Abstract
An ionic liquid hybrid monolithic capillary column was prepared within 7 min via photoinitiated free-radical polymerization of an ionic liquid monomer (1-butyl-3-vinylimidazolium-bis[(trifluoromethyl)sulfonyl]imide); VBIMNTF2) and a methacryl substituted polyhedral oligomeric silsesquioxane (POSS-MA) acting as a cross-linker. The effects of composition of prepolymerization solution and initiation time on the porous structure and electroosmotic flow (EOF) of monolithic column were investigated. The hybrid monolith was characterized by scanning electron microscopy and FTIR. Owing to the introduction of a rigid nanosized POSS silica core and ionic liquids with multiple interaction sites, the monolithic column has a well-defined 3D skeleton morphology, good mechanical stability, and a stable anodic electroosmotic flow. The hybrid monolithic stationary phase was applied to the capillary electrochromatographic separation of various alkylbenzenes, phenols, anilines and polycyclic aromatic hydrocarbons (PAHs). The column efficiency is highest (98,000 plates/m) in case of alkylbenzenes. Mixed-mode retention mechanisms including hydrophobic interactions, π-π stacking, electrostatic interaction and electrophoretic mobility can be observed. This indicates the potential of this material in terms of efficient separation of analytes of different structural type.

Preparation of a mixed-mode ionic liquid hybrid monolithic column via photoinitiated polymerization of methacryl substituted polyhedral oligomeric silsesquioxane (POSS-MA) and 1-butyl-3-vinylimidazolium-bis[(trifluoromethyl)sulfonyl]imide (VBIMNTF2) ionic liquid for use in capillary electrochromatography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Monolithic columns have emerged as an excellent substitute to conventional particle-packed columns, due to their higher permeability, lower flow resistance, comparable efficiency and fritless [1,2,3]. Polymer-based and silica-based monolithic columns have attracted comprehensive attention and widely applied in HPLC and microseparation. Particularly, the incorporation of monolith in capillary liquid chromatography (CLC) and capillary electrochromatography (CEC) offers additional advantages, such as low consumption in the sample and mobile phase, and good compatibility with efficient detection. However, a significant difference exists between these two types of monoliths in terms of the mechanical stability, the preparation process and the column efficiency for small molecules and biopolymers [4, 5]. Great efforts have been made to improve separation performance and mechanical properties of monoliths [6]. Since the first introduction in CEC by Malik’s group [7], the hybrid monolith prepared via sol–gel chemistry has been well developed by employing different organofunctional alkoxysilanes [8, 9]. It combines both merits of polymer-based and silica-based monolithic columns, including easy fabrication, satisfied separation efficiency, wide pH tolerance and better resistance to organic solvents [10]. To overcome the limitation originated from the limited types of organic-trialkoxysilanes and lengthy preparation, the Zou group [11, 12] proposed an “one-pot” approach by using organic monomers and alkoxysilanes simultaneously to fabricate the functionalized hybrid column in one step.
Polyhedral oligomeric silsesquioxane (POSS) is a kind of cagelike silsesquioxane with the general formula of (RSiO1.5)n (n ≥ 6), where R represents a range of organofunctionalized groups [13, 14]. It can be regarded as the smallest organic-silica hybrid nanoparticle with sizes from 1 to 3 nm in diameter. The reactive organofunctionalized groups arounding inherent polyhedral silicon-oxygen nanostructured skeleton provide the possibility for POSS to incorporate into polymeric networks. This will result in dramatic improvement in polymer properties, including physical, mechanical stability and oxidation resistance [15, 16]. A series of organic-silica hybrid monolithic columns based on POSS with different reactive groups (methyl acrylate group, acrylic ester group, vinyl group or epoxy group) as monomers or crosslinkers have been fabricated for chromatographic separation via free radical copolymerization, thiol-based reactions or ring-opening polymerization [7, 12, 14, 17,18,19,20]. POSS-based hybrid monoliths exhibited higher mechanical stability and good pH tolerance in comparison with other hybrid monoliths. Furthermore, the preparation process was relatively simpler than the sol–gel method, since no hydrolysis and condensation reaction of siloxane was needed. However, to enhance separation capability of analytes with polar or charged property, a time-consuming selection and optimization of functional monomers and composition of the pre-polymerization mixture were required, due to the low reaction activity and highly hydrophobic nature of most of POSS-based monomers. Sometimes surfactants should be added to promote the compatibility between hydrophobic monomer and aqueous prepolymerization mixture.
Ionic liquids (ILs) play an essential role in the development of cost-effective and environmental-safe extraction and separation processes [21]. Vinyl-functionalized ionic liquids (ILs) were especially exploited to prepare POSS-containing hybrid monolithic columns for HPLC [19]. The resulting POSS-IL hybrid monoliths exhibited less swelling propensity and multiple interactions, owing to the unique properties of ILs, including high thermal stability, low vapour pressure, tuneable viscosity, as well as high solvating capacity for organic compounds [22, 23]. A poly(POSS-MA-co-VBTA) column was prepared for CLC by thermally initiated free radical copolymerization of vinylbenzyltrimethyl-ammonium chloride (VBTA) and POSS-MA [24]. This column exhibited multiple retention mechanisms for both polar and nonpolar analytes, including cation exchange interaction, hydrophilic, π–interactions and hydrogen-bonding interaction. Xu et al. [25] utilized 1-vinyl-3- (per-fluorobenzyl)-imidazolium bromide (VIMPFP) and POSS-MA to fabricate a penta-fluorobenzyl imidazolium-modified POSS hybrid monolithic column (POSS-VIMPFP) for HPLC via “one-pot” thermally free radical polymerization, with the highest column efficiency of 72,000 plates/m. Mixed-mode retention mechanisms including hydrophobic interaction, π–π stacking, dipole-dipole interaction, anion-exchange and electrostatic interaction were observed for various kinds of compounds, such as alkylbenzenes, nucleosides and the halogenated compounds. By in-situ thermally copolymerization of 1-vinyl-3-octylimidazolium bromide (VOI) and POSS-MA, Qiao et al. [26] fabricated a high efficient imidazolium embedded C8 hybrid monolithic column for RP CLC separation of alkylbenzenes, polycyclic aromatic hydrocarbon and aromatic amines. To improve the potentially hypercrosslink degree of the monolith, they exploited a symmetric diene imidazolium ionic liquid (AVI) as both the cross-linker and organic monomer to polymerize with the POSS monomer in free radical polymerization [27]. The highest column efficiency of 151,000 plates/m was achieved for the CLC separation of alkylbenzenes on periodic imidazolium-bridged POSS-AVI hybrid monolithic column.
All the above-mentioned POSS-IL hybrid monoliths were fabricated by thermally initiated polymerization, which is temperature sensitive, energy consumption and would be finished in a rather long time, usually over 12 h. As a result, the preparation efficiency and column repeatability would be largely affected by the fabrication procedure. The control of spatial location of the monolith in capillary is also difficult in thermally fabrication [28]. Instead, photoinitiated polymerization shortens the preparation time to a large extent, and employs low-boiling-point organic solvents as porogenic solvents at room temperature [29]. Several reports [30] showed that both the separation time and the column efficiencies of photoinitiated polymerization monolithic columns were higher than that of thermally initiated columns, mainly due to the improved internal structure and permeability of photoinitiated monoliths. These good merits make the photoinitiated polymerization an excellent candidate to be used in fast preparation of hybrid monolithic columns. However, the POSS-based hybrid monolithic column fabricated by combining functionalized ionic liquid via photoinitiated polymerization has not been reported yet.
In this work, an “one-pot” method was established for rapid fabrication of POSS-VBIM hybrid monolithic column via photoinitiated free radical copolymerization of POSS-MA and ionic liquid in a porogenic system containing 1-propanol and PEG200. POSS-MA acted as a multifunctional crosslinker that not only formed the monolith skeleton by both homopolymerization and coupling with vinyl-based IL monomers, but also prevented the generation of gel-like micropores. A multiple interaction and strong EOF, which originated from the VBIMNTF2 monomer, can be observed on the monolith. The preparation process was careful optimized, and the surface morphology, mechanical property and EOF characteristic of the monolithic columns were characterized. The chromatographic performance of the resulting monolith was systematic evaluated in CEC.
Experimental
Chemicals and materials
Methacryl substituted polyhedral oligomeric silsesquioxane reagent (cage mixture, n = 8, 10, 12; POSS-MA) was purchased from Hybrid Plastic, Inc. (Hattiesburg, MS, USA, https://hybridplastics.com). 1-butyl-3-vinylimidazolium-bis[(trifluoromethyl)sulfonyl]imide (VBIMNTF2) was obtained from Shanghai Chengjie Chemical Co., Ltd. (China, http://www.cjil.com.cn). 3-Methacryloxypropyltrimethoxysilane (γ-MAPS), poly(ethylene glycol) 200 (PEG 200) and nonylphenol were purchased from Sigma (USA, https://www.sigmaaldrich.com). 2,2-Dimethoxy-2-phenylacetophenone (DMPA) was purchased from J&K Chemical (Beijing, China, http://www.jkchemical.com). 1-Propanol, aniline, sulfonamides, 1-aminonaphthalene, 4-nitroaniline, HPLC-grade methanol (MeOH) and acetonitrile (ACN) were all obtained from Shanghai Chemical Reagents Corporation (China, http://www.sinoreagent.com). Bisphenol A and octyl phenol were purchased from Alfa Aesar (Shanghai, China, https://www.alfa.com). Benzene, naphthalene, phenanthrene, fluorene were obtained from Aladdin (Shanghai, China, http://www.aladdin-e.com). Thiourea, toluene, ethylbenzene, n-propylbenzene, n-butylbenzene and n-amylbenzene were obtained from Tokyo Chemical Industry (Shanghai, http://www.tcichemicals.com). All reagents were analytical grade. The ultrapure water used in the experiments was purified by Milli-Q water system (Millipore, Bedford, MA, USA, http://www.merckmillipore.com). UV-transparent polyimide coating fused silica capillaries of 50 μm I.D. (375 μm O.D.) were purchased from Yongnian Optic Fiber Plant (Hebei, China, http://www.rui-feng.com).
Apparatus and methods
CEC experiments were performed on a Trisep™ 2010GV CEC system (Unimicro Technologies, Pleasanton, CA, USA, http://www.unimicrotech.com) equipped with a high-voltage power supply (+20 kV~ −20 kV), a solvent gradient delivery module, a micro fluid manipulation module, a variable wavelength UV–Vis detector (190~600 nm). The micro fluid manipulation module consists of a six-port injector for sample injection and a four-port valve as a splitter. After the mobile phase pumped by LC micropump enters into six-port inject valve, it will deliver the sample that injected in the internal sample loop of injector to the splitter. Through the splitter, one part of mobile phase will flow into the capillary column, while the other part flows to a blank capillary that connecting with a backpressure regulator. Backpressure regulator can maintain the pressure at both ends of the separation column and adjust the amount of mobile phase that flowing into the separation column. A high voltage was applied to the outlet of column, while the inlet of column was connected to the splitter and grounded. A HW-2000 chromatographic data work station of HW-2000 (Shanghai Qianpu software company, Shanghai, China, http://www.qianpu.com) was employed for chromatographic data acquisition and analysis. Various volume ratios of acetonitrile and aqueous trimethylamine/phosphoric acid (TEAP) buffer were used as mobile phase for CEC separations. All CEC measurements were performed at room temperature.
The polymerization was irradiated in a Spectrolinker™ XL-1500A UV cross-linker (365 nm, Spectronics Corporation, USA, http://www.spectroline.com). A Nova NanoSEM 230 field emission scanning electron microscope (FEI Company, USA, https://www.fei.com) was employed to characterize the porous morphology of monoliths. The Fourier transform infrared spectras (FT-IR) of the monoliths in KBr were recorded by a Nicolet 6700 spectrometer (Thermo Fisher, USA, https://www.thermofisher.com). Pore size distribution was measured by mercury intrusion porosimetry (MIP) on an AutoPore IV 9510 (Micromeritics instrument corp, U.S.A., http://www.micromeritics.com).
Preparation of POSS-VBIM hybrid monolithic column
Before the preparation of the POSS-VBIM hybrid monolithic column, the inner wall of the fused-silica capillary was silanol-activated and pretreated with a γ-MAPS/methanol (1:1, v/v) solution at 50 °C for 16 h to immobilize a layer of vinyl groups for anchoring monolith matrix. Then, the capillary was flushed by methanol and dried under nitrogen flow.
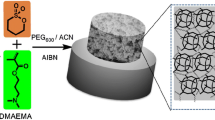
As shown in Fig. 1, POSS-MA and VBIMNTF2 were used as the precursors to fabricate hybrid monolith. The polymerization solution with different compositions (as listed in Table S1, Electronic Supplementary Material; ESM), consisting of POSS-MA, VBIMNTF2, PEG200, 1-propanol and DMPA, were mixed by ultrasonication to form a transparent homogeneous solution and then injected into the above-mentioned pretreated capillary. With both ends sealed with silicone rubbers, the capillary was irradiated by UV radiation at wavelength of 365 nm (120 mJ·cm−2) for 7 min. The resulting hybrid monolithic columns were connected to the pump and then rinsed with methanol to flush out residuals. The total length of the capillary monolithic column was 50 cm with an effective length of 25 cm. A detection window was made at the end of the continuous bed by removing about 2 mm of polyimide coating of the capillary. SEM characterization was conducted on the sample pieces (~1 cm) that cut from sections of the prepared monolithic column, and the cross-sectional views of the monoliths were obtained. Because the amount of in situ monoliths (i.e., from the capillaries) is not enough for porosimetry and FT-IR measurements, the bulk hybrid monoliths were prepared in a larger transparent vial (centrifuge tube) with the rest of the polymerization solution by mimicking the same polymerization conditions. Then the bulk monoliths were removed and cut into small pieces and grounded after the polymerization, followed by rinsing with methanol and vacuum dry (60 °C for 12 h).
Results and discussion
Synthesis of POSS-VBIM hybrid monolithic column
The POSS-VBIM hybrid monolithic column was prepared by an “one-pot” photoinitiated free-radical polymerization reaction between POSS-MA and VBIMNTF2 according to the process shown in Fig. 1. As expected, the morphology and permeability of hybrid monolithic column were highly depended on the composition of pre-polymerization solution, which is listed in Table S1 (see ESM). Several parameters such as the ratio of POSS-MA to VBIMNTF2, the porogenic solvent system and the UV irradiation time were careful optimized. The details for the optimization of synthetic conditions are illustrated in the ESM. Considering the appropriate permeability, stability and retention capability, a mixture of PEG200 and 1-propanol with a mass ratio of 46:54 was finally selected as the binary porogenic system. The column B that fabricated with a mass ratio of VBIMNTF2 to POSS-MA at 29:71 (Fig. S1 in ESM) and a UV irridiation time of 7 min was considered to be the optimum and finally selected for further CEC experiments.
Characterization of the column
The copolymerization between POSS-MA and VBIMNTF2 was verified by FT-IR spectroscopic analysis (Fig. S2, see ESM). Compared with the FT-IR spectras of POSS-MA (Fig. S2 B) and VBIMNTF2 ILs monomer (Fig. S2 C), the spectra of POSS-VBIM hybrid monolith (Fig. S2 A) clear shows the presence of strong adsorption bands at 1120 cm−1 (Si-O-Si), 1718 cm−1 (C=O) and 1353 cm−1 (C-N), originating from the introduction of POSS and VBIMNTF2 monomer, respectively. Fig. S2 A shows a peak at 1637 cm−1 that is assigned to the stretching vibration of C=C bond in the POSS-MA almost disappear. The intensity of the adsorption band of vinyl-based imidazolium ring at 1655 cm−1 (C=C), 1553 cm−1 and 1572 cm−1 (C=N) was remarkably decreased. These results confirmed the successfully homopolymerization of POSS-MA and the copolymerization of POSS-MA with VBIMNTF2 occurred in the synthesis.
The morphology of POSS-VBIM hybrid monolithic column (column B) was characterized by scanning electron microscope (SEM). As shown in Fig. S3 (see ESM), the uniform and continuous porous monolithic matrix with large throughpores (about 1–3 μm) benefits to the high permeability of resulting monolith, and no obvious cracking and shrinkage is observed. The monolith reveals a typical cauliflower-like globular structure and particle (< 0.5 μm) aggregates morphology, indicating the incorporation of POSS-MA to the polymer.
Pore size measurement by mercury intrusion porosimetry showed the existence of micrometer-large pores in hybrid monolith, with a total intrusion volume of 0.410 mL g−1 and a porosity of 33.8%. A lower specific surface area of hybrid monolith was also calculated as about 2 m2 g−1 according to nitrogen adsorption/desorption measurement of dry bulk monolith, possibly due to the absence of micropores in the dry state.
Mechanical stability of the column
The mechanical stability of the prepared hybrid monolith was evaluated by measuring the backpressure of monolith by pumping various mobile phases (ACN, methanol, ACN/water or water) at different flow rate. As shown in Fig. 2, good linear relationships between back pressure and flow rate of mobile phase are obtained when flow rate is increased from 0.001 to 0.015 mL min−1, indicating satisfied mechanical stability.
To examine the swelling propensity of monoliths, the permeability of resulting hybrid monolithic columns using various mobile phases were determined by the Darcy’s law [31] and listed in Table S2 (see ESM). The maximal permeability change (ΔK) of the monolith in different mobile of the monolith in different mobile phases was calculated to be 12.3%, which is much lower than that obtained from polymer monoliths. This result indicates a slighter swelling tendency of the prepared hybrid monolith. This can be attributed to the copolymerization of rigid POSS nanoparticles with Si-O-Si bond and soft monomer ILs, which offers the POSS-VBIM hybrid monolithic column a moderate rigidity and sufficient mechanical stability.
Reproducibility of hybrid monolith
The reproducibility of the prepared monolithic column was evaluated by measuring the relative standard deviations (RSDs) of the retention time and column efficiency of toluene as model analyte. The RSDs of run-to-run (n = 5), column-to-column (n = 5), and batch-to-batch (n = 5) were less than 1.4%, 2.2 and 2.9% for retention factor, and less than 1.8%, 2.9 and 3.4% for column efficiency, respectively. These results indicate that the POSS-VBIM hybrid monolithic column exhibit high repeatability and reproducibility in CEC, owing to the very small differences in the pore size of the monoliths generated by varying the intensity and time of UV cure in polymerization [32].
Chromatographic performance of the column
EOF of the POSS-VBIM monolithic column
In CEC, the magnitude of EOF which is related to the net surface charge density of all of the chargeable groups in stationary phase and the composition of mobile phase, can be calculated as:
Where Lt is the total length of the capillary column (cm), Ld is the effective length of the capillary column (cm), tm is the migration time of the void time marker (thiourea) (s), V is the separation voltage (kV). Figure 3 shows a relationship between the EOF and the buffer pH in the range of pH 2 to 10. At lower pH, the net surface charge of the monolith was determined by the imidazolium cation of the VBIMNTF2, because the ionization of residual silanol on the capillary wall was suppressed. The strongest anodic EOF generated on the monolith was obtained at pH 2, while a reduction in the EOF was observed as the buffer pH was increased, due to the increased ionization of the residual silanol moieties and the deprotonation of imidazolium groups at the higher pH. It is noteworthy that the EOF on the hybrid monolith is controllable by changing the buffer pH, and can even exist in strong basic condition.
Column efficiency
The column efficiency of the POSS-VBIM hybrid monolithic column was evaluated by changing the linear velocity of the mobile phase from 0.52 mm·s−1 to 1.32 mm·s−1, using thiourea and alkylbenzenes as model analytes. The Van Deemter curves of the monolithic columns (Fig. 4) shows the lowest plate height of ~10.2 μm for amylbenzene, corresponding to the column efficiency (theoretical plates, N) of ~ 98,000 plates/m. Figure 4 also indicates that the column efficiency for the strongly retained compounds (amylbenzene) on the hybrid monolith is higher than that of weakly retained compounds (toluene). The lower mass transfer resistance of amylbenzene in comparison with that of toluene (C-term 60 ms vs. 118 ms) can possibly be ascribed to the lack of gel-like micropores that are permeable to small molecules, due to the use of the multifunctional cross-linker in free radical polymerization [13].
Separation of alkylbenzenes
The retention behavior of hydrophobic compounds on POSS-VBIM monolithic column was investigated by using thiourea and alkylbenzenes as the model analytes. As shown in Fig. 5a, all of the analytes are baseline eluted within 16 min according to their hydrophobicity. The dependence of retention factor (k) of alkylbenzenes on the ACN content in the mobile phase is shown in Fig. 6. The retention factors of five alkylbenzenes decrease with an increase of ACN content from 40 to 80% in the mobile phase, demonstrating a typical reversed-phase retention mechanism that dominated by the hydrophobic interaction between hybrid monolith and analytes.
CEC chromatograms of alkylbenzenes and thiourea on (a) POSS-VBIM hybrid monolithic column B and (b) POSS monolith column G (without addition of ILs monomer). CEC conditions: mobile phase, ACN/10 mM TEAP buffer (pH 3) = 66/34 (v/v); separation voltage, +15 kV; supplementary pressure, 250 psi; pump flow rate, 0.05 mL min−1; detection wavelength, 214 nm. Analytes: 1. thiourea, 2. toluene, 3. ethylbenzene, 4. propylbenzene, 5. butylbenzene, 6. amylbenzene
Effect of the ACN content in the mobile phase on the retention factors of alkylbenzenes. CEC conditions: mobile phase, 10 mM TEAP buffer at pH 3.0 with different ACN content. Other conditions are the same as in Fig. 5
For comparison, the CEC chromatograms of alkylbenzenes on POSS-VBIM hybrid monolithic column (Column B, Table S1) and blank monolithic column polymerized by POSS-MA (Column G, Table S1) were recorded and evaluated. From the Fig. 5, we observe that the POSS-VBIM hybrid monolithic column exhibit far shorter retention time, sharper peak shapeand higher column efficiency than the blank column. The obvious enhancement of elution rate and RP retention of alkylbenzenes on column B can be attributed to the strong anodic EOF and π-interaction provided by the imidazolium group of VBIMNTF2 incorporated on the hybrid monolith. For the blank column G, however, only the hydrophobic interaction that provided by the POSS skeleton dominated the RP retention.
Separation of phenols
The POSS-VBIM hybrid monolithic column was used for separation of three weakly acidic and moderate polar phenolic compounds (bisphenol A, octylphenol and nonylphenol). As shown in Fig. 7a, good separation of three phenols is achieved at pH 3. The elution order is related to their polarity, and nonylphenol that possesses long carbon chain is eluted later, demonstrating a hydrophobic interaction supplied by the hybrid monolith. Since the dissociation of phenolic hydroxyl group on these analytes (pKa 10–11) are not occurred at examined pH, no obvious electrostatic effect is observed.
a Separation of phenols on the POSS-VBIM monolithic column in CEC. b Separation of anilines on the POSS-VBIM monolithic column in CEC. c Separation of PAHs on the POSS-VBIM monolithic column in CEC. CEC conditions: mobile phase, ACN/10 mM TEAP buffer (pH 3) = 70/30 (v/v) for (a); 50/50 (v/v) for (b); 66/34(v/v) for (c); separation voltage, +15 kV; supplementary pressure, 250 psi; pump flow rate, 0.05 mL ·min−1; detection wavelength, 214 nm for (a) and (b); 211 nm for (c) . Analytes: a 1. bisphenol A, 2. octylphenol, 3. nonylphenol. b 1. sulfonamide, 2. 4-nitroaniline, 3. aniline, 4. 1-aminonaphthalene. c 1. benzene, 2. naphthalene, 3. fluorene, 4. phenanthrene
Separation of basic aromatic amines
It is well known that basic compounds are prone to electrostatically attracted by silanol groups remaining on silica–based stationary phases, which leads to serious peak tailing in chromatographic separation. To test the CEC separation performance of the POSS-VBIM monolithic column, the retention behavior of four aromatic amines, including 4-nitroaniline (pKa 4.88), aniline (pKa 4.58), sulfonamide (pKa 10.50) and 1-naphthylamine (pKa 2.92) was investigated. At pH 3, all of the basic analytes were highly dissociated, except for the neutral 1-naphthylamine. The positively charged ionic liquid monomer provides anion exchanging sites and the anodic EOF. The electrostatic repulsion between positively charged analytes and the stationary phase weaken the retention of bases. In case of neutral analytes, the main interaction is the hydrophobic interactions and π stacking with the stationary phase. Therefore, as shown in Fig. 7b, baseline separation of four aromatic amines is achieved on the hybrid monolithic column with an elution order of sulfonamide <4-nitroaniline < aniline <1-aminonaphthalene.
Separation of polycyclic aromatic hydrocarbons
To evaluate the π interaction between the π-electron conjugated compounds and POSS-VBIM monolithic stationary phase, four hydrophobic polycyclic aromatic hydrocarbons (PAHs) were used as model analytes for CEC. Fig. 7c represents the chromatogram of four PAHs on hybrid monolith in CEC. The elution order of PAHs is benzene < naphthalene < fluorine < phenanthrene, which is well in agreement with their hydrophobicity and π-conjugation degree from low to high. The retention mechanism is suggested to be a combination of RP hydrophobic interaction with π-π effects between the stationary phase and analytes.
Conclusions
We describ a facile photoinitiated free radical polymerization procedure for ultrafast preparation of POSS-based ionic liquid hybrid monolithic column, utilizing POSS-MA as multifunctional crosslinker and VBIMNTF2 as charge-bearing monomer. The resulting hybrid monoliths have the merits of both polymer- and silica-based monoliths, such as good permeability and mechanical stability, wide pH tolerance and high column efficiency. These characteristics can be attributed to the introduction of POSS crosslinker that not only form the monolith skeleton but also provide the hydrophobic interaction for neutral targets. Besides, the POSS-VBIM monoliths containing the imidazolium groups show tuneable anodic EOF and mixed mode retention mechanism, including hydrophobic, electrostatic interaction, π-π stacking, and electrophoretic mobility as well. As a result, the hybrid monoliths exhibit good separation performance for various types of aromatic small molecules in CEC, such as alkylbenzenes, phenols, aromatic amines and PAHs, except for strong polar molecules. It is likely to be useful for the rapid separations of drugs, pesticides and some environmental pollutants with weak or moderate polarity in several modes using optimized chemistries. We will also focus attention on the potential application of the longer monolithic columns for the separation of complex biosamples such as protein in future works. Compared with the traditional thermal-initiated polymerization, photoinitiated polymerization can be rapidly completed within several minutes in the “one-pot” process. In addition, it avoids the tedious and energy-consuming procedures and the influence of temperature that would hamper the preparation reproducibility of column. It provides useful tool for fast fabrication of novel materials in widespread high efficient microseparation applications.
References
Hilder EF, Svec F, Fréchet JM (2004) Development and application of polymeric monolithic stationary phases for capillary electrochromatography. J Chromatogr A 1044:3–22. https://doi.org/10.1016/j.chroma.2004.04.057
Svec F (2009) My favorite materials: porous polymer monoliths. J Sep Sci 32:3–9. https://doi.org/10.1002/jssc.2008.00.530
Nischang I, Causon TJ (2016) Porous polymer monoliths: from their fundamental structure to analytical engineering applications. Trends Anal Chem 75:108–117. https://doi.org/10.1016/j.trac.2015.05.013
Jandera P (2013) Advances in the development of organic polymer monolithic columns and their applications in food analysis—a review. J Chromatogr A 1313:37–53. https://doi.org/10.1016/j.chroma.2013.08.010
Liu Z, Ou J, Zou H (2016) Click polymerization for preparation of monolithic columns for liquid chromatography. Trends Anal Chem 82:89–99. https://doi.org/10.1016/j.trac.2016.05.016
Svec F, Lv Y (2015) Advances and recent trends in the field of monolithic columns for chromatography. Anal Chem 87:250–273. https://doi.org/10.1021/ac504059c
Hayes JD, Malik A (2000) Sol−gel monolithic columns with reversed electroosmotic flow for capillary electrochromatography. Anal Chem 72:4090–4099. https://doi.org/10.1021/ac000120p
Zhang T, Ma C, Wu M, Ye Y, Chen H, Huang J (2013) Selective microextraction of carbaryl and naproxen using organic–inorganic monolithic columns containing a double molecular imprint. Microchim Acta 180:695–702. https://doi.org/10.1007/s00604-013-0990-y
Fang G, Qian H, Deng Q, Ran X, Yang Y, Liu C, Wang S (2014) A novel C18 reversed phase organic–silica hybrid cationic monolithic capillary column with an ionic liquid as an organic monomer via a “one-pot” approach for capillary electrochromatography. RSC Adv 4:15518–15525. https://doi.org/10.1039/C4RA00997E
Ou J, Liu Z, Wang H, Lin H, Dong J, Zou H (2015) Recent development of hybrid organic-silica monolithic columns in CEC and capillary LC. Electrophoresis 36:62–75. https://doi.org/10.1002/elps.201400316
Wu M, Wu R, Wang F, Ren L, Dong J, Liu Z, Zou H (2009) “One-pot” process for fabrication of organic-silica hybrid monolithic capillary columns using organic monomer and alkoxysilane. Anal Chem 81:3529–3536. https://doi.org/10.1021/ac9000749
Liu Y, Chen Y, Yang H, Nie L, Yao S (2013) Cage-like silica nanoparticles-functionalized silica hybrid monolith for high performance capillary electrochromatography via “one-pot” process. J Chromatogr A 1283:132–139. https://doi.org/10.1016/j.chroma.2013.01.112
Zhang H, Ou J, Liu Z, Wang H, Wei Y, Zou H (2015) Preparation of hybrid monolithic columns via “one-pot” photoinitiated thiol–acrylate polymerization for retention-independent performance in capillary liquid chromatography. Anal Chem 87:8789–8797. https://doi.org/10.1021/acs.analchem.5b01707
Zhang W, Müller AH (2013) Architecture, self-assembly and properties of well-defined hybrid polymers based on polyhedral oligomeric silsequioxane (POSS). Prog Polym Sci 38:1121–1162. https://doi.org/10.1016/j.progpolymsci.2013.03.002
Shen S, Ye F, Zhang C, Xiong Y, Su L, Zhao S (2015) Preparation of polyhedral oligomeric silsesquioxane based hybrid monoliths by thiol-ene click chemistry for capillary liquid chromatography. Analyst 140:265–271. https://doi.org/10.1039/C4AN01668H
Zhang H, Ou J, Wei Y, Wang H, Liu Z, Zou H (2016) A hybrid fluorous monolithic capillary column with integrated nanoelectrospray ionization emitter for determination of perfluoroalkyl acids by nano-liquid chromatography–nanoelectrospray ionization-mass spectrometry/mass spectrometry. J Chromatogr A 1440:66–73. https://doi.org/10.1016/j.chroma.2016.02.025
Ou J, Zhang H, Lin H, Dong J, Zou H (2013) Polyhedral oligomeric silsesquioxanes as functional monomer to prepare hybrid monolithic columns for capillary electrochromatography and capillary liquid chromatography. Anal Chim Acta 761:209–216. https://doi.org/10.1016/j.aca.2012.11.052
Liu Z, Ou J, Lin H, Wang H, Dong J, Zou H (2014) Preparation of polyhedral oligomeric silsesquioxane-based hybrid monolith by ring-opening polymerization and post-functionalization via thiol-ene click reaction. J Chromatogr A 1342:70–77. https://doi.org/10.1016/j.chroma.2014.03.058
Qiao X, Chen R, Yan H, Shen S (2017) Polyhedral oligomeric silsesquioxane-based hybrid monolithic columns: recent advances in their preparation and their applications in capillary liquid chromatography. Trends Anal Chem 97:50–64. https://doi.org/10.1016/j.trac.2017.08.006
Lin X, Wang X, Zhao T, Zheng Y, Liu S, Xie Z (2012) Electroneutral silica-based hybrid monolith for hydrophilic interaction capillary electrochromatography. J Chromatogr A 1260:174–182. https://doi.org/10.1016/j.chroma.2012.08.053
Soares B, Passos H, Freire CS, Coutinho JA, Silvestre AJ, Freire MG (2016) Ionic liquids in chromatographic and electrophoretic techniques: toward additional improvements in the separation of natural compounds. Green Chem 18:4582–4604. https://doi.org/10.1039/C6GC01778A
Sun P, Armstrong DW (2010) Ionic liquids in analytical chemistry. Anal Chim Acta 661:1–16. https://doi.org/10.1016/j.aca.2009.12.007
Tang S, Liu S, Guo Y, Liu X, Jiang S (2014) Recent advances of ionic liquids and polymeric ionic liquids in capillary electrophoresis and capillary electrochromatography. J Chromatogr A 1357:147–157. https://doi.org/10.1016/j.chroma.2014.04.037
Lin X, Zheng N, Wang J, Wang X, Zheng Y, Xie Z (2013) Polyhedral oligomeric silsesquioxane (POSS)-based multifunctional organic–silica hybrid monoliths. Analyst 138:5555–5558. https://doi.org/10.1039/C3AN01243C
Shan Y, Qiao L, Shi X, Xu G (2015) Preparation and evaluation of a novel hybrid monolithic column based on pentafluorobenzyl imidazolium bromide ionic liquid. J Chromatogr A 1375:101–109. https://doi.org/10.1016/j.chroma.2014.11.084
Zhang N, Zhang L, Qiao X, Wang Y, Yan H, Bai L (2015) Facile one-pot preparation of an imidazolium embedded C8 hybrid monolith using polyhedral oligomeric silsesquioxane for capillary liquid chromatography. RSC Adv 5:91436–91440. https://doi.org/10.1039/C5RA15823K
Qiao X, Zhang N, Han M, Li X, Qin X, Shen S (2017) Periodic imidazolium-bridged hybrid monolith for high-efficiency capillary liquid chromatography with enhanced selectivity. J Sep Sci 40:1024–1031. https://doi.org/10.1002/jssc.201601014
Walsh Z, Levkin PA, Abele S, Scarmagnani S, Heger D, Klán P, Diamond D, Paull B, Svec F, Macka M (2011) Polymerisation and surface modification of methacrylate monoliths in polyimide channels and polyimide coated capillaries using 660 nm light emitting diodes. J Chromatogr A 1218:2954–2962. https://doi.org/10.1016/j.chroma.2011.03.021
Weller A, Carrasco-Correa EJ, Belenguer-Sapiña C, Mauri-Aucejo AR, Amorós P, Herrero-Martínez JM (2017) Organo-silica hybrid capillary monolithic column with mesoporous silica particles for separation of small aromatic molecules. Microchim Acta 184:3799–3808. https://doi.org/10.1007/s00604-017-2404-z
Wang H, Ou J, Liu Z, Lin H, Peng X, Zou H (2015) Chromatographic efficiency comparison of polyhedral oligomeric silsesquioxanes-containing hybrid monoliths via photo- and thermally-initiated free-radical polymerization in capillary liquid chromatography for small molecules. J Chromatogr A 1410:110–117. https://doi.org/10.1016/j.chroma.2015.07.085
Stanelle RD, Sander LC, Marcus RK (2005) Hydrodynamic flow in capillary-channel fiber columns for liquid chromatography. J Chromatogr A 1100:68–75. https://doi.org/10.1016/j.chroma.2005.09.014
Lee D, Svec F, Fréchet JM (2004) Photopolymerized monolithic capillary columns for rapid micro high-performance liquid chromatographic separation of proteins. J Chromatogr A 1051(1–2):53–60. https://doi.org/10.1016/j.chroma.2004.04.047
Karout A, Pierre AC (2009) Silica gelation catalysis by ionic liquids. Catal Commun 10:359–361. https://doi.org/10.1016/j.catcom.2008.07.046
Liu C, Deng Q, Fang G, Liu H, Wu J, Pan M, Wang S (2013) Ionic liquids monolithic columns for protein separation in capillary electrochromatography. Anal Chim Acta 804:313–320. https://doi.org/10.1016/j.aca.2013.10.037
Shi X, Qiao L, Xu G (2015) Recent development of ionic liquid stationary phases for liquid chromatography. J Chromatogr A 1420:1–15. https://doi.org/10.1016/j.chroma.2015.09.090
Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (21375019), Special-funded Program on National Key Scientific Instruments and Equipment Development of China (2011YQ150072), and the Program for Changjiang Scholars and Innovative Research Team in University of China (IRT15R11).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 426 kb)
Rights and permissions
About this article
Cite this article
Zhang, B., Lei, X., Deng, L. et al. Ultrafast preparation of a polyhedral oligomeric silsesquioxane-based ionic liquid hybrid monolith via photoinitiated polymerization, and its application to capillary electrochromatography of aromatic compounds. Microchim Acta 185, 318 (2018). https://doi.org/10.1007/s00604-018-2847-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2847-x