follow-up. OS univariate analysis was carried out including each risk factor (surgery or biopsy) in a Cox regression model. Furthermore, the proportional hazard was always verified by the use of log(−log) curves. The results of the Cox regression were expressed by hazard ratios with its related confidence interval and related p value calculated by the Wald test.
Meta-analysis
In order to assess the heterogeneity of the data, the Higgins index (I2) was used and subsequently, DerSimonian and Laird random-effects model was applied. The graphical representation of the meta-analysis was performed by forest plot. To evaluate the heterogeneity and bias, the meta-regression and funnel plot followed by Egger’s linear regression test were analyzed, respectively.
Differences were considered significant at p < 0.05. Statistical analysis were performed with SPSS version 23 (SPSS Inc. SPSS® Chicago, IL, USA), with ProMeta version 2 (Internovi, Cesena, Italy) and GraphPadQuickCalcs software.
Results
Literature review
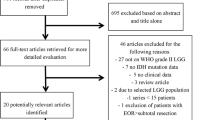
The search strategy is summarized in Online Resource 1. Discrepancies between first and second authors arose in 3% of cases during the study selection process, and in one case during the meta-analysis inclusion. Detailed information is provided in Online Resource 2. Studies included in our systematic review are summarized in Table 1. The search flow diagram is shown in Fig. 1. A total of 23 studies and 785 patients with M-HGGs were included in this study, 564 patients were reported to be MC or MF (270 and 294, respectively).
Quality score
In the “case-control” group, eight studies were graded as “high quality” and nine as “low quality.” For the “case series” group only two of six articles were scored as high quality. There was complete agreement between the two reviewers for 19 articles (10 rated as high quality). Discrepancies arose for four articles (all low quality), with the disagreement due to the following: (1) poorly defined patients inclusion criteria, (2) lack of demographic and clinical data, (3) small population included, and (4) no survival analysis. The final examination was performed by the third author who decided to rate all four articles as low quality. Detailed information in Online Resource 3.
M-HGGs prevalence rate, demographic data, and lesion characteristics
A summary of M-HGGs prevalence rate, demographic data, and lesion characteristics are provided in Table 2. In assessing M-HGGs prevalence rate, a total of 4764 patients with HGG were included. The overall M-HGGs prevalence rate was 19% (95% CI 13–26%). Meta-regression showed a significant increase of the effect size during the analyzed period (p = 0.003). Furthermore, the funnel plot followed by Egger’s linear regression test excludes publication bias (p = 0.413). The forest plot, the meta-regression, and the funnel plot are provided in Figs. 2, 3, and 4, respectively.
The mean age was 58.12 years (range 28–86), while the male/female ratio was 1.29. Focal deficits were the most frequent clinical presentation, reported in 44.10% of patients and ECOG 1–2 was the most frequent pre-operative clinical status, reported in 68.83% of patients. Overall, 83.61% of patients had at least one lesion in the frontal lobes. Basal ganglia, brainstem, corpus callosum, and the cerebellum were involved in less than 30% of patients. The mean number of lesions per patient was 2.67. At presentation, most patients had two lesions (75.76%), while the rest had three or more lesions. Tumors were unilateral in 67.84% of patients and synchronous in 86.45% of patients.
Histological examination was available for 445 patients and demonstrated glioblastoma (GBM) and anaplastic astrocytoma in 90.60 and 9.40% of cases, respectively.
When considering the prevalence rate of MF and MC tumors, 3363 and 4000 patients with HGG were, respectively, included. The MC prevalence rate was 6% (CI 95% 4–10%), whereas the MF prevalence rate was 11% (CI 95% 6–20%) (p < 0.0001). Meta-regression showed a non-significant increase of the effect size during the analyzed period for MF and MC tumors prevalence meta-analysis (0.802 and 0.318, respectively). Furthermore, the funnel plot followed by Egger’s linear regression test excludes publication bias for both analysis (0.425, 0.087).Seven studies provided radiological distinction of MC and MF gliomas based on MRI T2-weighted or FLAIR characteristics and two studies distinguished MC and MF tumors based on foci location. There were no statistically significant differences between MF and MC tumors in gender, lesion location, and histological type. Forest plots for MF and MC prevalence are provided in Online Resource 4 (Supplementary Figs. 1 and 2, respectively) and information regarding demographic data and lesion location is summarized in Online Resource 5.
Treatment of M-HGGs and post-operative complications
Data regarding M-HGGs treatment is summarized in Table 3. Biopsy was performed in 36.12% of patients, whereas STR and GTR were obtained in 41.33 and 22.54% of patients, respectively. Differences in surgical treatments rates were not statistically significant among sub-groups of GTR (detailed data in Online Resource 6). Multiple lesions resection was performed in 29.66% of cases. Overall, 58.82% of patients underwent concurrent radio-chemotherapy after surgical treatment. The most frequent chemotherapy drug was temozolomide (67.14%), whereas local radiotherapy (LRT) was the most common type of radiotherapy (28.63%). Post-operative complications were reported in 19.44% of patients.
For both MF and MC tumors, surgical resection was accomplished in almost 65% of patients (equally divided into GTR and STR), while the remaining patients underwent biopsy. Multiple resections were performed in 22.22% of patients with MF tumors, and in 35.94% of patients with MC lesions (p = 0.1117). Concurrent radio-chemotherapy was the prevalent adjuvant treatment associated with MF tumors (81.48% of patients), whereas no adjuvant therapy or chemotherapy alone were more common in MC tumors (50 and 16.23% of patients, respectively). Data regarding surgical and adjuvant treatments of MF and MC lesions is summarized in Online Resource 7.
M-HGGs survival analysis
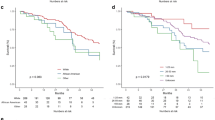
Data regarding overall survival was available in five articles, of which three scored as high-quality studies. The HR was calculated as the ratio of death probabilities per unit time having multiple cerebral lesions in an HGGs population. Results of this analysis showed an overall HR of 1.71 (95% CI 1.49–1.95, p < 0.0001). Forest plot is provided in Fig. 5. Meta-regression showed a non-significant decrease of the effect size during the analyzed period (p = 0.247). Furthermore, the funnel plot followed by Egger’s linear regression test excludes publication bias (p = 0.672). Meta-regression and funnel plot are provided in Online Resource 4 (Supplementary Figs. 3 and 4, respectively). Data regarding the impact of surgical resection on the OS of patients with MC lesions was available in two articles, both of which were rated as high-quality studies. Patients receiving biopsy presented a disadvantage on OS (HR 7.61, 95% CI 1.94–29.78, p = 0.004), compared with patients treated with GTR or STR. Our systematic review was not able to collect comparable series to assess the impact of surgical resection on MF population.
Forest plot of the HR of death for M-HGGS in HGGs population. HR, hazard ratio; W, weight; Sig., statistical significance (p < 0.05). The I2 = 0 indicate the lack of heterogeneity in the studies included in the meta-analysis. The publication bias was tested and excluded, as demonstrated by Egger’s linear regression test (p = 0,672)
Study heterogeneity
Heterogeneity of treatment effects across studies was evaluated using the I2 statistic, in which I2 > 50% suggests substantial heterogeneity [17]. For the prevalence studies, I2 values were above 50% for the overall M-HGGs, MF, and MC prevalence estimates. I2 values was less than 50% indicating lack of substantial heterogeneity for the M-HGGs survival analysis.
Discussion
Our systematic review and meta-analysis of the literature on the prevalence, treatment, and outcome of M-HGGs demonstrated a number of interesting findings. Overall, M-HGGs represented roughly 20% of HGGs population. MF and MC lesions represented 11 and 6% of HGGs population, respectively, with no difference in gender, histological type, and tumor location. Biopsy and subtotal resection were the most common surgical treatments for M-HGGs, with no differences between MF and MC gliomas. Multiple resections were uncommon and approximately 70% of patients underwent resection of a single lesion. Our study found that among patients with HGGs, the hazard ratio of death was higher in the subgroup of M-HGGs (HR of 1.71). In addition, the analysis of the two largest series of MC gliomas demonstrated a higher hazard ratio among patients receiving biopsy than among patients treated with surgical resection (SR or GTR) (overall HR 7.6).
Multiple high-grade gliomas, epidemiology, and radiological diagnosis
Multiple cerebral gliomatous foci were initially recognized in 1880 by Bradley [7]. In 1963, Batzdorf and Malamud provided radiological and pathological criteria to differentiate MF and MC lesions [5]. According to them, MF tumors are the result of tumor dissemination or growth via an established route, such as white matter tracts, CSF or local extension by satellite formation, and MC tumors are widely separated lesions with no connection between foci. Nowadays, a growing body of evidence corroborates the idea that HGGs multiplicity follows the trend of glioma cells to invade both adjacent and distant normal brain [4, 8]. This occurrence can be detected by changes in T2-weighted and FLAIR sequences outside the contrast-enhanced area, reflecting modifications in normal extracellular matrix due to invasion by glioma cells [16]. Hence, in current literature, the presence of connecting signal alteration in T2-weighted or FLAIR images is generally adopted to define MF lesions. Patil and Showalter [38, 47] defined multifocality as the presence of two distinct foci of the enhancing tumor, separated by a discrete distance (1 and 2 cm, respectively), in which a microscopic connection is presumed. Moreover, both authors did not provide a clear radiological characterization of MC lesions, defining them as “widely separated lesions” or lesions involving different hemispheres or lobes.
Our systematic review showed that only 7/23 studies had comparable MRI distinction between MF and MC tumors, based on T2-weighted and/or FLAIR characteristics. Furthermore, as underlined by Lasocki et al. [29], older studies included patients who were only investigated with CT scan, leading to an overlap between MF and MC populations due to neuroimaging inaccuracies. The aforementioned terminological discrepancies together with differences in neuroimaging technology explain the heterogeneous data available in current literature concerning the prevalence of multiple HGGs, which ranges from 0.5 to 35% [9, 15, 29, 38, 47, 51].
Our study reported an overall prevalence of 6 and 11% for MC and MF tumors, respectively, without statistically significant differences in gender, lesion location, and histological type. In parallel with radiological differences described previously, recent studies have underlined that MF and MC lesions had a common origin and peculiar genetic patterns [24] characterized by identical molecular profiles [1, 18]. Interestingly, for HGGs, cells were observed to move in isolation along white matter tracts and any changes to the adjacent environment might be very subtle during this migration [11]. Accordingly, no extensive extracellular matrix degradation is needed during this movement and it is plausible that MRI could not reliably detect isolated cells outside contrast-enhanced areas of the brain [12, 16]. Based on biological data previously exposed, it is conceivable that MF and MC tumors represent different features of the HGGs tendency to disseminate in surrounding and distant brain [4, 11, 16]. In our study, we found that multiple lesions occurred in 19% of patients with HGGs providing a comprehensive representation of the prevalence of M-HGGs in HGGs population. Additionally, our meta-regression showed a significant increase of effect size during the analyzed period (p < 0.0001). This result can be explained by the widespread diffusion of MRI with the resultant increase in recognition of multiple lesions. Furthermore, a growing awareness of M-HGGs, resulted in a greater research of histological confirmation in patients that would previously have been considered to have metastatic disease [29, 42, 51].
Surgical treatment
Although the role of surgery for HGGs has been clarified in the current literature, it remains controversial in patients with multiple gliomatous foci. Lacroix et al. [27] first demonstrated the relationship between extent of resection and OS. Subsequently, an expanding body of evidence confirmed that the extent of resection affects overall survival in patients with GBM [6, 25, 30, 44]. Nonetheless, class I evidence is scarce, due to both the logistical and ethical difficulty of organizing a randomized STR versus GTR trial. Furthermore, several authors underlined that the extent of surgical resection should be carefully balanced with pre- and post-operative neurological status, and that GTR only, and not STR, significantly improves OS [21, 23]. Much controversy exists in current literature over the optimal treatment of M-HGGs. In fact, some authors proposed that stereotactic biopsy should be the first approach for suspected M-HGGs, reporting that aggressive surgical resection does not improve overall survival and is associated with significant post-operative sequelae [13, 35]. Our systematic review showed that, in current literature, data concerning surgical management of M-HHGs is sparse and heterogeneous. When considering GTR as the resection of all contrast-enhanced area in post-operative MRI [54], only six studies provided adequate data. Nonetheless, no statistically significant differences arose between populations grouped by the different definition of GTR. Accordingly, STR was the most common surgical treatment among the M-HGGs population and GTR was achieved in roughly 20% of patients. Furthermore, biopsy was performed in more than one third of patients. In current literature, few series quantified the impact of the extent of resection in M-HGGs. Showalter et al. [47] reported that GTR strongly improved OS compared to STR and biopsy (HR 1.73 and 4.06, respectively). Similarly, Patil et al. [38] reported that patients who received GTR had a survival advantage compared to the others (median OS in months: 14 vs. 5, p = 0,004). On the other hand, Paulsson et al. [39] found no significant differences in OS related to the extent of resection in their series. It is important to underline that multivariate analysis was not performed in the included studies. Accordingly, it was not possible to analyze the impact of surgical resection on OS accounted to pre-operative performance status and lesions characteristics. In order to examine the role of surgical resection in patients with M-HGGs, we analyzed the two largest series of MC HGGs reported in the literature. Accordingly, both in the series of Salvati and di Russo [42, 43], cytoreductive surgery strongly affected OS revealing an almost eightfold statistically significant increase in mortality for patients receiving biopsy alone (overall HR 7.61, p = 0.004). Interestingly, Hassaneen et al. [15] presented 20 patients with M-HGGs treated by multiple craniotomies matched in a 1:1 ratio with patients with solitary glioblastoma. Their survival analysis demonstrated that GTR led to similar OS in patients with M-HGGs and in those with solitary GBMs. In addition, surgical resection via multiple craniotomies presented the same risk of morbidity than single craniotomy. Our findings showed a post-operative complication rate of roughly 20%, that is in agreement with previous surgical series dealing with solitary glioblastomas [13, 46, 50]. The choice of treatment is obviously related to clinical and radiological characteristics that our systematic review was not able to integrate in the analysis. Accordingly, the risk of bias is not negligible. In the current literature, there is no general consensus regarding the association between lesion multiplicity and a worse performance status [32, 39, 51]. Moreover, our work showed that in the M-HGGs population, ECOG ≤ 2 was the most common pre-operative clinical status. Accordingly, when patient status and lesion characteristics make it safe and feasible, cytoreduction should be considered because it improves overall survival. Further studies are required to determine the impact of surgical resection on OS when patient-related radiological and clinical characteristics are accounted for.
Adjuvant treatment
Standard post-operative treatment for HGGs includes conformal radiotherapy and concurrent and adjuvant chemotherapy with temozolomide [40]. Contrariwise, post-operative management of M-HGGs is still debated in the current literature and there is no consensus in chemo- and radiotherapy protocols. Our results showed that concurrent LRT plus temozolomide was the most common adjuvant treatment for patients with M-HGGs. When considering MF and MC tumors separately, significant differences arose between MF and MC tumors showing that, similarly to single HGGs, concurrent radio-chemotherapy was given in roughly 80% of patients with MF tumors. Otherwise, a consistent percentage of MC lesions (roughly 30%) did not undergo chemo- and radiotherapy. It should be emphasized that the aforementioned differences in adjuvant treatments were related to patient’s performance status, comorbidity, and lesion location [15, 38, 39]. Previously, radiation treatment for M-HGGs consisted of whole-brain therapy (WBRT) to control both local recurrence and distal spread, but this approach was challenged by the consistent pattern of failure in both purposes [47]. Our systematic review reported that less than 1% of patients with M-HGGs underwent WBRT, and this percentage referred to studies that changed their protocol during patient recruitment, or series collected when WBRT for HGGs was routinely administered [2, 20, 47]. Paulsson et al. [39] showed that, as in single HGGs, failure after treatment occurred locally in roughly 80% of cases. Furthermore, no significant differences in OS arose between patients with M-HGGs receiving WBRT or LRT post-operatively [47]. Accordingly, post-operative LRT and concomitant and adjuvant temozolomide should be recommended in the post-operative management of M-HGGs.
Relationship between multiple high-grade gliomas and overall survival
Glioblastoma is the most common primary brain tumor and overall survival after treatment remains unfavorable [14]. The current state of knowledge does not clarify whether lesion multiplicity at presentation is an independent predictor of a worse outcome. Studies investigating the outcome of patients with M-HGGs are sparse and often lead to contradictory conclusions on the effect of multiplicity on overall survival, especially when survival differences accounted for performance status, extent of resection, and MGMT status [15, 38, 51]. Only a few studies reported detailed data and hazard ratios for survival analysis. In our review, we estimated that the hazard ratio of death in patients with M-HGGs is roughly double compared with that of patients presenting a solitary glioblastoma (p < 0.0001). Nonetheless, our systematic review was not able to retrieve sufficient data to analyze the OS in both MF and MC tumors. In fact, discordant results are reported in the literature when the OS is analyzed separately for MF and MC tumors. In the surgical series of Hasseneen et al. [15], patients with MC tumors presented a median survival significantly longer than patients with MF HGGs. On the other hand, several authors [26, 29, 36, 47, 51] reported no significant difference in post-operative survival between patients with MC and MF glioblastomas. In addition, in some surgical series, MF and MC glioblastomas are analyzed together limiting the information available for a survival analysis. Even with these limitations, our study showed no significant differences between patients with MF and those with MC glioblastomas after surgical treatment. Accordingly, it seems that multiplicity similarly affects survival both in MF and MC glioblastomas, questioning the prognostic significance of this distinction.
Limitations of the study
Our study has several limitations. In order to report a more comprehensive representation of patients clinical status, we performed a conversion of KPS to ECOG. This might be an oversimplification because the comparison is more effective for good performance status, and gradually becomes less accurate with the decrease in grade [52]. Similarly, only 6/13 studies adopted the current definition of GTR (resection of all contrast-enhanced areas at post-operative MRI) [54]. Accordingly, even if no significant difference arose between sub-groups (Online Resource 6), the rate of GTR among M-HGGs could be over-estimated. The prevalence of M-HGGs could be underestimated because of the significant increase of effect size during the years of publication. Moreover, series included in our analysis are often small, retrospective, and involving heterogeneous populations. In addition, when considering MF and MC populations, only a few articles reported accurate data. With the aim to select the more representative sample avoiding selection bias, data was weighted on restricted, yet comparable populations. Nonetheless, the risk of exclusion bias is high and the reader should carefully weighed data with samples (Tables 2, 3 and Online Resources 5,7). Our analysis of hazard ratio of death for M-HGGs in HGGs population could be influenced by our inclusion criteria that excluded all series in which the mortality rate was not expressed with hazard ratios. Moreover, some of the studies excluded in survival analysis reported data only for patients with MF or MC tumors. Accordingly, even if this distinction is questionable, their samples should not be collected because they are not representative. Furthermore, our study was unable to comment on the influence of other predictive factors of worse outcome in M-HGGs population and the relationship with the extent of resection. It is important to point out that it was impossible to evaluate the effect of pre- and post-operative performance status, MGMT status, and IDH mutations on OS.
Conclusions
Our study demonstrated that M-HGGs are found in approximately 20% of patients with HGGs. Patients with multiple lesions are significantly more likely to have worse outcomes than those with a single lesion. No statistically significant differences were found in age, lesion location, histological type, and surgical management between patients with MF and MC HHGs, bringing into question the clinical utility of this distinction. Cytoreductive surgery improves the overall survival of patients with M-HGGs and consequently should be proposed when patient status and lesion characteristics make it safe and feasible. Further studies are required to determine patient-related prognostic factors and more effective treatment protocols for M-HGGs.
References
Akimoto J, Sasaki H, Haraoka R, Nakajima N, Fukami S, Kohno M (2014) A case of radiologically multicentric but genetically identical multiple glioblastomas. Brain Tumor Pathol 31:113–117. https://doi.org/10.1007/s10014-013-0157-x
Arcos A, Romero L, Serramito R, Santín JM, Prieto A, Gelabert M, Arráez MÁ, Ángel M (2012) Multicentric glioblastoma multiforme. Report of 3 cases, clinical and pathological study and literature review. Neurocirugia (Astur) 23:211–215. https://doi.org/10.1016/j.neucir.2012.06.004
Arvold ND, Tanguturi SK, Aizer AA, Wen PY, Reardon DA, Lee EQ, Nayak L, Christianson LW, Horvath MC, Dunn IF, Golby AJ, Johnson MD, Claus EB, Chiocca EA, Ligon KL, Alexander BM (2015) Hypofractionated versus standard radiation therapy with or without temozolomide for older glioblastoma patients. Int J Radiat Oncol Biol Phys 92:384–389. https://doi.org/10.1016/j.ijrobp.2015.01.017
Banan R, Hartmann C (2017) The new WHO 2016 classification of brain tumors—what neurosurgeons need to know. Acta Neurochir (Wien) 1–16. doi: https://doi.org/10.1007/s00701-016-3062-3
Batzdorf U, Malamud N (1963) The problem of multicentric gliomas. J Neurosurg 20:122–136. https://doi.org/10.3171/jns.1963.20.2.0122
Bloch O, Han SJ, Cha S, Sun MZ, Aghi MK, McDermott MW, Berger MS, Parsa AT (2012) Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg 117:1032–1038. https://doi.org/10.3171/2012.9.JNS12504
Bradley W (1880) Case of gliosarcomatous tumors of the brain. Proc Conn Med Soc
Cuddapah VA, Robel S, Watkins S, Sontheimer H (2014) A neurocentric perspective on glioma invasion. Nat Publ Gr 15:455–465. https://doi.org/10.1038/nrn3765
Djalilian HR, Shah MV, Hall WA (1999) Radiographic incidence of multicentric malignant gliomas. Surg Neurol 51:554–558. https://doi.org/10.1016/S0090-3019(98)00054-8
Fares Y, Younes M, Kanj A, Ruiz Barnes P, Muñiz J (2009) Multicentric glioma. P R Health Sci J 28:75–79
Giese A, Bjerkvig R, Berens ME, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21:1624–1636. https://doi.org/10.1200/JCO.2003.05.063
Globus J, Kuhlenbeck H (1944) The subependymal cell plate (matrix) and its relationship to brain tumors of the ependymal type. J. Neuropathol
Gulati S, Jakola AS, Nerland US, Weber C, Solheim O (2011) The risk of getting worse: surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg 76:572–579. https://doi.org/10.1016/j.wneu.2011.06.014
Hartmann C, Hentschel B, Simon M, Westphal M, Schackert G, Tonn JC, Loeffler M, Reifenberger G, Pietsch T, Von Deimling A, Weller M (2013) Long-term survival in primary glioblastoma with versus without isocitrate dehydrogenase mutations. Clin Cancer Res 19:5146–5157. https://doi.org/10.1158/1078-0432.CCR-13-0017
Hassaneen W, Levine NB, Suki D, Salaskar AL, de Moura LA, McCutcheon IE, Prabhu SS, Lang FF, DeMonte F, Rao G, Weinberg JS, Wildrick DM, Aldape KD, Sawaya R (2011) Multiple craniotomies in the management of multifocal and multicentric glioblastoma. Clinical article. J Neurosurg 114:576–584. https://doi.org/10.3171/2010.6.JNS091326
Hefti M, Von Campe G, Schneider C, Roelcke U, Landolt H, Article O, Hefti M, Von Campe G, Schneider C, Roelcke U, Landolt H (2010) Multicentric tumor manifestations of high grade gliomas: Independent proliferation or hallmark of extensive disease? Zentralbl Neurochir 71:103. https://doi.org/10.1055/s-0029-1241190
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ Br Med J 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Karlowee V, Amatya VJ, Hirano H, Takayasu T, Nosaka R, Kolakshyapati M, Yoshihiro M, Takeshima Y, Sugiyama K, Arita K, Kurisu K, Yamasaki F (2016) Multicentric glioma develops via a mutant IDH1-independent pathway: immunohistochemical study of multicentric glioma. Pathobiology:4–8. https://doi.org/10.1159/000447951
Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH (1948) The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer 1:634–656. https://doi.org/10.1002/1097-0142(194811)1:4<634::AID-CNCR2820010410>3.0.CO;2-L
Kato T, Aida T, Abe H, Ogata A, Nakamura N, Nagashima K, Kitaoka K (1990) Clinicopathological study of multiple gliomas—report of three cases. Neurol Med Chir (Tokyo) 30:604–609
Khan MB, Chakraborty S, Boockvar JA (2016) Gross total resection of glioblastoma improves overall survival and progression-free survival compared to subtotal resection or biopsy alone. Neurosurgery 79:N12–N13. https://doi.org/10.1227/01.neu.0000508600.08200.c1
Kong D-S, Kim J, Lee I-H, Kim ST, Seol HJ, Lee J-I, Park W-Y, Ryu G, Wang Z, Ma’ayan A, Nam D-H (2016) Integrative radiogenomic analysis for multicentric radiophenotype in glioblastoma. Oncotarget 7:11526–11538. 10.18632/oncotarget.7115
Kreth FW, Thon N, Simon M, Westphal M, Schackert G, Nikkhah G, Hentschel B, Reifenberger G, Pietsch T, Weller M, Tonn JC (2013) Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol 24:3117–3123. https://doi.org/10.1093/annonc/mdt388
Krex D, Mohr B, Appelt H, Schackert HK, Schackert G, Waziri AE, Bruce JN, Ph D, Schackert G, Al ET, Appelt H, Schackert HK, Schackert G, Waziri AE, Bruce JN (2003) Genetic analysis of a multifocal glioblastoma multiforme: a suitable tool to gain new aspects in glioma development. Neurosurgery 53:1377–1384. https://doi.org/10.1227/01.NEU.0000093426.29236.86
Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C (2011) Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro-Oncology 13:1339–1348. https://doi.org/10.1093/neuonc/nor133
Kyritsis AP, Levin VA, Alfred Yung WK, Leeds NE (1993) Imaging patterns of multifocal gliomas. Eur J Radiol 16:163–170. https://doi.org/10.1016/0720-048X(93)90063-S
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198. https://doi.org/10.3171/jns.2001.95.2.0190
Lasocki A, Gaillard F, Tacey MA, Drummond KJ, Stuckey SL (2016) The incidence and significance of multicentric noncontrast-enhancing lesions distant from a histologically-proven glioblastoma. J Neuro-Oncol 129:471–478. https://doi.org/10.1007/s11060-016-2193-y
Lasocki A, Gaillard F, Tacey M, Drummond K, Stuckey S (2016) Multifocal and multicentric glioblastoma: improved characterisation with FLAIR imaging and prognostic implications. J Clin Neurosci 31:92–98. https://doi.org/10.1016/j.jocn.2016.02.022
Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, Lillehei KO, Bernstein M, Brem H, Sloan A, Berger MS, Chang S, Tumor B (2003) Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the glioma outcomes project. J Neurosurg 99:467–473. https://doi.org/10.3171/jns.2003.99.3.0467
Lim DA, Cha S, Mayo MC, Chen M-HH, Keles E, Vanden Berg S, Berger MS (2007) Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-Oncology 9:424–429. https://doi.org/10.1215/15228517-2007-023
Liu Q, Liu Y, Li W, Wang X, Sawaya R, Lang FF, Yung WKA, Chen K, Fuller GN, Zhang W (2015) Genetic, epigenetic, and molecular landscapes of multifocal and multicentric glioblastoma. Acta Neuropathol 130:587–597. https://doi.org/10.1007/s00401-015-1470-8
Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (reprinted from annals of internal medicine). Phys Ther 89:873–880. https://doi.org/10.1371/journal.pmed.1000097
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C, Chaudhary N, Sagher O (2012) Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg 117:851–859. https://doi.org/10.3171/2012.8.JNS12234
Parsa AT, Wachhorst S, Lamborn KR, Prados MD, McDermott MW, Berger MS, Chang SM, Francisco S (2005) Prognostic significance of intracranial dissemination of glioblastoma multiforme in adults. J Neurosurg 102:622–628. https://doi.org/10.3171/jns.2005.102.4.0622
Patil CG, Eboli P, Hu J (2012) Management of multifocal and multicentric gliomas. Neurosurg Clin N Am 23:343–350. https://doi.org/10.1016/j.nec.2012.01.012
Patil CG, Yi A, Elramsisy A, Hu J, Mukherjee D, Irvin DK, Yu JS, Bannykh SI, Black KL, Nuño M, Angeles L (2012) Prognosis of patients with multifocal glioblastoma: a case-control study. J Neurosurg 117:705–711. https://doi.org/10.3171/2012.7.JNS12147
Paulsson AK, Holmes JA, Peiffer AM, Miller LD, Liu W, Xu J, Hinson WH, Lesser GJ, Laxton AW, Tatter SB, Debinski W, Chan MD (2014) Comparison of clinical outcomes and genomic characteristics of single focus and multifocal glioblastoma. J Neuro-Oncol 119:429–435. https://doi.org/10.1007/s11060-014-1515-1
R. Stupp, W. P. Mason MJ van den B et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma, N Engl J Med vol 352, no 10 987–996.
Russel D, Rubinstein S (1989) Pathol Tumours Nerv Syst 702:145–147. https://doi.org/10.1136/pgmj.35.410.702
di Russo P, Perrini P, Pasqualetti F, Meola A, Vannozzi R (2013) Management and outcome of high-grade multicentric gliomas: a contemporary single-institution series and review of the literature. Acta Neurochir 155:2245–2251. https://doi.org/10.1007/s00701-013-1892-9
Salvati M, Caroli E, Orlando ER, Frati A, Artizzu S, Ferrante L (2003) Multicentric glioma: our experience in 25 patients and critical review of the literature. Neurosurg Rev 26:275–279. https://doi.org/10.1007/s10143-003-0276-7
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62:753–764. https://doi.org/10.1227/01.neu.0000318159.21731.cf
Scott JG, Suh JH, Elson P, Barnett GH, Vogelbaum MA, Peereboom DM, Stevens GHJ, Elinzano H, Chao ST (2011) Aggressive treatment is appropriate for glioblastoma multiforme patients 70 years old or older: a retrospective review of 206 cases. Neuro-Oncology 13:428–436. https://doi.org/10.1093/neuonc/nor005
Selbekk T, Jakola AS, Solheim O, Johansen TF, Lindseth F, Reinertsen I, Unsgård G (2013) Ultrasound imaging in neurosurgery: approaches to minimize surgically induced image artefacts for improved resection control. Acta Neurochir 155:973–980. https://doi.org/10.1007/s00701-013-1647-7
Showalter TN, Andrel J, Andrews DW, Curran WJ, Daskalakis C, Werner-Wasik M (2007) Multifocal glioblastoma multiforme: prognostic factors and patterns of progression. Int J Radiat Oncol Biol Phys 69:820–824. https://doi.org/10.1016/j.ijrobp.2007.03.045
Stark AM, Van De Bergh J, Hedderich J, Mehdorn HM, Nabavi A (2012) Glioblastoma: clinical characteristics, prognostic factors and survival in 492 patients. Clin Neurol Neurosurg 114:840–845. https://doi.org/10.1016/j.clineuro.2012.01.026
Tanaka S, Meyer FB, Buckner JC, Uhm JH, Yan ES, Parney IF, Clinic M (2013) Presentation, management, and outcome of newly diagnosed glioblastoma in elderly patients. J Neurosurg 118:786–798. https://doi.org/10.3171/2012.10.JNS112268
Taylor MD, Bernstein M (1999) Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: a prospective trial of 200 cases. J Neurosurg 90:35–41. https://doi.org/10.3171/jns.1999.90.1.0035
Thomas RP, Xu LW, Lober RM, Li G, Nagpal S (2013) The incidence and significance of multiple lesions in glioblastoma. J Neuro-Oncol 112:91–97. https://doi.org/10.1007/s11060-012-1030-1
Verger E, Salamero M, Conill C (1992) Can Karnofsky performance status be transformed to the eastern cooperative oncology group scoring scale and vice versa? Eur J Cancer 28:1328–1330. https://doi.org/10.1016/0959-8049(92)90510-9
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2013) The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Hosp Res Inst 1–4. doi: https://doi.org/10.2307/632432
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, DeGroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, Van Den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. https://doi.org/10.1200/JCO.2009.26.3541
Acknowledgements
We thank Ms. Juliet Strachan and Ms. Anne-Marie Peduto for English revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Ethical approval
For this type of study, formal consent is not required.
Informed consent
The nature of this article did not require informed consent.
Electronic supplementary material
Online Resource 1
Literature search syntax (PDF 100 kb)
Online Resource 2
Discrepancies between authors in systematic review process (PDF 342 kb)
Online Resource 3
Discrepancies between authors in quality score assignment (PDF 328 kb)
Online Resource 5
Multifocal versus multicentric tumors: demographics data and lesion location (PDF 114 kb)
Online Resource 6
Comparison of surgical treatment rates in M-HGGs population (PDF 331 kb)
Online Resource 7
Surgical and adjuvant treatments of multifocal and multicentric HGGs (PDF 109 kb)
Rights and permissions
About this article
Cite this article
Di Carlo, D.T., Cagnazzo, F., Benedetto, N. et al. Multiple high-grade gliomas: epidemiology, management, and outcome. A systematic review and meta-analysis. Neurosurg Rev 42, 263–275 (2019). https://doi.org/10.1007/s10143-017-0928-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-017-0928-7