Abstract
An in-situ approach is described for synthesis of poly(sulfobetaine-co-polyhedral oligomeric silsesquioxane) [poly(sulfobetaine-co-POSS)] that can be used in a hybrid monolithic column as a hydrophilic liquid chromatography (HILIC) stationary phase. Synthesis involves (a) radical polymerization of octa(propyl methacrylate)-polyhedral oligomeric silsesquioxane (MA-POSS) and organic monomers such as dimethylaminopropyl methacrylate or vinyl imidazole, and (b) in-situ ring-opening quaternization between 1,4-butane sultone and the organic monomers. The sulfobetaine groups are generated in-situ monolith. This obviates the need for synthesis of sulfobetaine monomer previously. The pore size and permeability of the material can be tuned by using a binary porogenic system (polyethyleneglycol 600 and acetonitrile) and via the composition of the polymerization mixture. The optimized hybrid monolith owns its merits to the presence of POSS and sulfobetaine groups with good mechanical stability, the lack of residual silanol groups, and adequate hydrophilicity. The column filled with the monoliths was evaluated as a stationary phase for HILIC. Several kinds of polar compounds (including nucleosides, bases, phenols, aromatic acids and amides) were separated by using mobile phases with high organic solvent fractions in capillary liquid chromatography.

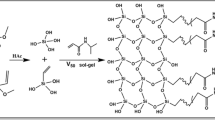
An in-situ approach is described for synthesis of poly(sulfobetaine-co-polyhedral oligomeric silsesquioxane) hybrid monolithic column for use in hydrophilic liquid chromatography. The optimized monolith owns good mechanical stability, the lack of residual silanol groups and adequate hydrophilicity. Baseline separation of several kinds of polar compounds is achieved on the column. MA-POSS: octa(propyl-methacrylate) polyhedral oligomeric silsesquioxane; DMAEMA: dimethylaminoethyl methacrylate; AIBN: azodiisobutyronitrile. Poly(DMABS-co-POSS): poly(N-(4-sulfobutyl)-N-methacryloxypropyl- N,N-dimethylammonium-betaine-co-polyhedral oligomeric silsesquioxane).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Monolithic stationary phases have been widely applied in drug separation [1], bioanalysis [2], proteomics [3, 4], and metabolomics [5] due to their facile preparation, fast mass transfer, low backpressure and easy modification [4, 6,7,8,9]. Monolithic station phase can be divided into four major groups according to the difference of separation mode, including reverse phase liquid chromatography (RPLC), hydrophilic interaction liquid chromatography (HILIC), and ion exchange (IEC), as well as affinity chromatography (AIC) [10,11,12]. The difference of separation mode of monolithic column depends heavily on the specific functional groups existing on the surface of monolith. Consequently, the functionalization of monolithic column was vital important for monolithic column synthesis.
For facile construction of functional monoliths, several kinds of strategies have been presented for introducing functional groups [10, 11, 13,14,15,16]. In past several decades, post-polymerization modification has become a common technique in the preparation of monolithic separation media with specific functional groups. Generally, two steps were often essential, including the synthesis of generic monolith with reactive groups, such as amine, epoxy and so on, and the post-modification processes. Several kinds of monolithic columns including IEC [17], AIC [18], HILIC [19], RPLC [20], and chrial chromatography [21], were fabricated by this method. Besides, a three-step approach has also been reported to synthesis a monolithic material with dual functionality by Kebe, S. I. et al. [22]. However, the multi-step operations and the time-consuming processes seriously limit the potential for actual use.
To simplify the synthesis step, co-polymerization of the functional monomers (commercial available or synthesized in laboratories) and cross-linker has been widely used to introduce functional groups in organic monolith. For example, RP monolith was synthesized by co-polymerization of poly(ethylene glycol) dimethacrylate (PEGDMA) [23, 24], butylmethacrylate (BMA)/ ethylenedimethacrylate (EDMA) [25], 1,2,4-trivinylcyclohexane/ pentaerythriol tetra(3-mercaptopropionate) [26], dipentaerythritol penta−/hexa-acrylate/ lauryl methacrylate [27] in presence of an appropriate porogen system. However, the lack of commercial functional monomers and the difficulty in monomer synthesis have impeded the further development of this strategy.
Structural stability was a critical issue that needs to be paid attention, because it determines the durability and pressure resistance of monolith. Hybrid monolithic columns which combine both merits of organic and inorganic monolithic column, are receiving more and more attention from researchers in separation materials [3, 4, 7]. “One pot” sol-gel route was firstly presented by Wu etc. [28], and it has been proved to be an ingenious way to construct functionalized hybrid monolithic column combinating the advantages of inorganic and polymer monolith. In this method, traditional sol-gel pre-polymerization solution was firstly mixed with polar functional monomer and initiator, then it was introduced into the confines of a capillary. The polycondensation of alkoxysilanes and the copolymerization of organic monomers with silane coupling agent were subsequently carried out at the proper reaction conditions, thus leading to the formation of the inorganic backbone and the introduction of organic functional groups synchronously. This method successfully overcomes the shortages of commercial functional silane in organic-silica hybrid monolith synthesized by a traditional way, and presents a new way to prepare the organic-silica hybrid monoliths by using a variety of available organic monomers. In subsequent years, many kinds of polar groups functionalized organic-silica hybrid monoliths including cation [8, 29], anion [30], saccharides [31], zwitterions [32, 33] and boronate affinity [13, 34] type, were synthesized through this method. As a strong polar zwitterions molecular, sulfo-betaine group has been widely applied in stationary phase modification to prepare polar monolithic stationary phase. According to different skeletons, they can be divided into organic skeleton and hybrid skeleton. Organic skeleton was synthesized by co-polymerization of cross linker (such as ethylene dimethacrylate (EDMA), N,N′-methylene-bisacrylamide) and sulfo-betaine monomer (such as N,N- dimethyl -N-acryloyloxyethyl-N-(3-sulfopropyl) ammonium betaine (SPDA), N,N-dimethyl -N-methacryloxyethyl-N-(3-sulfopropyl)ammonium betaine (SPE)) [35,36,37].The major limitation of these organic skeletons is that they generally have poor stability under high organic mobile phase, which can be overcome by introduction of inorganic skeleton as reported by our previous work [32, 38]. In these work, sulfo-betaine monomer (such as SPE, SPDA or homemade gemini type sulfo-betaine monomer) and initiator were added into traditional sol-gel pre-polymerization solution prepared by hydrolysis of tetramethyloxysilane and vinyltrimethoxysilane or γ-methacryloxypropyltrimethoxysilane (γ-MAPS) in an acetic acid solution. The sulfobetaine group was linked to the inorganic skeleton via radical and condensation polymerization. The monoliths showed good stability due to the existence of inorganic skeleton. Nevertheless, tedious operation and complex recipe optimization processes limited their application to some degree.
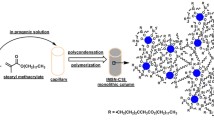
Here, as shown in Fig. 1, a novel “one-pot” method via an “in-situ functionalization strategy was proposed for synthesis of poly(sulfobetaine-co-POSS) hybrid monolith. In this method, octa(propyl-methacrylate) polyhedral oligomeric silsesquioxane (MA-POSS) was served as hybrid crosslinker, and dimethylaminoethyl methacrylate (DMAEMA) (or vinyl imidazole) and 1,4-butane sultone (1,4-BS) served as functionalized reagent. In the presence of porogen (PEG600/ACN) and initiator (azodiisobutyronitrile, AIBN), the monolith was synthesized by thermal initiated copolymerization of MA-POSS and DMAEMA. Synchronously, strong polarity function groups, sulfo-betaine groups were in situ generated via the ring opening quaternisation between DMAEMA and 1,4-BS. The recipe of pre-copolymerization mixture solution and polymerization temperature were investigated in detail. The optimized column was further examined by Infrared (FT-IR & IR) Spectroscopy, scan electron microscope (SEM), thermogravimetric analysis (TGA), as well as permeability test. The hydrophilic separation performance and the retention mechanism of the column were evaluated using five kinds of polar compounds including nucleosides, bases, phenols, aromatic acids and amides as probes. Furthermore, the separation of bovine serum albumin (BSA) digest was performed on the poly(sulfobetaine-co-POSS) hybrid monolithic column to evaluate the potential application in complex sample analysis.
Materials and experiments
Materials and chemicals
Dimethylaminoethyl methacrylate (DMAEMA) (≥98%), 1,3-propanesultone (1,3-PS, ≥98%), 1,4-BS (≥98%), N-(4-sulfobutyl)-N-methacryloxypropyl-N,N-dimethylammonium-betaine (DMABS, ≥98%), vinyl imidazole (VIDZ) (≥98%), AIBN (99%), 1-vinyl-3-(4-sulphobutyl)imidazolium betaine (VSBIB, ≥98%), dithiothreitol (DTT), iodoacetamide (IAA), acetonitrile (ACN, HPLC grade, ≥ 99.93%) and poly(ethylene glycol) (PEG, Mn = 600) were purchased from Aladdin Reagentshttps://www.aladdin-e.com/). MA-POSS (98%) was obtained from Aldrich (https://www.sigmaaldrich.com/canada-english.html). Fused-silica capillary column with 200 μm i.d. and 375 μm o.d were purchased from the Reafine Chromatography LTD (http://www.rui-feng.com/). All other reagents were of analytical grade and were used as received (https://www.reagent.com.cn/). All water was deionized using a Milli-Q synthesis A10 water purification system (http://www.milliq.cn/), and degassed by ultrasonic for 10 min prior to use.
Pretreatment of the fused-silica capillary
Prior to the fabrication of the poly(sulfobetaine-co-POSS) hybrid monolithic column, the fused-silica capillary was pretreated according to our previous method, and the programs of this method were as follows: 1). The fused-silica capillary (200 μm i.d., 365 μm o.d.) was sequentially rinsed with 1.0 M NaOH for 12 h, water for 4 h, 1.0 M HCl for 12 h, water for 4 h, and then dried by a nitrogen stream at room temperature to expose the surface silanol groups; 2). The fused-silica capillary was filled with a 20% γ-MAPS methanol (v/v) solution, and then reacted in a water bath at 50 °C for 12 h with both ends sealed to modified inner wall of capillary with vinyl groups. After that, the capillary was rinsed with methanol to remove residuals, and dried by a nitrogen stream. Both ends of capillary were sealed with silicone rubbers for further use.
The synthesis of poly(DMABS-co-POSS) hybrid monolithic column
Figure 1 illustrates the fabrication of poly(DMABS-co-POSS) hybrid monolithic column. Four steps were included in the synthesis of the monolithic column. Firstly, pre-polymerization solution was prepared by mixing DMAEMA, 1,4-BS, AIBN, and MA-POSS to form a homogeneous solution, and then dissolved oxygen was removed in a sonic bath for 10 min. Secondly, the modified capillaries were filled with pre-polymerization mixtures by a syringe, then both ends were sealed with silicone rubbers, and the mixtures were polymerized in a water bath at 60 °C for 12 h. Thirdly, the capillary columns were incubated at 70 °C for another 6 h to improve the efficiency of sulfonylation on surface of the monoliths. Finally, the monoliths were subsequently flushed with water and methanol to remove un-reacted monomers and porogen. Bulk monoliths for thermogravimetric analysis (TGA), infrared spectrum analysis and pore distribution measurements were prepared polymerization under equivalent conditions by mixing cross-linker, monomer, porogen and initiator in a centrifuge tube. After polymerization, the bulk monoliths were cut into small pieces, soaked in methanol to remove un-reacted monomers and porogen, and then dried by a nitrogen stream at room temperature.
A generic monolithic column was also prepared using MA-POSS as a monomer via a radical copolymerization strategy. To acquire an optimized POSS-based hybrid monolithic column, a variety of pre-polymerization mixture feed recipes and polymerization temperatures were investigated in detail.
Characterization of the monoliths
For the examination of the structure of the resulting monolithic columns, an optical microscopy equipped with a camera was used. Microscopy pictures were taken with a magnification of 40 times. In addition to optical microscopy, SEM images were obtained with a JEOL JSM-6490 SEM (JEOL, Tokyo, Japan) to observe the microscopic morphology of the monolithic columns.
For the bulk material, the pre-polymerization mixtures (with and without 1,4-BS) were polymerized in 5 mL centrifuge tubes at 60 °C water bath for 12 h. After polymerization, the bulk monoliths were cut into smaller pieces, extracted with water and ACN successively overnight and dried in a vacuum. The dried bulk monoliths were then analyzed by FT-IR spectrometer, Thermo gravimetric analyzer and mercury porosimetry to obtain the chemical structure and pore distribution information. FT-IR spectra were measured by Avatar 370 FT-IR spectrometer (Thermo Nicdet, China). The bulk monolithic matrixes were thoroughly mixed with KBr and pressed into a flaky form before FT-IR experiments. Pore size measurement of dry bulk monoliths was carried out on a PoreMasre GT-60 (Quantachrome, Boynton Beach, USA). Thermal gravimetric analysis was performed on a DTG-60 (Shimadzu Corporation, Japan).
Permeability tests of monolithic columns were performed on a Shimadzu 10AT pump. The precise flow rate was measured by determining the volume of effluent at the outlet of the monolithic column in a certain period of time. The permeability was calculated according to Darcy’s Law [39] by the equation, B0 = FηL/(πr2ΔP), where F (m3·s−1) is the flow rate, η is the viscosity of mobile phase (0.34 cP [40] for ACN/water = 92/8, v/v), L and r (m) are effective length and inner radius of the column, respectively, P (Pa) is the pressure drop of column.
Capillary liquid chromatography analysis
The chromatographic evaluations of hybrid monolithic columns were performed with a homemade HPLC system consisting of a binary LC-20 AD pump (Shimadzu, Tokyo, Japan), a UV detector (Cailu, Beijing, China) and a an Rheodyne Model 7725 injector (Cotati, Calif, USA) with a 20 μL sample loop. The outlet of the injector was connected to a T-union connector. One outlet was connected to the capillary monolithic column, and the other outlet to a generic capillary, which severed as splitting capillary, and the split ratio was set at about 1/200 by using 100-cm long generic capillary (75 μm i.d. and 365 μm o.d.). The outlet of monolith connected to a generic capillary (75 μm i.d. and 365 μm o.d.) by a short, small diameter Teflon Tube, and the detection cell was made by burning off 5 mm of polyimide coating on this generic capillary 10 cm from the inlet. The data were acquired at 214 nm as well as 254 nm, and processed by a chromatography workstation HW-2000 from Qianpu Software (Shanghai, China). All Chromatographic experiments were carried out at room temperature. The retention factor (k) was defined as (tR − t0)/t0, where tR represents the retention times of the analytes, and t0 represents the retention times of the dead volume marker. Due to co-elute with the unretained sample solvent in HILIC mode, toluene was selected as a dead time marker in our experiment. A binary solvent containing appropriate mixed ratio of ACN and H2O (or salt) was applied to study chromatographic behaviors of the resulting monoliths.
Results and discussion
The synthesis of poly(DMABS-co-POSS) hybrid monolithic column
The following parameters were optimized: (a) The amount of PEG600; (b) ACN; (c) 1,4-BS; (d) MA-POSS; (e) DMAEMA; (f) temperature. Respective data and Figures are given in the Electronic Supplementary Material. It was found that a mixture solution, including PEG600 (53.3 mg), ACN (106.7 mg), 1,4-BS (24 mg), MA-POSS (17.5 mg) and DMAEMA (20 mg), polymerized at 60 °C for 12 h and then at 70 °C for another 6 h, leading to the optimized monolith (as shown in Table S1).
The optimized monolith showed homogeneous monolithic matrix without any matrix-inner wall detaching phenomenon (Fig. 2a). The pore distribution measurement demonstrated that the centralized pore distribution of this monolith was in the range of 0.1~4 μm (Fig. S2), which would enhance the mass transfer efficiency, and be beneficial to separation performance.
To verify whether the betaine groups were introduced successfully, TGA and FT-IR were performed. TGA curve of poly(DMABS-co-POSS) hybrid monolith shows a similar stable zone and step of weight loss with generic monolith, when the temperature was below 400 °C (Fig. S3), corresponding to the decomposition of the organic polymer backbone. While the temperature was above 400 °C, the poly (DMABS-co-POSS) hybrid monolith was further broken down with a weight loss of 20%, but the generic monolith was decomposed any further. These TGA results indicated the organic-inorganic hybrid monolith was indeed obtained. The actual organic groups were further confirmed by the FT-IR analysis. As shown in Fig. 2b, the obvious absorption band at 1040 cm−1, 612 cm−1 and 530 cm−1 (-SO3) suggest the presence of sulfone groups synthesized by the ring-open quaternization reaction of DMAEMA with 1,4-BS. The absorption band at 1717 cm−1 was assigned to the -Si-O-Si- stretching vibration in POSS skeleton. A sharp intensity band around 1725 cm−1 was attributed to the stretching frequency of C=O on MA-POSS and DMAEMA. The board absorption band at 3445 cm−1 was assigned to the stretching vibration of -OH. The absorption band at 1476 cm−1 was assigned to the bending vibration of -CH2-. These results clearly confirmed that the sulfobetaine groups were successfully introduced in monolith with a higher proportion, which would provide the hybrid monolith with good hydrophilicity.
Characterization of column performance
Owing to the abundant hydrophilic groups and the homogeneous porous microstructure, the poly(DMABS-co-POSS) monolith was used for separation of hydrophilic analytes in capillary liquid chromatography. To evaluate the separation performance, a mixture sample containing toluene, allylthiourea, and thiourea was separated on the hybrid monolith. As shown in Fig. 3a, a baseline separation of these compounds with shape peaks was achieved using a mobile phase of ACN/water (90/10, v/v). The peaks were eluted according to their polarity in order from low to high (toluene < allylthiourea < thiourea), which significantly exhibited a typical HILIC retention mechanism. To evaluate the column performance, the separation of the mixture sample was performed on the hybrid monolith column by varying the line velocity of mobile phase. The plots of column efficiency for toluene, allylthiourea and thiourea with linear velocities ranging from 0.4–1.5 mm·s−1 are displayed in Fig. 3b. The hybrid monolith exhibited high column efficiencies. Notably, the highest column efficiency of 208,000 plates·m−1 for thiourea was achieved. Such high column efficiencies can be attributed to the high efficiency of mass transfer in homogeneous microstructure of the monolith, and the abundant sulfo-betaine groups.
a separation of toluene, allythiourea and thiourea on the poly(DMABS-co-POSS) hybrid monolithic column. b Van Demeter plots for toluene, allythiourea and thiourea. Separation conditions, column dimension, 25 cm-long, 200 μm i.d., 365 μm o.d, mobile phase, 90% ACN (v/v), detector wavelength, 214 nm, sample volume 20 μL (before split)
The run-to-run reproducibility was evaluated using a single poly(DMABS-co-POSS) hybrid monolithic column. As shown in Table S2, the relative standard deviations (RSDs) for retention time of analytes (toluene, allythiourea and thiourea) on the monolithic column were less than 2.3% for 5 runs. Both column-to-column and batch-to-batch reproducibility were also evaluated in terms of the RSDs for the retention times of three analytes, and RSDs were less than 4.2% (n = 5) and 5.6% (n = 3), respectively. These results indicated that the reproducibility of the poly(DMABS-co-POSS) hybrid monolithic columns was acceptable for quantitative analysis.
HILIC separation of polar molecular in capillary liquid chromatography
Several kinds of small polar analytes were used as probes to verify the hydrophilic separation performance of the monolithic column. Nucleosides are polar compounds of significant biological and pharmaceutical interest. They have been used extensively as model compounds for evaluating polar stationary phases in HILIC mode. Therefore, the mixture sample containing thymidine, adenosine, uridine, cytidine, inosine was used to evaluate the separation performance of monolithic column. Figure 4a shows the separation chromatogram of five nucleosides on the column, and excellent separation with baseline-separation and sharp peaks was achieved; The five nucleosides were eluted according to their polarities in order from low to high (toluene<thymidine< adenosine<uridine<cytidine<inosine). Figure 4b shows the plots of the retention factor with the ACN content in mobile phase, and all retention factors of five nucleosides were quickly increased with the increase of ACN content from 75%~95%. The highest retention factor of 6.8 was obtained for ionsine, which was much larger than those obtained on betaine-based monoliths reported in reference with similar conditions [32, 33]. These results demonstrated the excellent hydrophilicity and the potential of the hybrid monolith in analysis of nucleosides.
a Separation of nucleosides on the poly(DMABS-co-POSS) hybrid monolithic column. b The influence of ACN content in mobile phase on the retention factor of nucleosides. The concentrations of nucleosides was 120 ng·mL−1. Separation conditions: column dimension, 30 cm length, 200 μm i.d., 365 μm o.d., mobile phase used in A, 92% ACN aqueous solution; flow rate, 200 μL·min−1 (before split); detection wavelength, 214 nm, injection volume, 20 μL (before split)
The calibration plots for five nucleosides were also examined, and the results were summarized in Table S3. All the calibration plots exhibited good linear regressions in the range from 0.5 to 20 μg·mL−1, and the correlation coefficients of all the calibration plots were higher than 0.992, enabling the potential applications in quantitative analysis of nucleosides.
Apart from nucleosides, polar compounds, including bases, amides, phenols and aromatic acids are extensively adopted to evaluate hydrophilic separation performance of the monolith. Three bases were baseline separated using 90% ACN without any addition, and eluted according to the order of polarity from low to high (Fig. 5a), demonstrating the hydrophilic retention mechanism. Slight peak tailing and peak broadening suggested the existence of non-specific interactions between bases and monolithic surface. Amides and phenols were baseline separated with symmetrical and sharp peaks without any addictives in mobile phase (Fig. 5b and Fig. 5c), respectively. The performence was superior to our previous work [38]. Similarly, these compounds were also eluted according to their polarities in order from low to high. As shown in Fig. 5d, acidic compounds are also separated well, and the elution order is according to the difference of number and the position of the hydroxyl groups, as well as the difference of the substituted groups. It was notable that a mobile phase with 10 mM ammonium acetate was essential for the separation of charged acidic compounds, which suggested that electronic interactions can occur between the polymeric matrix and the charged compounds because of the existence of residual tertiary groups on the poly(DMABS-co-POSS) hybrid monolith. Besides, the effect of ACN content in the mobile phase on the retention factor was also investigated in detail. Retention factors (k) of bases, amides, phenols (except for pholoroglucinol), and aromatic acids increased with an increasing ACN concentration, when ACN concentration was larger than 50% (Fig. S4). The abnormal retention behavior of pholoroglucinol, when the ACN concentration was less than 80%, may be attributed to the unverified interaction between phenolic hydroxyl and the polar groups on the monolith surface. These results further confirmed the hydrophilic retention mechanism of the monolith.
Separation of bases (A, 90% ACN), amides (B, 90% ACN), phenols (C, 90% ACN), aromatic acids (D, 90% ACN with 10 mM ammonium acetate) on the poly(DMABS-co-POSS) hybrid monolithic column. The concentrations of bases were 150 ng·mL−1, and the concentrations of amides, phenols and aromatic acids were 80 ng·mL−1. Other separation conditions were the same as those shown in Fig. 4a except the ACN concentration
The effect of ammonium acetate concentration in mobile phase on retention factor was further examined in detail. As shown in Fig. 6a, with the increase of ammonium acetate concentration from 5 mM to 10 mM, the retention factors of nucleosides remain approximately constant, and in the range of 10~20 mM, slight increases of the retention factors are observed for five nucleosides. In contrast, as shown in Fig. 6b, significant decreases in retention factors of four substituted aromatic acids are observed as the increase of ammonium acetate concentration from 5 mM to 30 mM. This may be attributed to the ion exchange interaction between aromatic acids and the unreacted tertiary amine on poly(DMABS-co-POSS) hybrid monolith. Additionally, the effect of ammonium acetate concentration on retention factor for other three types of probe analytes, including amides, bases, and phenols, was also examined in detail. It was found that the retention factors of those analytes did not reduce as the increase of ammonium acetate concentration, but increased slightly (Fig. S5). The reason for these may need further detail investigation, due to the existence of complex interactions in HILIC mode. Therefore, the existence of ion exchange interaction suggested that the monolith had better separation ability for acidic analytes than other polar analytes.
Finally, the applicability of the poly(DMABS-co-POSS) hybrid monolith for separating complicated samples was evaluated by separation of a BSA digest with a gradient elution in capillary liquid chromatography. As shown in Fig. S6, at least 15 peaks were observed and baseline-separated, which enables the adaptability of the poly(DMABS-co-POSS) hybrid monolith for analysis of biological samples.
The synthesis of poly(VSBIB-co-POSS) hybrid monolithic column
To further validate the proposed strategy, another similar hybrid monolithic column was fabricated by using vinyl imidazole (VIDZ) instead of DMAEMA (Fig. S7). Due to the similar chemical property of VIDZ and DMAEMA, the synthetic recipes were optimized roughly based on rule displayed in Table S1, and the results are shown in Table S4, an optimized monolithic column with uniform structure and moderate permeability was obtained successfully (Fig. S8), when a recipe including MA-POSS (17.5 mg), 1,4-BS (28.8 mg), VIDZ (20 mg), AIBN (2 mg) and PEG600/ACN (195.5 mg, 1:3) was applied. The polymerization was performed at 60 °C for 12 h, and then at 70 °C for 6 h. The optimized monolithic column was further characterized by IR spectrum analysis. As shown in Fig. S9, the obvious absorption band at 1040 cm−1, 612 cm−1, 530 cm−1 (-SO3) indicate the successful introduction of sulfone groups. The absorption band at 3039 cm−1, 3135 cm−1 (assigned to the -C-H stretching vibration) and the weak absorption peak at 1630 cm−1, 1580 cm−1 (assigned to the skeletal vibration) suggest the presence of imidazole. The absorption band at 1717 cm−1 belongs to the -Si-O-Si- stretching vibration in POSS. A sharp intensity band around 1725 cm−1 is assigned to the stretching frequency of C=O on MA-POSS, and the board absorption band at 3445 cm−1 is assigned to the stretching vibration of -OH.
Separation of polar molecules
The separation performance of the column was studied in detail by using several kinds of polar moleculars as probe analyes. An excellent separation with sharp peaks was obtained for five nucleosides, which were eluted in order according to the polarity from weak to strong (toluene<thymidine <adenosine<uridine< cytidine< inosine) (Fig. 7a). Effect of organic solvent content in mobile phase on retention factor for five nucleosides was further examined, and the result indicated the retention behavior of nucleosides on the poly(VSBIB-co-POSS) hybrid monolithic column follow the typical HILIC retention mechanism (Fig. 7b). These results were consistent with those obtained on poly(DMABS-co-POSS) hybrid monolithic column. Much stronger retention can be obtained on poly(VSBIB-co-POSS) hybrid monolithic column when the same mobile phase was applied, possibly due to the higher polarity of 1-vinyl-3-(3-sulphopropyl) imidazolium betaine than 3-dimethyl (methacryloyloxyethyl) ammonium butane sulfonate. Finally, the poly(VSBIB-co-POSS) hybrid monolithic column was applied to the separation of other polar analytes. As shown in Fig. S10, well separation of bases, amides, phenols and aromatic acids were successfully achieved.
a Separation of nucleosides on the poly(VSBIB-co-POSS) hybrid monolithic column. The concentrations of nucleosides was 120 ng·mL−1. Column dimension, mobile phase (mobile phase, 90% ACN); (b) The influence of ACN concentration on the retention factor of nucleosides on poly(VSBIB-co-POSS) (solid icon) and poly(DMABS-co-POSS) hybrid monolithic column (hollow Icon). Other separation conditions were the same as shown in Fig. 4a
Comparison with other HILIC stationary phases
In the previous work, various nanomaterial-based HILIC systems have been successfully applied to separate similar or identical compounds. To further demonstrate the superiority of our method, we made a comparison between our method and previous reported literature. As shown in Table S5, high hydrophilicity and column efficiency in our system were obtained, although the RSDs of reproducibility are slightly highter than MSA Monoliths.
On the other hand, comparing to the traditional method, since the ring-opening reaction carries out in-situ, the two-step high-temperature process was applied to improve the conversion rate of ring-opening reaction, but it is difficult to ensure that 100% conversion rate was achieved. Besides, another limitation is that it is hard to prepare a monolithic column with a large inner diameter due to the swelling effect. Therefore, the maximum allowed injection volume is seriously limited than conventional packed columns.
Conclusion
To simplify the preparation of monoliths, a new strategy was proposed to synthesize the poly(sulfobetaine-co-POSS) hybrid monolith by combination of radical polymerization and ring open quaterisation reaction. Compared to traditional methods, this strategy represented a new way to prepare functionalized monoliths by in situ synthesis without purchase or synthesis of functional monomer in advance. The optimized column was fabricated with homogeneous bicontinuous porous microstructure, good permeability and mechanical stability by carefully optimizing the synthesis conditions. The selection of sulfonate reagent, butyrolactone sulfonate was the critical element for successful synthesis of hybrid column, due to the temperature sensitive in-situ synchronous sulfonated process. Highly effective introduction of sulfobetine groups enabled excellent separation ability for several kinds of small polar compounds, including nucleosides, phenols, amides, and bases as well as complex samples such as a BSA digest. The excellent separation ability and the feasibility of synthesis method suggested the hybrid monolithic column can be applied in routine laboratories and held great promise for potential application in separation of a variety of polar analytes.
References
Aboul-Enein HY, Imran A (2005) Determination of tadalafil in pharmaceutical preparation by HPLC using monolithic silica column. Talanta 65:276–280
Altun Z, Skoglund C, Abdel-Rehim M (2010) Monolithic methacrylate packed 96-tips for high throughput bioanalysis. J Chromatogr A 1217:2581–2588
Liu ZS, Ou JJ, Lin H, Liu ZY, Wang HW, Dong J, Zou HF (2014) Photoinduced thiol-ene polymerization reaction for fast preparation of macroporous hybrid monoliths and their application in capillary liquid chromatography. Chem Commun 50:9288–9290
Wu MH, Wu RA, Li RB, Qin HQ, Dong J, Zhang ZB, Zou HF (2010) Polyhedral oligomeric silsesquioxane as a cross-linker for preparation of inorganic-organic hybrid monolithic columns. Anal Chem 82:5447–5454
Maruška A, Kornyšova O (2006) Application of monolithic (continuous bed) chromatographic columns in phytochemical analysis. J Chromatogr A 1112:319–330
Chen YZ, Wang KY, Yang HH, Liu YX, Yao SZ, Chen B, Nie LH, Xu GM (2012) Synthesis of sulfo/vinyl biphasic silica hybrid monolithic capillary column and its application to on-column preconcentration for capillary electrochromatography. J Chromatogr A 1233:91–99
Liu YX, Chen YZ, Yang HH, Nie LH, Yao SZ (2013) Cage-like silica nanoparticles-functionalized silica hybrid monolith for high performance capillary electrochromatography via “one-pot” process. J Chromatogr A 1283:132–139
Lv XM, Tan WM, Chen Y, Chen YZ, Ma M, Chen B, Yao SZ (2016) Facile “one-pot” synthesis of poly(methacrylic acid)-based hybrid monolith via thiol-ene click reaction for hydrophilic interaction chromatography. J Chromatogr A 1454:49–57
Wu MH, Chen YZ, Wu RA, Li RB, Zou HF, Chen B, Yao SZ (2010) The synthesis of chloropropyl-functionalized silica hybrid monolithic column with modification of N,N-dimethyl-N-dodecylamine for capillary electrochromatography separation. J Chromatogr A 1217:4389–4394
Zheng C, Huang YP, Liu ZS (2011) Recent developments and applications of molecularly imprinted monolithic column for HPLC and CEC. J Sep Sci 34: 1988–2002
Ou JJ, Dong J, Dong XL, Yu ZY, Ye ML, Zou HF (2010) Recent progress in polar stationary phases for CEC. Electrophoresis 28:148–163
Tong S, Liu S, Wang H, Jia Q (2014) Recent advances of polymer monolithic columns functionalized with micro/Nanomaterials: synthesis and application. Chromatographia 77:5–14
Li HY, Liu Z (2012) Recent advances in monolithic column-based boronate-affinity chromatography. Trends Anal Chem 37:148–161
Song XL, Chen LX, Ma JP, Luo GA, Zhu HY, Ying RJ (2007) Recent Progress of research and application of monolithic chromatographic column. Phys Testing Chem Anal Part B: Chem Anal 43:12
Zhang ZB, Wu RA, Wu MH, Zou HF (2010) Recent progress of chiral monolithic stationary phases in CEC and capillary LC. Electrophoresis 31:1457–1466
Zhang ZB, Ou JJ, Lin H, Liu ZS, Zou HF (2013) Recent Progress in Preparation and Application of Organic-silica Hybrid Monolithic Columns. Chem J Chin Univ 34:2011–2019
Hutchinson JP, Hilder EF, Shellie RA, Smith JA, Haddad PR (2006) Towards high capacity latex-coated porous polymer monoliths as ion-exchange stationary phases. Analyst 131:215–221
Luo QZ, Zou HF, Xiao XZ, Guo Z, Kong L, Mao XQ (2001) Chromatographic separation of proteins on metal immobilized iminodiacetic acid-bound molded monolithic rods of macroporous poly(glycidyl methacrylate-co-ethylene dimethacrylate). J Chromatogr A 926:255–264
Guerrouache M, Pantazaki A, Millot MC, Carbonnier B (2015) Zwitterionic polymeric monoliths for HILIC/RP mixed mode for CEC separation applications. J Sep Sci 33:787–792
Guerrouache M, Carbonnier B, Vidal-Madjar C, Millot MC (2007) In situ functionalization of N -acryloxysuccinimide-based monolith for reversed-phase electrochromatography. J Chromatogr A 1149:368–376
Lin H, Ou JJ, Tang SW, Zhang ZB, Dong J, Liu ZS, Zou HF (2013) Facile preparation of a stable and functionalizable hybrid monolith via ring-opening polymerization for capillary liquid chromatography. J Chromatogr A 1301:131–138
Kebe SI, Boubaker MB, Guerrouache M, Carbonnier B (2016) Thiol-Ene click chemistry for the Design of Diol Porous Monolith with hydrophilic surface interaction ability: a capillary Electrochromatography study. New J Chem 40:6916–6923
Gu B, Armenta JM, Lee ML (2005) Preparation and evaluation of poly(polyethylene glycol methyl ether acrylate-co-polyethylene glycol diacrylate) monolith for protein analysis. J Chromatogr A 1079:382–391
Li Y, Tolley HD, Lee ML (2009) Poly[hydroxyethyl acrylate-co-poly(ethylene glycol) diacrylate] monolithic column for efficient hydrophobic interaction chromatography of proteins. Anal Chem 81:9416–9424
Geiser L, Eeltink S, Svec F, Fréchet JMJ (2007) Stability and repeatability of capillary columns based on porous monoliths of poly(butyl methacrylate-co-ethylene dimethacrylate). J Chromatogr A 1140:140–146
Chen LF, Ou JJ, Liu ZS, Lin H, Wang HW, Dong J, Zou HF (2015) Fast preparation of a highly efficient organic monolith via photo-initiated thiol-ene click polymerization for capillary liquid chromatography. J Chromatogr A 1394:103–110
Zhang H, Ou JJ, Wei Y, Wang HW, Liu ZS, Chen L, Zou HF (2015) A novel polymeric monolith prepared with multi-acrylate crosslinker for retention-independent efficient separation of small molecules in capillary liquid chromatography. Anal. Chim Acta 883:90–98
Wu MH, Wu RA, Wang FJ, Ren LB, Dong J, Liu Z, Zou HF (2009) "one-pot" process for fabrication of organic-silica hybrid monolithic capillary columns using organic monomer and alkoxysilane. Anal Chem 81:3529–3536
Yang HH, Chen YZ, Liu YX, Nie L, Yao SZ (2013) One-pot synthesis of (3-sulfopropyl methacrylate potassium)-silica hybrid monolith via thiol-ene click chemistry for CEC. Electrophoresis 34:510–517
Liang W, Wu M, Wang Q, Zhan J, Chen H (2016) Preparation of organic-silica hybrid monolith with anion exchange/hydrophilic interaction mixed-mode via epoxy–amine ring-opening polymerization using polyethylenimine as functional monomer. Chromatographia 79:1–7
Zhang ZB, Wu MH, Wu RA, Dong J, Ou JJ, Zou HF (2011) Preparation of perphenylcarbamoylated β-cyclodextrin-silica hybrid monolithic column with "one-pot" approach for enantioseparation by capillary liquid chromatography. Anal Chem 83:3616–3622
Chen YZ, Wang KY, Liu YX, Yang HH, Yao SZ, Chen B, Nie LH, Xu GM (2013) Improved sulfoalkylbetaine-based organic-silica hybrid monolith for high efficient hydrophilic interaction liquid chromatography of polar compounds. Electrophoresis 34:1877–1885
Lin H, Ou JJ, Zhang ZB, Dong J, Wu MH, Zou HF (2012) Facile preparation of zwitterionic organic-silica hybrid monolithic capillary column with an improved "one-pot" approach for hydrophilic-interaction liquid chromatography (HILIC). Anal Chem 84:2721–2728
Li Q, Lü C, Li H, Liu Y, Wang H, Wang X, Liu Z (2012) Preparation of organic-silica hybrid boronate affinity monolithic column for the specific capture and separation of cis-diol containing compounds. J Chromatogr A 1256:114–120
Liu C, Chen W, Yuan G, Yao X, Crommen J, Xu S, Jiang Z (2014) Influence of the crosslinker type on the chromatographic properties of hydrophilic sulfoalkylbetaine-type monolithic columns. J Chromatogr A 1373:73–80
Yuan G, Peng Y, Liu Z, Hong J, Xiao Y, Guo J, Smith NW, Crommen J, Jiang Z (2013) A facile and efficient strategy to enhance hydrophilicity of zwitterionic sulfoalkylbetaine type monoliths. J Chromatogr A 1301:88–97
Li F, Qiu D, Kang J (2017) Preparation of a Sulfoalkylbetaine-based Zwitterionic monolith with enhanced Hydrophilicity for capillary Electrochromatography separation applications. Chromatographia 80:1–7
Tan WM, Chang F, Shu Y, Chen Y, Liu JJ, Chen YZ, Ma M, Chen B (2017) The synthesis of Gemini-type sulfobetaine based hybrid monolith and its application in hydrophilic interaction chromatography for small polar molecular. Talanta 173:113–122
Wal SVD (1985) Low viscosity organic modifiers in reversed-phase HPLC. Chromatographia 20:274–278
Wode H, Seidel W (2010) Precise viscosity measurements of binary liquid mixtures of acetonitrile-water and 1,3-dimethyl-2-imidazolidinone-water. Zeitschrift für Elektrochemie, Berichte der Bunsengesellschaft für physikalische Chemie 98:927–934
Acknowledgements
This work was financially supported by the Natural Science Foundation of Hunan Province (2018JJ2251), the National Natural Science Foundation of China (21575040) and National Natural Science Foundation of China (81701356).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 5.78 mb)
Rights and permissions
About this article
Cite this article
Tan, W., Chen, Y., Xiong, X. et al. Synthesis of a poly(sulfobetaine-co-polyhedral oligomeric silsesquioxane) hybrid monolith via an in-situ ring opening quaternization for use in hydrophilic interaction capillary liquid chromatography. Microchim Acta 187, 109 (2020). https://doi.org/10.1007/s00604-019-4088-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-4088-z