Abstract
Helianthemum nummularium s.l. is a young, morphologically diverse species distributed from western Europe to the Caucasus and the Southern Urals in the east. We analysed the rps16-trnK plastid intergenic spacer sequences from 85 localities covering most of the range of H. nummularium. Thirteen haplotypes were very unevenly distributed throughout the range of the species, and exhibited a strong phylogeographic signal. The results confirm range expansions of H. nummularium from Mediterranean refugia northwards, but also show the major role of eastern European (the Caucasus and the Southern Urals) refugia in rapid postglacial colonization of east, north and central Europe. The plastid haplotypes form distinct clades, one representing an eastern European lineage with few haplotypes and the other representing a western European lineage with many haplotypes. Parallel to this split in haplotype diversity is the pronounced differentiation in morphological variation displayed by the taxa found in west and east Europe. We discuss the role of topography in generating differences in morphological and genetic diversity between these two groups. We also discuss the taxonomical status of Helianthemum arcticum, which is regarded as an endangered local endemic of the Kola Peninsula. Helianthemum arcticum appears to represent an outlying peripheral population of H. nummularium preserved since the last postglacial major range expansion of this species, and bears the same plastid haplotype as the bulk of east and north European populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Local relief is one of the main factors shaping biodiversity. Mountain ranges are important for diversity and speciation in temperate regions in two ways; they can act as a barrier to species migration during Quaternary climate fluctuations (Taberlet et al. 1998), and they can serve as refugia due to their complex topography and varied habitat types (Tribsch and Schönswetter 2003). The mountain ranges in western Europe (the Alps and the Pyrenees) form so-called ‘suture zones’, where different genetic lineages come into contact and possibly hybridize during periods of favourable climate conditions (Hewitt 2000). The altitudinal distribution of mountain and alpine species fluctuated with the Quaternary climate oscillations, which caused repeated contraction and expansion that could enhance differentiation among mountains (Kropf et al. 2003; Ronikier et al. 2008a). Many studies have documented high genetic differentiation of alpine species in western European mountain ranges (the Pyrenees, the Alps and the Carpathians) and surrounding areas (e.g. Stehlik et al. 2002; Tribsch and Schönswetter 2003; Ronikier et al. 2008a). The origin of this differentiation is often attributed to glacial refugia in ice-free areas in southern Europe (e.g. Iberian Peninsula, Italy and the Balkans), and to periglacial refugia north of the glaciated mountain areas of southern Europe (the Pyrenees, the Alps and the Carpathians) (Petit et al. 2003; Stewart and Lister 2001).
In contrast to western Europe, the relief of eastern Europe is more homogenous, with no prominent mountain ranges in its central part (the Caucasus and the Urals form the border of this region). The southern part of the East European plain was never glaciated during the late Quaternary climate fluctuations (Svendsen et al. 2004). Physical obstacles had less influence on range contractions and expansions of species during the climate oscillations than in western Europe. The risk of local refugia being locked in by mountain ranges during glacial maxima was low, so we would expect lower genetic differentiation between populations in eastern Europe than in western Europe. However, as far as we know, this has never been tested with appropriate sampling of plants from the entire distribution area (Hewitt 2004). Inclusion of samples from eastern Europe in phylogeographic studies is especially important, as this region was regarded as being an important source of genetic variation for plants in the western part of the continent (Taberlet et al. 1998). There have been few pan-European phylogeographic studies of plants, but all of them support the general idea of westward postglacial migrations (e.g. Palmé et al. 2003; Pyhäjärvi et al. 2008; Tollefsrud et al. 2009; Treier and Müller-Schärer 2011; Eidesen et al. 2013).
Helianthemum nummularium s.l. (Cistaceae) is a species aggregate that shows great morphological differentiation in Europe. Many taxa have been described on the basis of flower colour, petal and leaf size, and indumentum (Janchen 1909; Proctor and Heywood 1968). Most morphological variation is found in western Europe, displayed by a greater mosaic of taxa distributed in this area than in eastern Europe. This is in accordance with the greater impact by glaciation in western Europe during the Quaternary climate fluctuations (Svendsen et al. 2004). The phylogeography of the species in western Europe based on studies of plastid microsatellites has recently revealed genetic data that did not support the current taxonomical subdivision of the H. nummularium s.l. (Soubani et al. 2014). The most important key character (presence/absence of a dense cover of stellate hairs on the abaxial surface of the leaves) in the species complex turned out to be governed by a single Mendelian gene with two alleles (Widén 2015).
In this study, we extend the sampling of H. nummularium s.l. to comprise almost the entire species range of the yellow-flowered morphs in the species complex. We explore the phylogeography of the species based on plastid DNA sequences. We focus on the contrasts in genetic differentiation between the two regions, western and eastern Europe. We compared our results to the previous taxonomy of H. nummularium s.l. based on morphology and plastid microsatellites (Janchen 1909; Soubani et al. 2014). Based on our analysis of plastid DNA sequences in H. nummularium s.l., we can suggest scenarios of the biogeographical history of the species.
Materials and methods
Study model
Helianthemum Mill. is a young genus (Guzman and Vargas 2009), comprising several species complexes (Janchen 1907, 1909) that have not reached reproductive isolation (Widén 1986, 2015). Helianthemum nummularium s.l. is a European dwarf shrub characterized by 2n = 20. The morphological variation in this species is complex, where the indumentum characters in particular have been considered to be taxonomically important, and the species has been divided into several taxa, most of them in western Europe (Janchen 1909; Proctor and Heywood 1968). According to the ‘Flora Europaea’ (Proctor and Heywood 1968), the H. nummularium s.l. consists of eight subspecies (sometimes regarded as separate species (e.g. H. grandiflorum (Scop.) Lam. et DC., H. tomentosum (Scop.) Spreng in Yuzepchuk 1974, see also “Appendix”), five of which belong to a yellow-flowered group. Additional taxa have been regarded as important local endemic morphs, e.g. H. arcticum (Grosser) Janch. in the Kola Peninsula (Belousova et al. 2008).
Helianthemum nummularium s.l. is distributed from the Iberian Peninsula, through central and southern Europe, northward to Scotland, Denmark, central Sweden and southern Finland; scattered populations are found in the Caucasus and central Russia, extending to the Urals in the east and to Lake Ladoga in the north (Proctor 1956; Vasari and Vasari 1999). The species is listed as rare or decreasing in a number in the Red Data Books of many regions of central Russia (Prisyazhnyuk 2009), East Fennoscandia (Kotiranta et al. 1998) and Sweden (Artdatabanken 2015). In addition, one highly isolated population of H. nummularium s.l. is located on the southern coast of the Kola Peninsula, more than 500 km away from the nearest conspecific locality. This large (hundreds of individuals along several kilometres of coastline) and stable (definitely existing for more than a century; Yuzepchuk 1974) population is sometimes regarded as a separate and endangered endemic species (H. arcticum). It is included in the Red Data Book of the Russian Federation (Kostina 2008) and has been protected in the Kandalaksha State Nature Reserve since 1977.
The evolutionary history of this population is closely connected with the glacial history of the area, as the Kola Peninsula was ice-covered during the last (Weichselian) glacial maximum; the ice retreated from its southern shore as recently as approximately 13,000 years ago (Svendsen et al. 2004). The fossil pollen spectra suggest that H. nummularium s.l. was abundant in late-glacial European grasslands and virtually disappeared from the areas where post-glacial forests spread (e.g. Hirsch et al. 2015). Today, it is restricted to relatively small treeless refugia on base-rich rocks with thin soils (Proctor 1956, 1958; Vasari and Vasari 1999 and references therein).
According to Proctor and Heywood (1968), the two main subspecies of the yellow-flowered H. nummularium (L.) Mill. are subsp. nummularium and subsp. obscurum (Čelak.) Holub, with overlapping distributions; the latter is mainly found in western Europe, while the former covers the main distribution area of the species. H. nummularium subsp. nummularium is characterized by a dense cover of stellate hairs on the abaxial surface of the leaves, and subsp. obscurum has abaxial surfaces of the leaves without this key character. The two subspecies may occur together and, when they do, they cross freely; the diagnostic character—a dense cover of stellate hairs on the abaxial surface—is inherited as a Mendelian gene with a recessive allele for subsp. nummularium and a dominant allele for subsp. obscurum (Widén 2015). Besides the two main subspecies, the yellow-flowered part of the complex comprises three altitudinal subspecies mainly distributed in mountains of southern and central Europe (Proctor and Heywood 1968)—subsp. tomentosum (Scop.) Schinz & Thell., with large flowers and a dense cover of stellate hairs on the abaxial surface of the leaves, and two subspecies without the dense cover of stellate hairs—subsp. glabrum (Koch) Wilczek with more or less glabrous leaves and subsp. grandiflorum (Scop.) Schinz & Thell., with large flowers.
Recent studies (Soubani et al. 2014) have shown that the variation patterns of indumentum, along with the leaf and petal shape and plastid haplotype variability do not correlate with the subdivision of H. nummularium into subspecies. Morphological variation of H. nummularium s.l. has been shaped by hybridization, selection and by historical processes, such as range fluctuations during Pleistocene glaciations (Soubani et al. 2014). The species origin was attributed to the Mediterranean. The Alps and the surrounding areas constituted the centre of genetic diversity, and northern areas displayed lower diversity. The main refugia were assumed to be located in central and southern Europe (around the Alps and the Balkans). There was a refugium in eastern Europe, and an assumed partial colonization of Scandinavia from it, but this hypothesis could not be tested in that study because of the absence of samples from the eastern part of the continent (Soubani et al. 2014).
In the present study, we analysed five taxa of H. nummularium from western Europe (all yellow-flowered morphs in Proctor and Heywood 1968) and four taxa from eastern Europe [subsp. nummularium, subsp. obscurum, subsp. glabrum (= H. nitidum Clem.) and H. arcticum]. We added the endemic morph H. arcticum, even though the species status of H. arcticum seems to be doubtful, since it differs from H. nummularium s.s. only by reduced indumentum of leaves, peduncles and sepals (Yuzepchuk 1974).
Sampling
We used plant material of H. nummularium s.l. collected in the field or cultivated in a greenhouse at Lund University from seeds collected in the field. All the populations sampled are listed in Table 1. Plants were determined to subspecies level, according to Proctor and Heywood (1968) (Table 1 in Soubani et al. 2014). We analysed one plant per population except for H. arcticum, where we sampled three plants from different parts of its population (indicated as 6, 11 and 12 in Table 1; the taxonomical status and morphological variation of this morph were one of the original foci of the study). Leaf samples from all the plants were dried in silica gel prior to DNA analysis, and one plant per population was pressed as a voucher. Voucher specimens are deposited at the Herbaria of Moscow State University (MW), Russia and Lund University (LD), Sweden. In this study, we also included additional specimens of H. nummularium from the Herbarium of the Main Botanical Garden in Moscow (MHA), Russia. Altogether we sampled 85 localities (Table 1), covering almost the entire range of H. nummularium (Proctor 1956), and including six taxa (H. nummularium subspp. nummularium, tomentosum, obscurum, grandiflorum, glabrum and the putative species H. arcticum). One-third of the studied populations (Table 1) were also included in the study of plastid microsatellites in Soubani et al. (2014). Two herbarium samples of H. ledifolium (L.) Mill. from Azerbajdzhan were included in the study as an outgroup (following Guzman and Vargas 2009 and Parejo-Farnés et al. 2013).
DNA isolation and sequencing
DNA was extracted from dehydrated leaf material using the CTAB method (Doyle and Doyle 1987). We sequenced the chloroplast intergenic spacer rps16-trnK (Takahashi et al. 2005). Polymerase chain reactions (PCR) were conducted in 20 µl reaction volumes containing 4 µl of Ready-to-Use PCR MaGMix (200 µM of each dNTP, 1.5 mM MgCl2, 1.5 U SmarTaqDNA Polymerase and reaction buffer; Dialat Ltd., Moscow, Russia), 15 µl deionised water, 3.4 pmol of each primer and 1 µl of template DNA of unknown concentration. PCR cycling was performed with a MJ Research PTC-220 DNA Engine Dyad Thermal Cycler (BioRad Laboratories, USA) with the following parameters: initial denaturation for 5 min at 95 °C followed by 35 cycles of 30 s at 95 °C, 30 s at 50 °C, and 2 min at 72 °C, ending with 10 min extension at 72 °C. Double-stranded PCR products were checked on agarose gel and purified using centrifugation with a solution of ammonium acetate in ethanol. Sequencing was performed in both directions using ABI PRISM BigDye® Terminator v 3.1 Kit (Applied Biosystems) according to the manufacturer’s manual, except that we used 10 µl reaction volumes, and further analysis was carried out using 3130 and 3500 Genetic Analyzers (Applied Biosystems). The GenBank accession numbers of the sequences are JQ927012–JQ927212 (http://www.ncbi.nlm.nih.gov/genbank/) (cf. Online Resources 1, 2).
Data analyses
The sequences were aligned manually using BioEdit 7.0.5.3 (Hall 1999). Conventional phylogenetic methods may perform poorly in revealing intraspecific relationships (Jakob and Blattner 2006), so we carried out statistical parsimony analysis using the network algorithm described in Templeton et al. (1992), and implemented in the TCS v. 1.21 program (Clement et al. 2000). This method estimates an unrooted haplotype network and a 95 % plausible set of all haplotype lineages in that network. Since the alignment contained multiple gaps of various lengths, we performed separate analyses with and without gap data included. In the latter case, gaps were treated as missing data, otherwise they were coded using the simple method of indel coding (Simmons et al. 2001) as implemented in the GapCoder software (Young and Healy 2003).
Rooting of the network was performed using sequences of H. ledifolium as an outgroup. H. ledifolium has been shown to belong to a sister clade of the H. nummularium clade in two independent phylogenetic surveys of the genus Helianthemum and other Cistaceae (Guzman and Vargas 2009; Parejo-Farnés et al. 2013). We performed two separate analyses using the NJ algorithm as implemented in the SplitsTree4 ver. 4.9.1 program (Huson and Bryant 2006), and independently using the ML analysis as implemented in the Treefinder program (Jobb 2011). In the latter case, a substitution model was assessed by means of the same program.
AMOVA analyses were calculated in Arlequin 3.1 (Excoffier et al. 2005). Geographic groups were defined in terms of three major haplotype lineages revealed by the statistical parsimony analysis.
Correspondence between subspecies identity of H. nummularium s.l. and plastid DNA haplotype was tested with Pearson’s Chi-square test with simulated p value, appropriate for correspondence tables with many zeroes, as in our case, p value was calculated by Monte Carlo simulation based on 2000 replicates. The test was performed in statistical environment R 2.9.2 (R Development Core Team 2011).
Results
The rps16-trnK sequences were 570–699 positions long. The alignment was 809 positions long and contained seven parsimony informative sites and six singletons within the ingroup. The outgroup differed from the ingroup by mutations in 13 sites. The alignment included ten gaps 1–217 positions long, two of them in poly-A and poly-T sequences. For the purpose of the analysis they were coded using simple indel coding method in the GapCoder program as 17 indels.
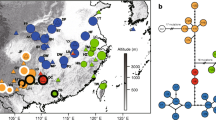
Analysis of the rps16-trnK alignment in TCS without outgroup, with indels treated as missing data, revealed 17 haplotypes, four of which were not in the data and represented hypothetical extinct haplotypes. The TCS program calculated the 95 % limit of parsimony of 13 mutational steps, and connected all the haplotypes into a single network (Fig. 1a). The outgroup was too distant genetically to be included in the network (not shown). When indels were included in the analysis, the program revealed 30 haplotypes, ten of which were not in the data and represented hypothetical extinct haplotypes. In this case, the haplotypes A, C, E, and G revealed in the previous analysis were split into 2–4 closely related haplotypes. However, the analysis introduced eight loops into the network caused by homoplasy (Crandall and Templeton 1993), obscuring the relationships of these additional haplotypes (Fig. 1b).
Geographic distribution of these split haplotypes did not add much to the analysis either. Within haplotype A, haplotype A1 is restricted to the Caucasus, but A2 occurs both in the Caucasus, the Urals, and in Ulyanovsk Province on the East European Plain. The A3 haplotype is represented by a single specimen from the Urals and may constitute a product of local diversification. Within the major haplotype C, C2 is represented by a single specimen from southern Germany, while all the other specimens belong to the haplotype C1. The haplotype E1 occurs in Slovakia, northern Italy, and Belgium; E2 is represented by a single specimen from the Pyrenees; E3 is represented by two specimens from southern slopes of the Alps in northern Italy; E4 also belongs to a single specimen from the Pyrenees. Consequently, no clear geographic pattern can be seen in these split haplotypes, and the relationships between them cannot be easily interpreted.
Among the haplotypes derived from the haplotype G, the G1 is known from a single specimen from Bulgaria, while all the other specimens bear the haplotype G2 and occur in Greece. This may reflect some geographical diversification at a local scale. However, relationships between haplotypes G1 and G2, and their relations to haplotypes A1 and A2, are unresolved.
We assume that variation in indel length and composition in H. nummularium is strongly homoplasic. For this reason, the initial network based on the analysis with all the gaps treated as missing data was used for all further analyses and calculation of genetic indices. However, one major incongruence between the trees is worth mentioning and should be kept in mind, i.e. the position of the haplotype D and its derivative haplotype M. In the tree based on nucleotide substitution data only, the clade is internal, i.e. positioned deep inside the network. The inclusion of indel data in the analysis changes position of this clade to tip, though relations between haplotypes D and E are retained.
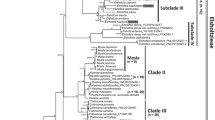
To root the network, we performed NJ and ML analyses on a matrix comprising 13 ingroup haplotypes and the outgroup. The Treefinder program assessed the most likely substitutional model as TVM+G. In both cases the resulting trees were topologically identical. The analysis connected the outgroup with the internal haplotype I (Fig. 2), which appears to be basal to the rest of the haplotypes. The root position of the haplotype I is also supported by its deep internal position in the network. With its only derivative tip haplotype F, haplotype I forms a basal clade from which two major evolutionary lineages originate. The eastern lineage 1 includes the clade AGCH distributed mostly in eastern Europe, the Caucasus and the Balkan Peninsula, but also penetrating into central Europe. The western lineage 2 includes two other clades (DM and ELBJK) distributed in central and western Europe.
The results of AMOVA are given in Table 2. Most of the variation is due to differences between clades, i.e. evolutionary lineages, which indicate their rather wide divergence.
We found no correspondence between subspecies identity of H. nummularium plants and their haplotypes [Table 3, Chi-square test: N = 85, p = 0.17, (Chi square) = 76.63]: plants referred to one subspecies were characterized by different haplotypes (usually quite distant) and each non-single haplotype was associated with different subspecies. Different subspecies in a given area tended to share the same haplotype (e.g. populations 112 and 128 in southern Sweden and populations 3021 and REV in the Pyrenees: Table 1). The three H. arcticum plants sampled in different parts of a population from the Kola Peninsula were characterized by haplotype C. This haplotype is found in many plants of subsp. nummularium and obscurum, but only in a few plants of subsp. glabrum and grandiflorum (Table 3).
The contrast in haplotype diversity between western and eastern Europe is striking. Dividing Europe in two halves with different glacial histories along an arbitrary line from the southeastern Baltic to the western Black Sea gives twelve haplotypes in western Europe but only two in eastern Europe. This means 2.4 haplotypes per taxon and 0.5 (or 0.75 if H. arcticum is included in H. nummularium subsp. nummularium) for western and eastern Europe, respectively.
Discussion
We revealed a clear spatial pattern of plastid DNA haplotypes in H. nummularium s.l. in Europe, which is largely consistent with the earlier independent phylogeographic analyses of plastid microsatellites of western European samples (Soubani et al. 2014). As discussed further below, plant populations from the plains of eastern Europe are characterized by low morphological and genetic diversity compared with mountainous western Europe. Based on the analysis of variation in one plastid marker, we discuss alternative scenarios of refuge areas and migration routes of H. nummularium—hypothesizes that will have to be tested in the future using independent nuclear markers (cf. Després et al. 2003; Petit et al. 2005).
Taxonomic implications
We revealed up to seven not closely related plastid haplotypes in a single subspecies of H. nummularium s.l., and several haplotypes shared among up to five taxa (cf. Soubani et al. 2014). The sharing of haplotypes among taxa may be due to introgression or incomplete lineage sorting from a polymorphic ancestor (Wolfe and Elisens 1995; Comes and Abbott 2001). Both of these processes occur mostly between young and reproductively not completely isolated taxa (such as representatives of subgen. Helianthemum Willk., radiating in Pleistocene; Guzman and Vargas 2009; Parejo-Farnés et al. 2013), and distinguishing them in phylogenetic analyses is often impossible (Jakob and Blattner 2006). The maintenance of shared ancient polymorphisms along with constantly growing effective population size is suggested by the small number of hypothetically extinct haplotypes in the network, resulting in the fixation of nearly all newly arising haplotypes. Local introgression may be indicated by the tendency of different morphological subspecies to share the same haplotype in a given region.
Although sharing of several plastid haplotypes among a multitude of species has been shown (e.g. Jakob and Blattner 2006; Hathaway et al. 2009; Christe et al. 2014), we believe that the current splitting of H. nummularium into taxonomic units of any rank, especially of specific level (Yuzepchuk 1974), seems unjustified, as morphological characters that were treated as diagnostic are not stable but correlated with ecological conditions (Kupatadze 1978; Soubani et al. 2014). Furthermore, the most important key character in the species, a dense cover of stellate hairs on the abaxial surface of the leaves, has turned out to be governed by a single Mendelian gene (Widén 2015). The conclusion of low taxonomical rank of morphs in H. nummularium also applies to ‘H. arcticum’, whose morphology lies within the variation range of subsp. nummularium (Widen, unpubl.) and which is not genetically distinct in terms of plastid DNA haplotype variation. The isolated occurrence of H. arcticum on the Kola Peninsula can be explained by the postglacial history of H. nummularium (Jalas 1980).
Phylogeography
Helianthemum nummularium is a young species (Guzman and Vargas 2009), which was split into a western and an eastern lineage early in its evolution during the Pleistocene. We could not date the split with our methods, but this probably occurred before the LGM.
The internal root haplotype I, together with its nearest derivative F, is found in two populations in the Balkan Peninsula, suggesting a Mediterranean origin for the species (cf. Soubani et al. 2014), a pattern typical for members of the Cistaceae family (Guzman and Vargas 2009). This also supports the idea of the Balkans as one of the main sources of genetic diversity in Europe (Hewitt 1999, 2000).
The eastern lineage covers a vast area of east, north and south-central Europe. This lineage was split into two clades; haplotype H (now confined to southern Italy) and the more widespread clade ACG. It may be speculated that the earliest H. nummularium migration was directed from the Balkans eastwards (haplotype G and/or haplotype A), colonizing the Caucasus, and further to the north of the Southern Urals and Ulyanovsk region. This migration was rapid, as no mutations accumulated apart from a few deletions. Unfortunately, we do not have samples from Asia Minor, but it seems likely that the Caucasus may have been colonized via this region. The time of this eastward expansion may be estimated as one of the glacial maxima, since it encompassed mostly territory only partially covered with ice in high mountains.
The origin and the migration routes of haplotype C are less obvious. This haplotype could have originated in the Caucasus or in the eastern part of the Russian Plain region, and then expanded northward and westward to the late- and post-glacial landscapes of the Russian Plain and onward to central Europe, including southern slopes of the Alps and central Italy. An alternative hypothesis to explain the current distribution of haplotype C could be an origin in the Balkans or in the Italian peninsula and a successive expansion into central Europe and northward and eastward to the post-glacial landscape of the Russian Plain.
Irrespective of origin and direction of migration, the expansion of haplotype C must have occurred very recently, probably after the last glacial maximum (LGM), and rapidly, since no mutations have accumulated apart from a few locally occurring deletions. This eastern lineage of H. nummularium should also have colonized northern Europe, including southern Sweden and eastern Denmark, as was suggested earlier for H. nummularium (Soubani et al. 2014, 2015) and for several other plant species (e.g. Van Rossum and Prentice 2004 and references therein).
Although palaeobotanical data for eastern Europe are very scanty, fossil pollen of Helianthemum spp. was reported for periglacial shrub tundra expanded in the north of central and eastern Europe (Hirsch et al. 2015). This includes the Kola Peninsula (Kultina and Spiridonova 1972) and southernmost Sweden in the Bølling-Allerød interstadial complex approximately 11–12,000 years BP (van Kolfschoten et al. 2008).
Scattered distribution of modern northeasterly populations of H. nummularium is consistent with their proposed relict status, as they were virtually displaced by post-glacial forests (Proctor 1958); this is proved by fossil pollen findings in that area (Vasari and Vasari 1999). Relict character of stony slopes where H. nummularium grows in Southern Karelia was postulated more than a century ago (Litvinov 1902), and the unique edaphic properties of Cape Turij (the Kola Peninsula) are also favourable for a number of relict species (Bubenets et al. 1993). The occurrence of several relict species in Cape Turij and the fact that H. nummularium lacks mechanisms for current long-distance dispersal (the seeds drop to the ground close to the parent and lack any morphological adaptation to long-distance dispersal; Widén, personal observation) makes a recent long-distance dispersal unlikely. Consequently, we found no genetic differentiation of isolated peripheral populations of H. nummularium—this is difficult to explain without knowledge of their evolutionary history (cf. Lesica and Allendorf 1995; Eckert et al. 2008).
The western lineage of H. nummularium covers a more restricted area than the eastern lineage. The distribution of haplotype D and its descendant M covers areas north of the central European mountains that have been suggested as periglacial refugia for several temperate plant species (Stewart and Lister 2001; Ronikier et al. 2008b). This lineage (haplotype D) colonized western Denmark, a region that was partly ice-free during the glacial maximum (Tzedakis et al. 2013). The other haplotypes in the western lineage are distributed across western Europe (haplotype E and its descendants B, L, K and J). Haplotype B is distributed along the basin of the River Rhone and along the northern slopes of the Alps, as well as south of the Alps. The distribution of haplotype B coincides with that of haplotype H14, identified on the basis of plastid microsatellites in Soubani et al. (2014), a haplotype that surrounds the Alps.
The observed high genetic differentiation in western Europe is partly explained by the existence of the well-known ‘suture zone’ located in the Alps, which was covered by ice during the LGM (Hewitt 2000). The distribution of the two haplotypes of H. nummularium on the northern (haplotype B) and southern (haplotype C) slopes of the Alps, i.e. along the Alpine arc, is consistent with distributions of other calcicolous species, explained by availability of calcareous bedrock in these mountains (Alvarez et al. 2009).
A secondary contact zone detected in Denmark is probably explained by the uneven melting of the LGM ice cap, which allowed eastern and western lineages to meet. This contact zone can be traced in the morphology of present-day populations of H. nummularium in southern Scandinavia. The distributions of the almost exclusive morph in Denmark (subsp. obscurum) and the most common morph in southern Sweden (subsp. nummularium) constitute a wide hybrid zone in southwestern Sweden, where plants of the two taxa cross freely (Soubani et al. 2015; Widén 2015). The western haplotype D, represented by subsp. obscurum, reached the ice-free areas in western Denmark, while the eastern haplotype C in the form of subsp. nummularium, arrived via more easterly routes. The distributions of taxa and haplotypes in the hybrid zone today do not coincide with each other (cf. Soubani et al. 2015), probably due to “chloroplast capture” (Jakob and Blattner 2006; Hathaway et al. 2009). Similar contact zones between western and eastern postglacial migration routes have previously been described for several species in Scandinavia (Malm and Prentice 2002; Tyler et al. 2002; Van Rossum and Prentice 2004; Nordström and Hedrén 2008; Hathaway et al. 2009; Prentice et al. 2011), outside of the current distribution range of H. nummularium.
The morphological variation of plants represented by the haplotypes A and C in the east is nearly homogenous, and the plants in this area are mostly recognized as one subspecies (subsp. nummularium). This is in sharp contrast to the haplotypes in western and southern Europe, where variation in morphology has been divided into five subspecies. The altitudinal fluctuations of the ice cap, for instance in the Alps, probably created a lot of local reductions and expansions in population size and diverse opportunities for adaptation to new habitats. Morphs with large flowers and leaves within different haplotype lineages may have been adapted repeatedly to mountain and alpine habitats. It is significant that three of the five western European subspecies are found in the Alpine belt (subsp. grandiflorum, glabrum and tomentosum). A single dominant mutation in the nuclear genome that changed the phenotype from plants with a dense cover of stellate hairs on the abaxial surface of the leaves to plants without this cover (or vice versa) may have been fixed in small populations once or repeatedly (a mutation from subsp. nummularium to subsp. obscurum probably occurs today, see Soubani et al. 2015).
Genetic and morphological diversity of H. nummularium in western Europe are shaped not only by older dispersal from southern refugia as was shown for many other species (Hewitt 2000, 2004) but also by more recent colonization from eastern Europe—the area that is still ignored in most phylogeographic reconstructions. In contrast, eastern and northern Europe virtually lacks both morphological and genetic variation among populations of H. nummularium. This may be regarded as a signature of rapid and recent colonization of these territories following the melting of glaciers, with subsequent extinction of many of these populations after forest vegetation became established and suitable habitats disappeared in most of the area.
Conclusions and future perspectives
We found a very distinct pattern in molecular and morphological variation in H. nummularium throughout Europe. Our results clearly show the importance of phylogeographic analyses of the entire species range for recovering species history. Questions remain about glacial refuges and postglacial migration routes, so areas not covered in the present study (e.g. southernmost Italy, western parts of the Balkans, Turkey and Sierra Nevada) should be included to give a deeper understanding of the evolution of H. nummularium. Moreover, to further understand the complex pattern of genetic and morphological differentiation of the H. nummularium s.l., closer examinations of introgression between different haplotypes of the western lineage and between western and eastern lineages are needed, using other molecular methods (Després et al. 2003; Petit et al. 2005) as well as morphometric analysis.
References
Alvarez N, Thiel-Egenter C, Tribsch A, Holderegger R, Manel S, Schönswetter P, Taberlet P, Brodbeck S, Gaudeul M, Gielly L, Küpfer P, Mansion G, Negrini R, Paun O, Pellecchia M, Rioux D, Schüpfer F, Van Loo M, Winkler M, Gugerli F, IntraBioDiv Consortium (2009) History or ecology? Substrate type as a major driver of spatial genetic structure in Alpine plants. Ecol Lett 12:632–640. doi:10.1111/j.1461-0248.2009.01312.x
Artdatabanken (2015) Rödlistade arter i Sverige 2015. Artdatabanken SLU, Uppsala
Belousova AV, Miliutina ML, Shilin NI, Mezhnev AP, Semenov VB, Sobolev NA, Varlygina TI (2008) Reference information: conservation of plants, animals and their habitats in Western Europe and Russia (by the example of Bern Convention, Directive on the Conservation of Wild Birds and Directive on the Conservation of Natural Habitats and Wild Fauna and Flora). Ministry of Natural Resources of Russian Federation, Moscow
Bubenets VN, Pokhilko AA, Tsaryova VT (1993) Vascular plants of Cape Turij. In: Konstantinova NA (ed) Floristic and geobotanical investigation in Murmansk Region [in Russian]. KNC RAN Publ, Apatity, pp 45–73
Christe C, Caetano S, Aeschimann D, Kropf M, Diadema K, Naciri Y (2014) The intraspecific genetic variability of siliceous and calcareous Gentiana species is shaped by contrasting demographic and re-colonization processes. Molec Phylogen Evol 70:323–336. doi:10.1016/j.ympev.2013.09.022
Clement M, Posada D, Crandall KA (2000) TCS: a computer program to estimate gene genealogies. Molec Ecol 9:1657–1659. doi:10.1046/j.1365-294x.2000.01020.x
Comes HP, Abbott RJ (2001) Molecular phylogeography, reticulation and lineage sorting in Mediterranean Senecio sect. Senecio (Asteraceae). Evolution 55:1943–1962
Crandall KA, Templeton AR (1993) Empirical tests of some predictions from coalescent theory with applications to intraspecific phylogeny reconstruction. Genetics 134:959–969
Després L, Gielly L, Redoutet B, Taberlet P (2003) Using AFLP to resolve phylogenetic relationships in a morphologically diversified plant species complex when nuclear and chloroplast sequences fail to reveal variability. Molec Phylogen Evol 27:185–196. doi:10.1016/S1055-7903(02)00445-1
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Eckert CG, Samis KE, Lougheed SC (2008) Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Molec Ecol 17:1170–1188. doi:10.1111/j.1365-294X.2007.03659.x
Eidesen PB, Ehrich D, Bakkestuen V, Alsos IG, Gilg O, Taberlet P, Brochmann C (2013) Genetic roadmap of the Arctic: plant dispersal highways, traffic barriers and capitals of diversity. New Phytol 200:898–910. doi:10.1111/nph.12412
Excoffier L, Laval LG, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Guzman B, Vargas P (2009) Historical biogeography and character evolution of Cistaceae (Malvales) based on analysis of plastid rbcL and trnL–trnF sequences. Org Diversity Evol 9:83–99. doi:10.1016/j.ode.2009.01.001
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hathaway L, Malm JU, Prentice HC (2009) Geographically congruent large-scale patterns of plastid haplotype variation in the European herbs Silene dioica and S. latifolia (Caryophyllaceae). Bot J Linn Soc 161:153–170. doi:10.1111/j.1095-8339.2009.01003.x
Hewitt GM (1999) Post-glacial re-colonization of European biota. Biol J Linn Soc 68:87–112. doi:10.1111/j.1095-8312.1999.tb01160.x
Hewitt GM (2000) The genetic legacy of the Quaternary ice ages. Nature 405:907–913. doi:10.1038/35016000
Hewitt GM (2004) Genetic consequences of climatic oscillations in the quaternary. Philos Trans Ser B 359:183–195. doi:10.1098/rstb.2003.1388
Hirsch F, Schneider A, Nicolay A, Blaszkiewicz M, Kordowski J, Noryskiewicz AM, Tyszkowski S, Raab A, Raab T (2015) Late Quaternary landscape development at the margin of the Pomeranian phase (MIS 2) near Lake Wygonin (Northern Poland). Catena 124:28–44. doi:10.1016/j.catena.2014.08.018
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Molec Biol Evol 23(2):254–267. doi:10.1093/molbev/msj030
Jakob SS, Blattner FR (2006) A chloroplast genealogy of Hordeum (Poaceae): long-term persisting haplotypes, incomplete lineage sorting, regional extinction, and the consequences for phylogenetic inference. Molec Biol Evol 23(8):1602–1612. doi:10.1093/molbev/msl018
Jalas J (1980) Helianthemum nummularium (L.) Miller—Päivännouto. In: Jalas J (ed) Suuri kasvikirja, III. Otava, Helsinki, pp 93–95
Janchen E (1907) Helianthemum canum (L.) Baumg. und seine nächsten verwandten. Abh Zool Bot Ges Österreich 4:1–67
Janchen E (1909) Die Cistaceen Österreich-Ungarns. Mitt Naturwiss Vereins Univ Wien 7:1–124
Jobb G (2011) TREEFINDER version of March 2011. Munich Germany. Distributed by the author. Available at: http://www.treefinder.de
Kostina VA (2008) Helianthemum arcticum (Grosser) Janch. In: Trutnev YP (ed) Red data book of Russian Federation (plants and fungi) [in Russian]. KMK Press, Moscow, pp 178–179
Kotiranta H, Uotila P, Sulkava S, Peltonen DL (1998) Red data book of East Fennoscandia. Finnish Mus Nat Hist, Helsinki
Kropf M, Kadereit JM, Comes HP (2003) Differential cycles of range contraction and expansion in European high mountain plants during the Late Quaternary: insights from Pritzelaho alpine (L.) Kuntze (Brassicaceae). Molec Ecol 12:931–949. doi:10.1046/j.1365-294X.2003.01781.x
Kultina VV, Spiridonova EA (1972) Findings of Helianthemum Mill. pollen in interstadial deposits of Valday glaciation in the north–west of Russian Plain. Bot Zhurn (Moscow & Leningrad) 57:1240–1252
Kupatadze GA (1978) A quantitative analysis of the Caucasian group “Helianthemum nummularium” and the taxonomic importance of quantitative characters [in Russian with English summary]. Byull Moskovsk Obshch Isp. Prir Otd Biol 83:98–107
Lesica P, Allendorf FW (1995) When are peripheral populations valuable for conservation? Conserv Biol 9:753–760. http://www.jstor.org.ludwig.lub.lu.se/stable/2386984
Litvinov DJ (1902) About the relict character of stony slopes in European Russia [in Russian]. Trav Mus Bot Acad St Petersburg 1:76–109
Malm JU, Prentice HC (2002) Immigration history and gene dispersal: allozyme variation in Nordic populations of the red campion, Silene dioica (Caryophyllaceae). Biol J Linn Soc 77:23–34. doi:10.1046/j.1095-8312.1999.00079.x
Nordström S, Hedrén M (2008) Genetic differentiation and postglacial migration of the Dactylorhiza majalis ssp. traunsteineri/lapponica complex into Fennoscandia. Pl Syst Evol 276:73–87. doi:10.1007/s00606-008-0084-1
Palmé AE, Su Q, Rautenberg A, Manni F, Lascoux M (2003) Postglacial recolonization and cpDNA variation of silver birch, Betula pendula. Molec Ecol 12:201–212. doi:10.1046/j.1365-294X.2003.01724.x
Parejo-Farnés C, Albaladejo RG, Juan Arroyo J, Aparicio A (2013) A phylogenetic hypothesis for Helianthemum (Cistaceae) in the Iberian Peninsula. Bot Complutensis 37:83–92. doi:10.5209/rev_BOCM.2013.v37.42272
Petit RJ, Aguinagalde I, de Beaulieu JL, Bittkau C, Brewer S, Cheddadi R, Ennos R, Fineschi S, Grivet D, Lascoux M, Mohanty A, Muller-Starck G, Demesure-Musch B, Palmé A, Martin JP, Rendell S, Vendramin GG (2003) Glacial refugia: hotspots but not melting pots of genetic diversity. Science 300:1563–1565. doi:10.1126/science.1083264
Petit RJ, Duminil J, Fineschi S, Hampe A, Salvini D, Vendramin GG (2005) Comparative organization of chloroplast, mitochondrial and nuclear diversity in plant populations. Molec Ecol 14:689–701. doi:10.1111/j.1365-294X.2004.02410.x
Prentice HC, Andersson S, Månsby E (2011) Mosaic variation in allozyme and plastid DNA markers in the European ranges of Silene vulgaris and its partially sympatric relative S. uniflora (Caryophyllaceae). Bot J Linn Soc 166:127–148. doi:10.1111/j.1095-8339.2011.01128.x
Prisyazhnyuk VE (2009) Red data list of rare and endangered species of animals and plants, particularly protected in Russia, Draft Version [in Russian]. All-Russian Research Institute of Nature Protection, Moscow
Proctor MCF (1956) Helianthemum Mill. J Ecol 44:675–692
Proctor MCF (1958) Ecological and historical factors in the distribution of the British Helianthemum species. J Ecol 46:349–371
Proctor MCF, Heywood VH (1968) Helianthemum. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (eds) Flora Europaea, 2nd edn. Cambridge University Press, Cambridge, pp 286–291
Pyhäjärvi T, Salmela MJ, Savolainen O (2008) Colonization routes of Pinus sylvestris inferred from distribution of mitochondrial DNA variation. Tree Genet Genomes 4:247–254. doi:10.1007/s11295-007-0105-1
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available at: http://www.R-project.org/
Ronikier M, Cieslak E, Korbecka G (2008a) High genetic differentiation in the alpine plant Campanula alpine Jacq. (Campanulaceae): evidence for glacial survival in several Carpathian regions and long-term isolation between the Carpathians and the Alps. Molec Ecol 17:1763–1775. doi:10.1111/j.1365-294X.2008.03664.x
Ronikier M, Costa A, Aquilar JF, Feliner GN, Küpfer P, Mirek Z (2008b) Phylogeography of Pulsatilla vernalis (L.) Mill. (Ranunculaceae): chloroplast DNA reveals two evolutionary lineages across central Europe and Scandinavia. J Biogeogr 35:1650–1664. doi:10.1111/j.1365-2699.2008.01907.x
Simmons MP, Ochoterena H, Carr TG (2001) Incorporation, relative homoplasy, and effect of gap characters in sequence-based phylogenetic analyses. Syst Biol 50(3):454–462. doi:10.1080/10635150120427
Soubani E, Hedrén M, Widén B (2014) Phylogeography of the European rock rose Helianthemum nummularium (Cistaceae): incongruent patterns of differentiation in plastid DNA and morphology. Bot J Linn Soc 176:311–331. doi:10.1111/boj.12209
Soubani E, Hedrén M, Widén B (2015) Genetic and morphological differentiation across a contact zone between two postglacial immigration lineages of Helianthemum nummularium (Cistaceae) in southern Scandinavia. Pl Syst Evol 301:1499–1508. doi:10.1007/s00606-014-1170-1
Stehlik I, Schneller JJ, Bachmann K (2002) Immigration and in situ glacial survival of the low-alpine Erinus alpinus (Scrophylariaceae). Biol J Linn Soc 77:87–103. doi:10.1046/j.1095-8312.2002.00094.x
Stewart JR, Lister AM (2001) Cryptic northern refugia and the origin of modern biota. Trends Ecol Evol 16:608–613. doi:10.1016/S0169-5347(01)02338-2
Svendsen JI, Alexanderson H, Astakhov VI, Demidov I, Dowdeswell JA, Funder S, Gataullin V, Henriksen M, Hjort C, Houmark-Nielsen M, Hubberten HW, Ingólfsson Ó, Jakobsson M, Kjær KH, Larsen E, Lokrantz H, Lunkka JP, Lyså A, Mangerud J, Matiouchkov A, Murray A, Möller P, Niessen F, Nikolskaya O, Polyak L, Saarnisto M, Siegert C, Siegert MJ, Spielhagen RF, Stein R (2004) Late Quaternary ice sheet history of northern Eurasia. Quaternary Sci Rev 23:1229–1271. doi:10.1016/j.quascirev.2003.12.008
Taberlet P, Fumagalli L, Wust-Saucy AG, Cosson JF (1998) Comparative phylogeography and postglacial colonization routes in Europe. Molec Ecol 7:453–464. doi:10.1046/j.1365-294x.1998.00289.x
Takahashi S, Furukawa T, Asano T, Terajima Y, Shimada H, Sugimoto A, Kadowaki K (2005) Very close relationship of the chloroplast genomes among Saccharum species. Theor Appl Genet 110:1523–1529. doi:10.1007/s00122-005-1990-z
Templeton AR, Crandall KA, Sing CF (1992) A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics 132:619–633
Tollefsrud MM, Sønstebø JH, Brochmann C, Johnsen Ø, Skrøppa T, Vendramin GG (2009) Combined analysis of nuclear and mitochondrial markers provide new insight into the genetic structure of North European Picea abies. Heredity 102:549–562. doi:10.1038/hdy.2009.16
Treier U, Müller-Schärer H (2011) Differential effects of historical migration, glaciations and human impact on the genetic structure and diversity of the montane pasture weed Veratrum album L. J Biogeogr 38:1776–1791. doi:10.1111/j.1365-2699.2011.02516.x
Tribsch A, Schönswetter P (2003) Patterns of endemism and comparative phylogeography confirm palaeoenvironmental evidence for Pleistocene refugia in the Eastern Alps. Taxon 52:477–497
Tyler T, Prentice HC, Widén B (2002) Geographic variation and dispersal history in Fennoscandian populations of two forest herbs. Pl Syst Evol 233:47–64. doi:10.1007/s006060200054
Tzedakis PC, Emerson BC, Hewitt GM (2013) Cryptic or mystic? Glacial tree refugia in northern Europe. Trends Ecol Evol 28:696–704. doi:10.1016/j.tree.2013.09.001
van Kolfschoten T, Markova A, Simakova A, Puzachenko A (2008) The ecosystems during the Bølling-Allerød interstadial complex (BAIC). In: Markova A, van Kolfschoten T (eds) Evolution of the European ecosystems during pleistocene–holocene transition (24–8 k year BP) [in Russian with English summary and figure captions]. KMK Sci Press, Moscow, pp 473–478
Van Rossum F, Prentice HC (2004) Structure of allozyme variation in Nordic Silene nutans (Caryophyllaceae): population size, geographical position and immigration history. Biol J Linn Soc 81:357–371. doi:10.1111/j.1095-8312.2003.00301.x
Vasari Y, Vasari A (1999) Helianthemum nummularium (L.) Mill. in the Karelian Republic, Russian Federation. Memoranda Soc Fauna Fl Fenn 75:39–47
Widén B (1986) Biosystematics in the Helianthemum oelandicum complex on Öland. Symb Bot Upsal 27:53–60
Widén B (2015) Genetic basis of a key character in Helianthemum nummularium. Pl Syst Evol 301:1851–1862. doi:10.1007/s00606-015-1198-x
Wolfe AD, Elisens WJ (1995) Evidence of chloroplast capture and pollen mediated gene flow in Penstemon sect. Peltanthera (Scropulariaceae). Syst Bot 20:395–412
Young ND, Healy J (2003) GapCoder automates the use of indel characters in phylogenetic analysis. BMC Bioinformatics 4:6. doi:10.1186/1471-2105-4-6
Yuzepchuk SV (1974) Helianthemum Adans. In: Shishkin BK (ed), Flora of the USSR, v 15 (translated from Russian), Israel Program for Scientific Translations, Jerusalem, pp 248–260
Acknowledgments
P.V. dedicates this paper to the memory of the deceased Dr. A. S. Koryakin, deputy director of Kandalaksha Nature Reserve, who always inspired our studies of northern nature. We are grateful to C. Gilli, P. Mejias, U. Meve, D. Reich, R. Natcheva and T. Reitalu who provided some plant material, to L. Abramova, V. Antonov, P. Kurochkin and N. Sajfullina (Shulgan-Tash Nature Reserve) for help in the field, to A. Kuznetsov for his help in the lab, and three anonymous referees for comments on the manuscript. We are also grateful to the seed exchange programme among botanical gardens in Europe. We acknowledge CommunicAID for language review and revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The work was supported by Sovremennoje Estestvoznanije Foundation, the Biodiversity Program of the Russian Academy of Sciences, a Grant from the Ministry of Science and Education, Russian Federation, no. 16.51811.7076, a Grant from the Russian Fund for Basic Research, no. 15-29-02486, and a Grant from the Swedish Research Council, no. 60290501.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Hervé Sauquet.
Appendices
Appendix
Some names used for the yellow-flowered morphs in the Helianthemum nummularium s.l. according to the International Plant Names Index (IPNI), Janchen (1909), Proctor and Heywood (1968) and Yuzepchuk (1974).
IPNI | Janchen (1909) | Yuzepchuk (1974) | Proctor and Heywood (1968) |
|---|---|---|---|
H. nummularium Mill. | H. nummularium (L.) Dunal. | H. nummularium (L.) Dunal. | H. nummularium (L.) Mill. subsp. nummularium |
H. obscurum Pers. | H. hirsutum (Thuill.) Mérat. | H. hirsutum (Thuill.) Mérat. | H. nummularium subsp. obscurum (Čelak.) Holub |
H. grandiflorum DC | H. grandiflorum (Scop.) Lam. et DC. | H. grandiflorum (Scop.) Lam. et DC | H. nummularium subsp. grandiflorum (Scop.) Schinz & Thell. |
H. glabrum Kerner | H. nitidum Clem. | H. nitidum Clem. included in H. grandiflorum | H. nummularium subsp. glabrum (Koch) Wilczek |
H. tomentosum Gray | H. tomentosum (Scop.) Spreng. | H. tomentosum (Scop.) Spreng | H. nummularium subsp. tomentosum (Scop.) Schinz & Thell. |
H. arcticum Janch. | H. arcticum (Grosser) Janch. included in H. nummularium | H. arcticum (Guss.) Juz. included in H. nummularium subsp. nummularium |
Information on Electronic Supplementary Material
Online resource 1. Matrix of sequence data for haplotypes.
Online resource 2. Matrix of sequence data for individual samples.
Rights and permissions
About this article
Cite this article
Volkova, P.A., Schanzer, I.A., Soubani, E. et al. Phylogeography of the European rock rose Helianthemum nummularium s.l. (Cistaceae): western richness and eastern poverty. Plant Syst Evol 302, 781–794 (2016). https://doi.org/10.1007/s00606-016-1299-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-016-1299-1