Abstract
Fargesia, a temperate woody bamboo genus, is one of the largest genera and constitutes a taxonomically problematic group due to unusual life cycles and the rarity of flowering. We explored phylogenetic relationships within Fargesia and its allies based on sequence data from three cpDNA regions (matK, psbA-trnH and trnL-trnF) and one nuclear region (nrITS). A representative sample of 49 species, including 36 Fargesia and nine Yushania, were sampled, and maximum parsimony, maximum likelihood and Bayesian inference were used to reconstruct the phylogeny of Fargesia. The results suggest that Fargesia is polyphyletic, with F. crassinoda and F. damuniu in the Thamnocalamus clade, F. ampullaris, F. semiorbiculata, F. gyirongensis and F. collaris in the Drepanostachyum + Himalayacalamus clade, and the rest of species of Fargesia and all sampled species of Yushania in a Fargesia + Yushania clade, which was further divided into weakly supported Fargesia spathe and non-spathe clades. Species in the Fargesia spathe clade have the derived “spathe-like leaf sheath syndrome,” which may have evolved as a result of an adaptive advantage of compressed inflorescences in colder temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bamboos (Poaceae: Bambusoideae) have rich species diversity (Bamboo Phylogeny Group 2012). Based on molecular systematic studies, Bambusoideae is divided into three tribes: Arundinarieae, Bambuseae and Olyreae, representing temperate woody, tropical woody and herbaceous bamboos, respectively (Bouchenak-Khelladi et al. 2008; Sungkaew et al. 2009; Bamboo Phylogeny Group 2012). Most members of the Bambusoideae are woody species (Bamboo Phylogeny Group 2012). The majority of the woody bamboo species have been frequently described without knowledge of the flowers (Wang and Ye 1980; Stapleton 1997; Li et al. 2006; Viana et al. 2011) due to the long blooming intervals from several to 120 or even 150 years (Janzen 1976; Campbell 1987; Geng and Wang 1996). Thus, vegetative features are significant in identifying these infrequently flowering plants (Soderstrom 1981; Keng and Wen 1989; Stapleton 1997; Stapleton et al. 2009). The current infrageneric taxonomy is mainly based on the varied vegetative features, especially for the woody bamboos distributed in China (e.g., Yi 1996; Li et al. 2006). Since the vegetative characters used for identification are likely to be unstable in most cases, it is difficult to argue if they truly reflect the evolutionary history of these species (Guo et al. 2001). Therefore, the roles of vegetative and reproductive characteristics in evolutionary relationships of the Chinese genera remain unclear (Zhang et al. 2012; Yang et al. 2013). Fargesia is a good system to address this issue as it is one of the largest alpine woody bamboo genera. Furthermore, Fargesia species have various morphological characteristics, wide distributions and an infrageneric classification based on vegetative features (Yi 1988a).
Fargesia, a member of the tribe Arundinarieae (Bamboo Phylogeny Group 2012; Kellogg 2015), comprises about 80–100 species (Yi 1996; Li et al. 2006; Yi et al. 2008; Kellogg 2015) and is distributed mainly in mountains from 1400 to 3800 m in the eastern Qinghai-Tibetan Plateau and southwest China, the eastern Himalayas, Burma and Vietnam (Yi 1996; Li et al. 2006; Yi et al. 2008).
Fargesia was first described by Franchet (1893) based on F. spathacea Franchet, and contained only one species until the 1980s, when Yi (1983a, b, c, 1985a, b, c, 1986, 1988a, b, 1989) and others (Lu 1981; Wang and Ye 1981; Keng 1987; Yi and Shao 1987; Wen 1984, 1989; Yi and Long 1989) published 70 new species in < 10 years, and nine species were transferred into Fargesia from other genera. Ninety to 100 Fargesia species are currently recognized in the literature (Li et al. 2006; Yi et al. 2008; Kellogg 2015).
After Fargesia, some allied genera, including Sinarundinaria (Nakai 1935), Yushania (Keng 1957) (ca. 80 species, Li et al. 2006), Chimonocalamus (Hsueh and Yi 1979), Ampelocalamus (Chen et al. 1981), Drepanostachyum, Himalayacalamus (Keng 1983) and Borinda (Stapleton 1994b), were described, among which Sinarundinaria and Borinda were later shown to be synonymous with Fargesia (Wang and Ye 1980; Keng 1984, 1987; Yi 1982, 1988a, 1996; Guo et al. 2001, 2002; Guo and Li 2004; Li et al. 2006). Although Fargesia was accepted by many authors (Wang and Ye 1980; Keng 1983; Yi 1982, 1988a, 1996; Yi et al. 2008; Bamboo Phylogeny Group 2012), the publication of these genera made the classification of Fargesia more controversial, with different opinions appearing. There were mainly three point of views. First, Fargesia was recognized as a separate genus (Keng 1982, 1983; Soderstrom and Ellis 1987; Yi 1988a, 1996; Yi et al. 2008), and at least part of the species in Yushania, Chimonocalamus, Ampelocalamus, Drepanostachyum and Himalayacalamus were considered to be included in Fargesia (Chao et al. 1980; Hsueh and Li 1987; Soderstrom and Ellis 1987; Chao and Renvoize 1989; Yi 1988a, 1996). Second, Fargesia was either included in Thamnocalamus (Soderstrom 1979a, b; Chao et al. 1980; Soderstrom and Ellis 1982; Clayton and Renvoize 1986; Chao and Renvoize 1989; Demoly 1991) or Arundinaria (Soderstrom and Ellis 1988). Demoly (2005) even moved the species of Fargesia into Thamnocalamus, Drepanostachyum or Yushania. The third opinion partly accepted Fargesia, with the removal of some species in Yi’s (1988a) infrageneric classification into Thamnocalamus, Drepanostachyum or Himalayacalamus (Stapleton 1994c; Ohrnberger 1996; Li et al. 2006). The boundary of Fargesia is not well defined possibly because the identifications of those genera were based mainly on vegetative characteristics. It is well known that compared with the characteristics of flowers and inflorescences, vegetative characteristics are more affected by environment factors, and some labile traits exist among genera (Guo et al. 2001). Because of the controversial classification of Fargesia, it is reasonable to doubt the monophyly of Fargesia as suggested by previous molecular studies (Guo and Li 2004; Triplett and Clark 2010; Zeng et al. 2010).
Yi (1988a) established an infrageneric classification of Fargesia based on vegetative characteristics including two sections and six series among which only two sections were accepted (Yi et al. 2008), but this infrageneric classification has not yet been tested by molecular phylogenetics. It is difficult to determine the position of those species due to the lack of description of the flowers, especially the species in section Sphaerigemma that were put in Fargesia (Yi 1996). Regardless of the inflorescences or floral characters, the vegetative character-based infrageneric classification might result in instable generic/infrageneric classifications because of extensive morphological parallelism or homoplasy in morphology.
Fargesia has been included in several molecular systematic studies (Kelchner and Clark 1997; Zhang and Clark 2000; Bouchenak-Khelladi et al. 2008; Peng et al. 2008; Hodkinson et al. 2010; Triplett and Clark 2010; Zeng et al. 2010; Zhang et al. 2012; Yang et al. 2013; Ma et al. 2014). Sequence data from the nuclear internal transcribed spacer region (nrITS) have shown that some species of Fargesia nested within Yushania (Guo et al. 2001, 2002) and that Fargesia might be polyphyletic (Guo et al. 2002). Based on nrITS and GBSSI sequence data, Guo and Li (2004) showed that Fargesia and Yushania species form a monophyletic group except for F. fractiflexa, and again Fargesia species nested with Yushania species in different clades. However, the focus of these studies has been to resolve the intergeneric relationships within tribe Arundinarieae, but not the infrageneric phylogeny of Fargesia. The infrageneric phylogeny of Fargesia has not been resolved because of limited sampling in previous studies.
Fargesia species play an important role in the ecosystem and also as the main food of giant pandas and some other rare animals (Yi 1985a, b; McNeely 1999). In this study, representative species from each series and section of Fargesia (sensu Yi 1988a) and Yushania were sampled. Chloroplast matK, psbA-trnH, trnL-trnF and nuclear nrITS sequence data were used to (1) evaluate the boundary of this genus; (2) provide an infrageneric phylogenetic framework; and (3) investigate the evolutionary pattern of floral characters.
Materials and methods
Taxon sampling
Thirty-six species of Fargesia from Yi’s (1988a) infrageneric classification, including species transferred to Drepanostachyum, Himalayacalamus or Thamnocalamus by other authors, and nine species of Yushania were selected as members of the ingroup. Two species of Thamnocalamus and two species of Pleioblastus were selected as the outgroup. Fresh leaves from each individual were sampled in the field and immediately placed on silica gel and stored at room temperature for DNA extraction.
All vouchers were deposited in the herbarium of Shaanxi Normal University (SANU, Xi’an, China). The identification of bamboo species was based on Flora Republicae Popularis Sinicae (Yi 1996), Flora Yunnanica (Li 2003), Flora of China (Li et al. 2006), The Bamboos of Nepal and Bhutan (Stapleton 1994a, b, c) and Iconographia Bambusoidearum Sinicarum (Yi et al. 2008).
DNA extraction, PCR amplification and DNA sequencing
Total DNA was extracted from silica gel-dried leaves (0.3 g) using a modified CTAB method (Doyle and Doyle 1987). Three cpDNA gene regions, matK (Bouchenak-Khelladi et al. 2008), psbA-trnH (Ruiz-Sanchez et al. 2008) and trnL-trnF (Taberlet et al. 1991), and the nuclear ribosomal ITS (White et al. 1990) were isolated in the accessions (Table 1).
PCR amplifications were carried out using ABI Veriti TM 96-well Thermal cycler (Applied Biosystems, CA, USA) in total volumes of 25 μL. Each reaction contained 0.25 μL Takara LA Taq, 2.5 μL 10 × PCR Buffer, 2.5 μL MgCl2 (25 mM), 4 μL of dNTPs (Takara Biomedical Technology, Beijing, China), 1 μL of each primer (10 μM), 1 μL of template DNA (20–30 ng/μL) and 12.75 μL H2O. The PCR products were subsequently purified through ethanol precipitation and sequenced by Beijing Dingguo Changsheng Biotechnology Co. Ltd. (Beijing, China). Several ITS sequences contained some nucleotide positions with double peaks, so we confirmed the sequences via cloning with pMD™19-T vector cloning kit (Takara Biomedical Technology, Beijing, China) following the manufacturer’s protocols, and re-scanned and edited as necessary to accurately identify polymorphic positions.
Phylogenetic analyses
The sequences were assembled and manually edited in Geneious R9.0.2 (Kearse et al. 2012). To check whether the obtained sequence was the target sequence, we performed a homology search using BLAST (The National Center for Biotechnology Information, NCBI). Sequence divergences between taxa and base frequencies (G + C content) were determined using MEGA v.5.05 (Tamura et al. 2011).
Phylogenetic analyses were conducted using separate and combined data sets. Gaps were coded using simple indel coding (Simmons and Ochoterena 2000) in SeqState (Müller 2005) (Online Resource 1). Congruence between datasets was tested with the incongruence length difference test (ILD) (Darlu and Lecointre 2002) implemented in PAUP v4.0b10 (Swofford 2002) as the partition homogeneity test.
Maximum parsimony (MP) phylogenetic analyses were performed using PAUP v.4.0b10 (Swofford 2002). Maximum parsimony bootstrap (MPB) analyses (Felsenstein 1985) were carried out with 1000 random addition sequences and tree bisection-reconnection (TBR) branch swapping. A full heuristic bootstrap was conducted for maximum parsimony with 1000 bootstrap replicates, each with 100 random addition sequence replicates. Akaike information criterion (Posada 2008) calculations, implemented in jModelTest v.2.1.4 (Darriba et al. 2012), were used to select the appropriate model of sequence evolution for the different datasets. Bayesian inference (BI) was conducted using MrBayes v.3.2.6 (Ronquist and Huelsenbeck 2003). The Markov chain Monte Carlo algorithm was implemented with two parallel runs, each with four independent chains that were set to run for 4 million generations and to sample every 1000 generations. Convergence was assessed using the average standard deviation and the potential scale reduction factor (PSRF). Convergence of runs was also checked using Tracer v 1.6 (Rambaut et al. 2014) to assess the effective sample size (ESS) > 200 for all parameters. The first 25% of the trees was discarded as a burn-in. ML analysis was run in RA × ML-HPC ver.8 on XSEDE in the Cipres Science Gateway (Miller et al. 2010). Rapid bootstrap analysis with 1000 replications was performed using GTR-GAMMA estimation to assess branch support (Stamatakis 2006).
The approximately unbiased (AU) test was performed in CONSEL (Shimodaira and Hasegawa 2001; Shimodaira 2002) to test phylogenetic hypotheses (e.g., the monophyly of Yushania).
Reconstruction of character-state evolution
Vegetative features of Fargesia, Thamnocalamus, Drepanostachyum and Yushania are similar, but the inflorescences in these taxa vary. In particular, the inflorescences of some species are subtended by leaf sheaths, which expand to different levels. Inflorescence types were coded as follows: spathe-like leaf sheath syndrome (0: compressed and unilateral inflorescences are subtended by a series of spathe-like leaf sheaths, of which the topmost is longer than the inflorescence), non-expanding leaf sheaths (1: open inflorescences are subtended by non-expanding leaf sheaths) and slightly expanded leaf sheaths (2: open inflorescences are somewhat subtended but never spathe-like expanded leaf sheaths). Stigma types were coded as follows: pistil with three stigmas (0), pistil with two stigmas (1). To reconstruct ancestral states, we used the “trace character history” option with parsimony ancestral states in Mesquite v.2.75 (Maddison and Maddison 2011). These two reproductive characters were optimized on the ML phylogenetic tree obtained from the combined data.
Average temperature data were downloaded from WorldClim (http://www.worldclim.org/) for current conditions (interpolations of observed data, representative of 1960–1990) at 2.5-min grids and exported from DIVA-GIS v.7.5 (Hijmans et al. 2012) based on the latitude and longitude of the sampling point. The 3D-scatter line was made to reflect the fluctuation of average temperature along with the latitude and distribution elevation using Origin v 9.1 (Seifert 2014).
Results
Phylogenetic analyses
The characteristics of the alignment matrix of three plastid regions and nrITS sequences are summarized in Table 2. The ILD test indicated that cpDNA sequences were congruent (P = 0.100 between matK and psbA-trnH, P = 0.080 between trnL-trnF and the combined matK and psbA-trnH), and likewise, the ILD test indicated the combined cpDNA and nrITS sequences (P = 0.50) were also congruent. The best evolutionary model estimated for each dataset was TIM1 + I, TrN + I + G and TIM3 + I+G for the cpDNA, nrITS and combined dataset, respectively. Different methods of phylogenetic analysis were used based on the separate and the combined cpDNA and nrITS sequences.
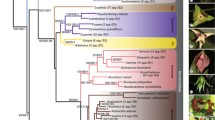
In all the phylogenetic trees, F. crassinoda (= T. spathiflorus var. crassinodus) and F. damuniu together with two Thamnocalamus species formed the Thamnocalamus clade; four species, including F. ampullaris (= D. ampullare), F. semiorbiculata (= D. semiorbiculatum), F. gyirongensis (= H. falconeri) and F. collaris (= H. collaris) formed a clade in most of the trees, and all species in this clade were transferred to Drepanostachyum or Himalayacalamus, so the clade was accepted as the Drepanostachyum + Himalayacalamus clade in previous studies (Ohrnberger 1996; Demoly 2005; Stapleton et al. 2005; Li et al. 2006); and the remaining Fargesia species and all Yushania species formed a Fargesia + Yushania clade, except in the cpDNA tree, in which the Drepanostachyum + Himalayacalamus clade and Fargesia + Yushania clades were not well resolved. The species in the Fargesia + Yushania clade divided into the Fargesia spathe and the non-spathe clades, and each of them divided into smaller clades, but with weak or no bootstrap support (Fig. 1).
50% majority-rule consensus tree based on the combined data of cpDNA (matK, psbA-trnH, trnL-F) and nrITS. Tree length = 164, CI = 0.610, RI = 0.829. The bootstrap values (≥ 50%) from 1000 replicates are plotted along the branches. Numbers above and below branches are bootstrap values of MP and ML analysis and PP of Bayesian analysis, respectively
The Fargesia spathe clade also resolved as monophyletic based on nrITS sequences (Online Resource 2) but formed a polytomy based on plastid data (Online Resource 3). Two main subclades were resolved in the Fargesia spathe clade, one including F. decurvata, F. robusta and F. rufa, and the other including F. demissa, F. obliqua and F. qinlingensis (Fig. 1). The non-spathe clade was subdivided into subclades B1 and B2 (Fig. 1) with moderate statistical support (MPB = 62, MLB = 57, PP = 1.00). Subclades B1 consisted of the most species of Fargesia and all species of Yushania, but the statistical support was low. Yushania species did not form a monophyletic group but collapsed with Fargesia species in subclades B1 (Fig. 1). Subclades B2 consisted of four species with moderate bootstrap values (MPB = 87, MLB = 74, PP = 1.00), including F. canaliculata, F. membranacea, F. fractiflexa and F. fungosa.
Based on the AU test, our data failed to reject the monophyly of the Fargesia spathe clade as sister to the non-spathe clade (p = 0.987), but rejected the monophyly of the non-spathe clade + Drepanostachyum + Himalayacalamus clade (p < 0.001) (Online Resource 3). The AU test also rejected the monophyly of Yushania as sister to the remainder of Fargesia (p = 0.013).
Morphological characteristic and geographic distribution
Species in the Thamnocalamus, Drepanostachyum and Fargesia + Yushania clades are similar in their vegetative features, but differ in inflorescence features. In the Thamnocalamus clade, the racemes are lateral and terminal on the front nodes of the flowering branches and the leaf sheaths on the front nodes are spathe-like. In the Drepanostachyum + Himalayacalamus clade, the inflorescences are fascicled on the upper nodes of culms and branches, and the nodes in lower parts of inflorescences are compressed and wrapped by scaly sheaths at the base.
Species in the Fargesia + Yushania clade have inflorescences at the tips of leafy branches. Species in the Fargesia spathe clade have pistils with three stigmas (rarely two such as in F. spathacea) and the spathe-like leaf sheath syndrome where the inflorescences are compressed, unilateral and subtended by a series of leaf sheaths which expand as spathes, of which the uppermost spathe is generally longer than the inflorescence (Fig. 3). Bamboos in the Fargesia spathe clade bloom from March to May (rarely June, December) (Yi 1985b, 1996, 2000; Li et al. 2006; Yi et al. 2007; Lei et al. 2011), and occupy the northern part of the distribution of the Fargesia + Yushania clade, including Sichuan, southern Gansu, Shaanxi and Ningxia, northwestern Hunan, western Henan and Hubei and northern Chongqing (Geng and Wang 1996; Yi et al. 2008; Yi and Zhu 2012) (Fig. 2).
In the non-spathe clade, species which had descriptions of flowers and inflorescences share the following features: Open inflorescences are subtended by more or less expanded but never spathe-like leaf sheaths that are always shorter than the inflorescence, or part of the inflorescences on a culm are unilateral, such as in F. edulis (Yi 1988a, 1996), F. fungosa (Yi 1985c, 1996) and F. subflexuosa (Zhang and Ren 2016); the pistil has two stigmas (rarely two to three in Y. violascens or three in F. grossa)(Yi 1988a, 1996) (Fig. 3). Species in the non-spathe clade bloom from April to August (rarely to September) and occupy the southern part of the distribution of the Fargesia + Yushania clade, including southern Sichuan, Yunnan, southeastern Tibet and Taiwan, as well as India, Nepal, Bhutan, Burma, Vietnam, the Philippines, Malaysia and Sri Lanka (Stapleton 1994b; Yi 1996; Yi et al. 2008) (Fig. 2).
Morphological character evolution optimized onto the tree from the analysis of combined data. a Evolution of the leaf sheaths of inflorescence and b the number of stigmas. Note that spathe-like leaf sheath syndrome represents the inflorescences are compressed, unilateral and subtended by a series of leaf sheaths which expand as spathes, of which the uppermost is generally longer than the inflorescence
To better understand the evolution of inflorescences in Fargesia, floral types were mapped onto the MP tree (Fig. 3). The ancestral condition was inferred as open inflorescences subtended by non-expanding leaf sheaths. The spathe-like leaf sheath syndrome is the exclusive inflorescence type seen among the Fargesia spathe clade. Although we considered the distribution elevation of sampling points, there were significant upward trends of average temperature with the decreasing latitude during the winter, namely from November to February (Fig. 4). Species in the Fargesia spathe clade tend to occupy the northern part of the distribution of the Fargesia + Yushania clade, where species are distributed at higher latitudes than species of the non-spathe clade, which are distributed in the southern part; the environment of the Fargesia spathe clade is colder than that of the non-spathe clade.
Discussion
Fargesia (sensu Yi 1988a) as a polyphyletic group
Several phylogenetic studies have been conducted that the monophyly of Fargesia (sensu Yi 1988a) was problematic, as the boundary of Fargesia is confused with Yushania (Guo et al. 2002; Guo and Li 2004; Wang et al. 2017), Chimonocalamus (Yang et al. 2013; Wang et al. 2017), Bashania (Zhang et al. 2012; Wang et al. 2017) and Phyllostachys (Ma et al. 2014) based on limited taxon sampling and various gene markers. Both the current and previous molecular phylogenetic studies demonstrate that the classification of Fargesia will need to be re-evaluated.
The results from the present study suggest that Fargesia (Yi 1988a) is polyphyletic with F. crassinoda (= Thamnocalamus spathiflorus var. crassinodus) and F. damuniu in the Thamnocalamus clade, F. semiorbiculata (= D. semiorbiculatum), F. collaris (= H. collaris), F. gyirongensis (= Himalayacalamus falconeri) and F. ampullaris (= Drepanostachyum ampullare) in the Drepanostachyum + Himalayacalamus clade and the remaining Fargesia and all species of Yushania in the Fargesia + Yushania clade.
Fargesia crassinoda was described by Yi (1983b) without description of inflorescences and later transferred to Thamnocalamus as T. spathiflorus (Trin.) Munro var. crassinodus (Yi) Stapleton (Stapleton 1994b). This treatment was supported by previous molecular systematic studies (Guo et al. 2001, 2002; Guo and Li 2004) and accepted by Ohrnberger (1999) and Li et al. (2006). Fargesia damuniu was described by Yi et al. (2007), but lacked description of flowers and inflorescences, and placed in section Fargesia (Yi et al. 2008). The present study suggests that both F. crassinoda and F. damuniu are in the same clade with the two Thamnocalamus species included in this investigation.
Because of a lack of description of flowers and inflorescences, F. ampullaris, F. canaliculata, F. collaris, F. fractiflexa, F. gyirongensis, F. membranacea and F. semiorbiculata were placed in Fargesia section Sphaerigemma by Yi (1985a, b, 1988a, 1992, 1996). Later, F. collaris and F. gyirongensis were transferred into Himalayacalamus as H. collaris (T.P.Yi) Ohrnb. and H. gyirongensis (T.P.Yi) Ohrnb. (Ohrnberger 1996), respectively; F. fractiflexa, F. ampullaris, F. semiorbiculata and F. membranacea were transferred into Drepanostachyum as D. fractiflexum (T.P.Yi) D.Z.Li (2003), D. ampullare (T.P.Yi) Demoly (Demoly 2005), D. semiorbiculatum (T.P.Yi) Stapleton and D. membranaceum (T.P.Yi) D.Z.Li (Stapleton et al. 2005), respectively. Li et al. (2006) accepted all the transferred names except H. gyirongensis, which was treated as a synonym of H. falconeri (Hook.f. ex Munro) Keng f. in the Flora of China (Li et al. 2006). On the one hand, only F. gyirongensis (= H. falconeri) has had its flowers and inflorescences described (Keng 1983), and we collected the flowers and inflorescences of F. semiorbiculata (= D. semiorbiculatum); these flowers and inflorescences were different from Fargesia species and further supported the inclusion of D. semiorbiculatum in Drepanostachyum (Zhang and Ren 2016). On the other hand, Fargesia ampullaris, F. collaris, F. gyirongensis and F. semiorbiculata should be transferred into Drepanostachyum or Himalayacalamus, and other species in section Sphaerigemma should be retained in Fargesia. Our phylogenetic trees support the opinion that Himalayacalamus should be merged into Drepanostachyum (Soderstrom and Ellis 1987; Li 1997; Wang 1997), whereas others treated Himalayacalamus as a distinct genus closely related to Drepanostachyum (Stapleton 1994b; Ohrnberger 1999; Zhang et al. 2012). Additional phylogenetic research sampling more species in those genera will be necessary to disentangle their relationships and help make taxonomic revisions.
Consistent with morphological data, the present analyses corroborate the placement of the Thamnocalamus clade and the Drepanostachyum + Himalayacalamus clade, which is consistent with previous studies (Guo and Li 2004; Zhang et al. 2012; Yang et al. 2013; Ma et al. 2014; Attigala et al. 2016; Zhang et al. 2016; Wang et al. 2017). Several species which were treated as the members of Fargesia seems more reasonable to transfer into Thamnocalamus clade and the Drepanostachyum + Himalayacalamus clade. Thus, Fargesia (sensu Yi 1988a) is polyphyletic.
Phylogenetic relationships between Fargesia and Yushania
Yushania was considered the most closely allied genus to Fargesia, although it differs in having pachymorphic rhizomes with very long, symmetrical necks (Keng 1957; Wang and Ye 1980; Keng 1984, 1987; Stapleton 1994a; Yi 1982, 1985a, 1988a, 1996; Li et al. 2006) and inflorescences without subtending spathes (Wang et al. 2017). Chao et al. (1980) treated Yushania as a synonym of Sinarundinaria, which was subsequently shown to be a synonym of Fargesia, and this was also followed by Clayton and Renvoize (1986, 1989). The molecular systematic results on the relationship between these two genera are confusing. Neither Fargesia nor Yushania is monophyletic, and species in these two genera nest together in different clades with low to strong support under varying sampling strategies and gene markers (Guo et al. 2001, 2002; Guo and Li 2004; Wang et al. 2017). Several studies suggested that the phylogenetic relationships between these two genera were not resolved (Zeng et al. 2010; Zhang et al. 2012; Yang et al. 2013). Our results suggested that Yushania and Fargesia were indeed closely related. Further work including additional taxa is required to resolve relationships between the two genera.
Species in the Fargesia + Yushania clade share the following morphological features: rhizomes pachymorphic with short or long necks; inflorescences are on top of leafy branches, subtended by a series of leaf sheaths which expand to varying degrees, or even to spathes, and are not scaly, as in the Drepanostachyum + Himalayacalamus clade. Although the pulvini at the base of the spikelet stalks were considered an identifying characteristic of Yushania (Stapleton 1994b), they exist in most of Yushania species except Y. lineolata, and do not exist in Fargesia except in F. yunnanensis (Yi 1996). Therefore, this characteristic is labile in these two genera. The tessellation in the leaf venation was also considered a unique characteristic of Yushania (Stapleton 1994b), but this characteristic seems to not be critical to distinguish the two genera.
Our data also revealed that the Fargesia + Yushania clade is divided into a Fargesia spathe clade and a non-spathe clade. Previous molecular studies showed that species with the spathe-like leaf sheath syndrome formed a clade which is sister to other genera (Zhang et al. 2012, 2016; Wang et al. 2017), and some researchers treated F. spathacea, F. nitida and F. murielae as Fargesia (s.s.) (Guo and Li 2004). However, Fargesia species without the spathe-like leaf sheath syndrome nested with Yushania, Phyllostachys, Chimonocalamus and other genera (Zhang et al. 2012, 2016; Wang et al. 2017). Thus, in order to understand the phylogenetic relationships within the Fargesia + Yushania clade, more taxa from other related genera should be considered in further research before making any taxonomic conclusions.
Evolution of the spathe-like leaf sheath syndrome as an adaptation to low temperature
Yi (1988a) divided Fargesia into section Sphaerigemma, which have culm buds that are ovoid or pyramidal, thick and appressed with two early deciduous prophylls, and section Fargesia, which have culm buds that are long ovoid, thin and unappressed with two late deciduous prophylls. Section Sphaerigemma was divided into series Collares, which has unequal, i.e., thick and thin, branches at the same node and series Ampullares, which has equal branches. Section Fargesia was divided into four series: series Murielae with oblong or oblong-elliptical culm sheaths, while series Fargesia, series Angustissimae and series Yunnanenses have long triangular or oblong-triangular culm sheaths. This subdivision was accepted by Yi (1996), and Yi et al. (2008) accepted only the subdivision of two sections.
Moreover, previous molecular studies showed that Fargesia (sensu Yi 1988a) might be a polyphyletic group with other closely related genera under limited sampling taxa and different markers (Guo et al. 2001, 2002; Guo and Li 2004; Zeng et al. 2010; Ma et al. 2014; Zhang et al. 2016; Wang et al. 2017). Our analyses with expanded Fargesia species found that the shape of the culm buds and the shape of the culm sheaths used by Yi (1988a) as the identity characteristic of sections and series, respectively, were inconsistent with the division of Fargesia (Fig. 1). Therefore, the morphological feature-based subdivision of Fargesia (sensu Yi 1988a) is not objective. These vegetative characteristics may not be a reliable diagnostic character for the delineation of the Fargesia + Yushania clade.
Interestingly, floral characters to some extent support the revision of the classification of the Fargesia + Yushania clade as indicated by molecular data. The ancestral inflorescences in the Fargesia + Yushania clade are open inflorescences with varying degrees of expansion but never spathe-like leaf sheaths, while the ancestral inflorescence types in the Fargesia spathe clade are compressed, unilateral and subtended by a series of persistent and delicate leaf sheaths that expand as spathes. Members of the Fargesia spathe clade occupy the northern part of the distribution, where it is colder in the spring than in the south (Figs. 2, 4). The temperature from November to February might influence flower bud differentiation. As shown in Lin et al. (2015), the flower buds of Bambusa multiplex initiated in October, differentiated into spikelets by March or April and the inflorescences bloomed from April to May (Lin et al. 2015). Changes in the size, color and structure of spathes have been reported as adaptive responses to temperature (Li et al. 2013). Furthermore, the structure of spathes could have influenced insect pollination as demonstrated in other plants (Ørgaard and Jacobsen 1998). Compared with the non-spathe clade, this derived spathe-like leaf sheath syndrome in the Fargesia spathe clade can be considered a “key innovation” or morphological novelty that allowed these species to adapt to the cold weather in the north, because this type of morphological novelty can open new adaptive zones (Nitecki 1990). In other words, the inflorescences of species in the Fargesia spathe clade might have proceeded via an acquisition of the spathe-like leaf sheath syndrome to protect young flowers from damage caused by low temperatures. Nevertheless, further studies are needed to test this hypothesis based on a broad taxonomic and geographic sampling.
In conclusion, our analyses suggested that recovered clades of Fargesia (Yi 1988a) and its closely related genera conflicted significantly with traditional morphological classification. All species of Yushania nested with most Fargesia species and formed Fargesia + Yushania clade. Only nine species of Yushania were examined in our study; therefore, more taxa will be required to be included to resolve relationships between the two genera. We also found that the spathe-like leaf sheath syndrome could be an adaptive advantage for species in the Fargesia spathe clade in cold environment. The tangled relationships recovered among these taxa demonstrate that the floral characters may be a reliable diagnostic character. Nevertheless, further analyses including more taxa from Fargesia and other related genera are required to understand the evolution of the different components of floral morphology.
References
Attigala L, Wysocki WP, Duvall MR, Clark LG (2016) Phylogenetic estimation and morphological evolution of Arundinarieae (Bambusoideae: Poaceae) based on plastome phylogenomic analysis. Molec Phylogen Evol 101:111–121. https://doi.org/10.1016/j.ympev.2016.05.008
Bamboo Phylogeny Group (2012) An updated tribal and subtribal classification for the Bambusoideae (Poaceae). In: Gielis J, Potters G (eds) Proceedings of the 9th World Bamboo congress. World Bamboo Organization, Antwerp, pp 3–27
Bouchenak-Khelladi Y, Salamin N, Savolainen V, Forest F, Van der Bank M, Chase MW, Hodkinson TR (2008) Large multi-gene phylogenetic trees of the grasses (Poaceae): progress towards complete tribal and generic level sampling. Molec Phylogen Evol 47:488–505. https://doi.org/10.1016/j.ympev.2008.01.035
Campbell JJN (1987) The history of sino-himalayan bamboo flowering, droughts and sun-spots. J Bamboo Res 6:1–15
Chao CS, Renvoize SA (1989) A revision of the species described under Arundinaria (Gramineae) in Southeast Asia and Africa. Kew Bull 44:349–367. https://doi.org/10.2307/4110809
Chao CS, Chu CD, Hsiung WY (1980) A revision of some genera and species of Chinese bamboos. Acta Phytotax Sin 18:20–36
Chen SL, Sheng GY, Wen TH (1981) Ampelocalamus-A new genus of Chinese Bamboo. Acta Phytotax Sin 19:332–334
Clayton WD, Renvoize SA (1986) Genera Graminum, grasses of the world. Kew Bull Addit Ser XIII Her Majesty’s Stationery Office, London
Darlu P, Lecointre G (2002) When does the incongruence length difference test fail? Molec Biol Evol 19:432–437. https://doi.org/10.1093/oxfordjournals.molbev.a004098
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Meth 9:772. https://doi.org/10.1038/nmeth.2109
Demoly JP (1991) Recensement des bambous cultivés en Europe. Bambou Bull Assoc Europeene du Bambou Section France 8:20–28
Demoly JP (2005) Nouvelles combinaison snomenclaturales. Bambou Bull Assoc Europeene du Bambou Section France 46:6–8
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. https://doi.org/10.2307/2408678
Franchet MA (1893) Fargesia, nouveau genre de Bambuseés de la Chine. Bull Mens Soc Linn Paris 2:1067–1069
Geng PC, Wang CP (1996) Flora Reipublicae Popularis Sinicae. Science Press, Beijing
Guo ZH, Li DZ (2004) Phylogenetics of the Thamnocalamus group and its allies (Gramineae: Bambusoideae): inference from the sequences of GBSSI gene and nrITS spacer. Molec Phylogen Evol 30:1–12. https://doi.org/10.1016/S1055-7903(03)00161-1
Guo ZH, Chen YY, Li DZ, Yang JB (2001) Genetic variation and evolution of the alpine bamboos (Poaceae: Bambusoideae) using DNA sequence data. J Pl Res 114:315–322. https://doi.org/10.1007/PL00013993
Guo ZH, Chen YY, Li DZ (2002) Phylogenetic Studies on Thamnocalamus group and its allies (Poaceae: Bambusoideae) based on nrITS sequence data. Molec Phylogen Evol 22:20–30. https://doi.org/10.1006/mpev.2001.1039
Hijmans RJ, Guarino L, Mathur P (2012) DIVA-GIS, Version 7.5, Manual. Available at: http://www.diva-gis.org
Hodkinson TR, Chonghaile GN, Sungkaew S, Chase MW, Salamin N, Stapleton CMA (2010) Phylogenetic analyses of plastid and nuclear DNA sequences indicate a rapid late Miocene radiation of the temperate bamboo tribe Arundinarieae (Poaceae, Bambusoideae). Pl Ecol Diversity 3:109–120. https://doi.org/10.1080/17550874.2010.521524
Hsueh CJ, Li DZ (1987) New taxa of Bambusoideae from Sichuan and Yunnan, with discussion on concepts of related genera. J Bamboo Res 6:16–19
Hsueh CJ, Yi TP (1979) Two new genera of Bambusoideae from S. W. China, 1. Chimonocalamus Hsueh et Yi. Acta Bot Yunnan 1:74–81
Janzen DH (1976) Why bamboos wait so long to flower. Annual Rev Ecol Syst 7:347–391. https://doi.org/10.1146/annurev.es.07.110176.002023
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kelchner SA, Clark LG (1997) Molecular evolution and phylogenetic utility of chloroplast rpl16 intron in Chusquea and the Bambusoideae (Poaceae). Molec Phylogen Evol 8:385–397. https://doi.org/10.1006/mpev.1997.0432
Kellogg EA (2015) Flowering plants, monocots, Poaceae. In: Kubitzki K (ed) The families and genera of vascular plants. Spinger, Basel, pp 1–416. https://doi.org/10.1007/978-3-319-15332-2
Keng PC (1957) One new genus and two new species of Chinese bamboos. Acta Phytotax Sin 6:355–360
Keng PC (1982) A revision of the genera of bamboos from the world. I. J Bamboo Res 1:1–19
Keng PC (1983) A revision of the genera of bamboos from the world. III. J Bamboo Res 2:11–27
Keng PC (1984) A revision of the genera of bamboos from the world. V. J Bamboo Res 3:22–42
Keng PC (1987) On the nomenclature of the high-alpine bamboos from China. J Bamboo Res 6:11–17
Keng PC, Wen TH (1989) A preliminary study on bamboo classification according to the vegetative characters. J Bamboo Res 8:17–29
Lei KM, Zhang Y, Xiao WY, Cai YS, Ze J, Liu Y, Sun HG, Zeng T (2011) Characteristics of the flowering Fargesia nitida populations in Jiuzhaigou. J Sichuan Forest Sci Technol 32:92–95
Li DZ (1997) The flora of China Bambusoideae project-problems and current understanding of bamboo taxonomy in China. In: Chapman GP (ed) The Bamboos. Academic Press, London, pp 61–81
Li DZ (2003) Flora yunnanica, vol. 9. Science Press, Beijing
Li DZ, Guo ZH, Stapleton CMA (2006) Fargesia. In: Wu ZY, Raven PH (eds) Flora of China (Poaceae). Science Press, Beijing, pp 74–96
Li CH, Huang SR, Wang LL, Wang C, Yang GS (2013) Relationship between temperature, accumulation of mineral elements and “green ear” of anthurium spathe. Chin J Trop Agric 33:3–7
Lin SY, Li J, Zhao R, Xu Q, Ding YL (2015) A research on the flowering biological characteristics of Bambusa multiplex in Nanjing City. J Nanjing Forest Univ 39:52–56
Lu JL (1981) The new species of Bambusoideae from China. J Henan Coll Agric 74:70–79
Ma PF, Zhang YX, Zeng CX, Guo ZH, Li DZ (2014) Chloroplast phylogenomic analyses resolve deep-level relationships of an intractable bamboo Tribe Arundinarieae (Poaceae). Syst Biol 63:933–950. https://doi.org/10.1093/sysbio/syu054
Maddison WP, Maddison DR (2011) Mesquite: a modular system for evolutionary analysis, version 2.75. Available at: http://mesquiteproject.org. Accessed 30 Sept 2011
McNeely JA (1999) Biodiversity and bamboo genetic resources in Asia: in situ, community-based and ex situ approaches to conservation. Chin Biodivers 7:38–51
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the gateway computing environments workshop (GCE). New Orleans, US, pp 1–8
Müller K (2005) SeqState. Appl Bioinformatics 4:65
Nakai T (1935) Novitates Bambusacearum in imperio Japonico recentissime detectae III. J Jap Bot 11:1
Nitecki MH (1990) Evolutionary innovations. University of Chicago Press, Chicago
Ohrnberger D (1996) The bamboos of the world: introduction to the work. Blackwell Ltd., Beaver House, England, p 14
Ohrnberger D (1999) The bamboos of the world: annotated nomenclature and literature of the species and the higher and lower taxa. Elsevier Science, Amsterdam
Ørgaard M, Jacobsen N (1998) SEM study of surface structures of the spathe in Cryptocoryne and Lagenandra (Araceae: Aroideae: Cryptocoryneae). Bot J Linn Soc 126:261–289. https://doi.org/10.1006/bojl.1997.0136
Peng S, Yang HQ, Li DZ (2008) Highly heterogeneous generic delimitation within the temperate bamboo clade (Poaceae: Bambusoideae): evidence from GBSSI and ITS sequences. Taxon 57:799–810
Posada D (2008) jModelTest: phylogenetic model averaging. Molec Biol Evol 25:1253–1256. https://doi.org/10.1093/molbev/msn083
Rambaut A, Suchard MA, Xie D, Drummond AJ (2014) Tracer, version 1.6. Available at: http://beast.bio.ed.ac.uk/Tracer
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Ruiz-Sanchez E, Sosa V, Mejía-Saules MT (2008) Phylogenetics of Otatea inferred from morphology and chloroplast DNA sequence data, and recircumscription of Guaduinae (Poaceae: Bambusoideae). Syst Bot 33:277–283. https://doi.org/10.1600/036364408784571644
Seifert E (2014) Origin Pro 9.1: scientific data analysis and graphing software-software review. J Chem Inf Model 54(5):1552. https://doi.org/10.1021/ci500161d
Shimodaira H (2002) An approximately unbiased test of phylogenetic tree selection. Syst Biol 51:492–508. https://doi.org/10.1080/10635150290069913
Shimodaira H, Hasegawa M (2001) CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics 17:1246–1247. https://doi.org/10.1093/bioinformatics/17.12.1246
Simmons MP, Ochoterena H (2000) Gaps as characters in sequence-based phylogenetic analyses. Syst Biol 49:369–381. https://doi.org/10.1093/sysbio/49.2.369
Soderstrom TR (1979a) The bamboozling Thamnocalamus. Garden 3:22–27
Soderstrom TR (1979b) Another name for the umbrella bamboo. Brittonia 31:495. https://doi.org/10.2307/2806007
Soderstrom TR (1981) Some evolutionary trends in the Bambusoideae (Poaceae). Ann Missouri Bot Gard 68:15–47. https://doi.org/10.2307/2398809
Soderstrom TR, Ellis RP (1982) Taxonomic status of the endemic South African bamboo, Thamnocalamus tessellatus. Bothalia 14:53–67. https://doi.org/10.4102/abc.v14i1.1135
Soderstrom TR, Ellis RP (1987) The position of bamboo genera and allies in a system of grass classification. In: Soderstrom TR, Hilu KW, Campbell S, Barkworth ME (eds) Grass systematics and evolution. Institution Press, Washington, DC, pp 225–238
Soderstrom TR, Ellis RP (1988) The woody bamboos (Poaceae: Bambuseae) of Sri Lanka: a morphological–anatomical study. Smithsonian Contr Bot 72:1–75. https://doi.org/10.5479/si.0081024X.72
Stamatakis A (2006) RA × ML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Stapleton CMA (1994a) The bamboos of the Nepal and Bhutan Part I: Bambusa, Dendrocalamus, Melocanna, Cephalostachyum, Teinostachyum, and Pseudostachyum (Gramineae: Poaceae, Bambusoideae). Edinburgh J Bot 51:1–32. https://doi.org/10.1017/S0960428600001682
Stapleton CMA (1994b) The bamboos of the Nepal and Bhutan Part II: Arundinaria, Thamnocalamus, Borinda, and Yushania (Gramineae: Poaceae, Bambusoideae). Edinburgh J Bot 51:275–295. https://doi.org/10.1017/S0960428600000883
Stapleton CMA (1994c) The bamboos of the Nepal and Bhutan Part III: Drepanostachyum, Himalayacalamus, Ampelocalamus, Neomicrocalamus and Chimonobambusa (Gramineae: Poaceae, Bambusoideae). Edinburgh J Bot 51:301–330. https://doi.org/10.1017/S0960428600001815
Stapleton CMA (1997) Morphology of woody bamboos. In: Chapman GP (ed) The Bamboos. Academic Press, London, pp 167–251
Stapleton CMA, Li DZ, Xia NH (2005) New combinations for Chinese bamboos (Poaceae, Bambuseae). Novon 15:599–601
Stapleton CMA, Chonghaile GN, Hodkinson TR (2009) Molecular phylogeny of Asian woody bamboos: Review for the Flora of China. Bam Sci Cult: J Amer Bamboo Soc 22:5–25
Sungkaew S, Stapleton CMA, Salamin N, Hodkinson TR (2009) Non-monophyly of the woody bamboos (Bambuseae; Poaceae): a multi-gene region phylogenetic analysis of Bambusoideae. J Pl Res 122:95–108. https://doi.org/10.1007/s10265-008-0192-6
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0 Beta. Sinauer Ass. Inc., Sunderland
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Pl Molec Biol 17:1105–1109. https://doi.org/10.1007/BF00037152
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molec Biol Evol 28:2731–2739. https://doi.org/10.1093/molbev/msr121
Triplett JK, Clark LG (2010) Phylogeny of the temperate bamboos (Poaceae: Bambusoideae: Bambuseae) with an emphasis on Arundinaria and allies. Syst Bot 35:102–120. https://doi.org/10.1600/036364410790862678
Viana PL, Filgueiras TS, Paiva EAS (2011) A new combination in Aulonemia (Poaceae: Bambusoideae: Bambuseae) based on floral analysis, anatomical features, and distribution. Brittonia 63:102–112. https://doi.org/10.1007/s12228-010-9138-0
Wang CP (1997) A proposal concerning a system of classification of Bambusoideae from China. J Bamboo Res 16:1–6
Wang CP, Ye GH (1980) On the problems of the classification of Chinese bamboos with creeping rhizomes. Acta Phytotax Sin 18:283–291
Wang ZP, Ye GH (1981) Miscellaneous notes on Chinese Bambusoideae. J Nanjing Univ (Nat Sci) 1:91–108
Wang XQ, Ye XY, Zhao L, Li DZ, Guo ZH, Zhuang HF (2017) Genome-wide RAD sequencing data provide unprecedented resolution of the phylogeny of temperate bamboos (Poaceae: Bambusoideae). Sci Rep 7:11546. https://doi.org/10.1038/s41598-017-11367-x
Wen TH (1984) New taxa of Bambusoideae in China (I). J Bamboo Res 3:23–47
Wen TH (1989) Some new bamboos from Southern Yangtze River. J Bamboo Res 8:13–24
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Yang HM, Zhang YX, Yang JB, Li DZ (2013) The monophyly of Chimonocalamus and conflicting gene trees in Arundinarieae (Poaceae: Bambusoideae) inferred from four plastid and two nuclear markers. Molec Phylogen Evol 68:340–356. https://doi.org/10.1016/j.ympev.2013.04.002
Yi TP (1982) A revision of the genera of Fargesia group in China. J Sichuan Forest Sci Technol 2:54–59
Yi TP (1983a) New taxa of Bamboosoideae from Xizang (Tibet), China. J Bamboo Res 2:28–46
Yi TP (1983b) New species of Fargesia Franchet and Yushania Keng f. from Tibet. J Bamboo Res 2:18–53
Yi TP (1983c) A new species of bamboo form island Hainan, China. Bull Bot Res 3:151–154
Yi TP (1985a) Classification and distribution of the food bamboos of the Giant Panda (I). J Bamboo Res 4:11–27
Yi TP (1985b) Classification and distribution of the food bamboos of the Giant Panda (II). J Bamboo Res 4:20–45
Yi TP (1985c) New taxa of bamboo from China. Bull Bot Res 5:121–137
Yi TP (1986) A new species of Fargesia from Sichuan. Acta Bot Yunnan 8:48–50
Yi TP (1988a) A study on the genus Fargesia from China. J Bamboo Res 7:1–119
Yi TP (1988b) Four new species of bamboo from South Yunnan, China. Acta Bot Yunnan 10:437–443
Yi TP (1989) Two new species of bamboo from Southwest Sichuan. Acta Bot Yunnan 11:35–38
Yi TP (1992) New bamboos of Fargesia and Chimonobambusa from Sichuan. Acta Bot Yunnan 14:135–138
Yi TP (1996) Fargesia, Yushania. In: Geng PC, Wang ZP (eds) Flora reipublicae popularis sinicae. Science Press, Beijing, pp 387–560
Yi TP (2000) A new species of Fargesia from northeastern Sichuan, China. Acta Bot Yunnan 22:251–254
Yi TP, Long TL (1989) Two new species of bamboos for Giant Panda. J Bamboo Res 8:30–36
Yi TP, Shao JX (1987) New taxa of Fargesia from Shaanxi. J Bamboo Res 6:42–45
Yi TP, Zhu XB (2012) A new species and two combinations of Bambusoideae (Poaceae). J Sichuan Forest Sci Technol 33:8–11
Yi TP, Shi JY, Yang L (2007) Alpine new bamboos from Sichuan, Tibet and Chongqing, China. Bull Bot Res 27:515–520
Yi TP, Shi JY, Ma LS (2008) Iconographia bambusoidearum sinicarum. Science Press, Beijing
Zeng CZ, Zhang YX, Triplett JK, Yang JB, Li DZ (2010) Large multi-locus plastid phylogeny of the tribe Arundinarieae (Poaceae: Bambusoideae) reveals ten major lineages and low rate of molecular divergence. Molec Phylogen Evol 56:821–839. https://doi.org/10.1016/j.ympev.2010.03.041
Zhang WP, Clark LG (2000) Phylogeny of classification of the Bambusoideae (Poaceae). In: Jacobs SWL, Everett JE (eds) Grasses: systematics and evolution. CSIRO, Collingwood, pp 35–42
Zhang YQ, Ren Y (2016) Supplementary description of flower and flowering branch for four Fargesia species and one Drepanostachyum species (Bambusoideae, Poaceae), and notes on their taxonomy. Nordic J Bot 34:565–572. https://doi.org/10.1111/njb.00975
Zhang YX, Zeng CX, Li DZ (2012) Complex evolution in Arundinarieae (Poaceae: Bambusoideae): incongruence between plastid and nuclear GBSSI gene phylogenies. Molec Phylogen Evol 63:777–797. https://doi.org/10.1016/j.ympev.2012.02.023
Zhang XZ, Zeng CX, Ma PF, Haevermans T, Zhang YX, Zhang LN, Guo ZH, Li DZ (2016) Multi-locus plastid phylogenetic biogeography supports the Asian hypothesis of the temperate woody bamboos (Poaceae: Bambusoideae). Molec Phylogen Evol 96:118–129. https://doi.org/10.1016/j.ympev.2015.11.025
Acknowledgments
This study was funded by the National Natural Science Foundation of China (Grant No. 31570221).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of “Phylogeny of Fargesia (Poaceae: Bambusoideae) and infrageneric adaptive divergence inferred from three cpDNA and nrITS sequence data” declare that they have no conflict of interest.
Additional information
Handling Editor: Yunpeng Zhao.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Information on Electronic Supplementary Material
Information on Electronic Supplementary Material
Online resource 1. Nucleotide sequence alignment matrix of 4 combined marker datasets (psbA-trnH, matK, trnL-trnF, nrITS) in the Nexus format.
Online resource 2. 50% majority-rule consensus tree based on the nrITS data.
Online resource 3. 50% majority-rule consensus tree based on the combined cpDNA data (matK, psbA-trnH, trnL-F).
Rights and permissions
About this article
Cite this article
Zhang, YQ., Zhou, Y., Hou, XQ. et al. Phylogeny of Fargesia (Poaceae: Bambusoideae) and infrageneric adaptive divergence inferred from three cpDNA and nrITS sequence data. Plant Syst Evol 305, 61–75 (2019). https://doi.org/10.1007/s00606-018-1551-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-018-1551-y