Abstract
Molybdenum oxide quantum dots (MoOx QDs) were synthesized by a one-pot method and used as a versatile probe in an electrochemiluminescent (ECL) immunoassay of the non-small cell lung cancer biomarker cytokeratin 19 fragment 21–1 (CYFRA21-1) as a model analyte. The MoOx QDs exhibited stable and strong cathodic green ECL, with an emission peak at 535 nm, in the presence of K2S2O8 within the potential range of −2.0 to 0 V. On exposure to CYFRA21-1, the ECL decreases because of the immunoreaction between CYFRA21-1 and its antibody which generates a barrier for electron transfer. The determination of CYFRA21-1 with favorable analytical performances was successfully realized under the optimal conditions. ECL decreases linearly in the 1 pg mL−1 to 350 ng mL−1 CYFRA21-1 concentration range, and the detection is as low as 0.3 pg mL−1. Excellent recoveries from CYFRA21-1-spiked human serum indicate that the assay can be operated under physiological conditions.

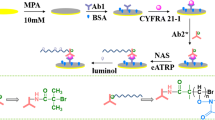
Schematic representation of the fabrication of molybdenum oxide quantum dots (MoOx QDs) and the electrochemiluminescent (ECL) immunoassay based on the use of the MoOx QDs ECL probe for cytokeratin 19 fragment 21–1 (CYFRA21-1).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrochemiluminescent (ECL) immunoassays provide an ultrasensitive and highly selective tool for detecting disease-related biomarkers. The key challenge of ECL immunosensor fabrication is the selection of a proper luminescent material [1]. Apart from traditional ones (luminol and Ru complexes), various nanomaterial-based ECL luminophores with high ECL efficiency, such as CdSe quantum dots (QDs) [2, 3], Ag2Se@CdSe [1], CdTe [4], CeO2 [5], ZnO/CdS [6], N-CQDs [7], and GO-g-C3N4 [8], have been successfully explored and used to fabricate biosensors for analytical applications. Among these nano-luminophores, QD-based ECL emitters have received much attention in view of their unique size-dependent electrochemical properties and ECL parameter tunability [9]. Although various QD-based ECL emitters, especially IIB-VIA type QDs, have been extensively designed for and applied in bioanalysis [4], innovative, stable, and highly efficient QD-based ECL luminophores for ECL sensors are still highly sought after. MoOx QDs have been extensively studied in view of their superior electronic properties, high photostability, low toxicity, and excellent chemical stability [10,11,12,13,14], and have been widely employed in various fields [10, 15,16,17]. However, the ECL properties and related applications of MoOx QDs remain underexplored.
We synthesized water-soluble MoOx QDs by a one-pot environmentally friendly method and characterized them as a potential ECL luminophore. The results demonstrate that these QDs exhibit excellent cathodic ECL properties in the presence of K2S2O8 as a co-reactant, and the origin of this behavior was discussed in detail. Subsequently, MoOx QDs were employed to fabricate a universal ECL immunosensing platform, the performance of which was evaluated for cytokeratin 19 fragment 21–1 (CYFRA21-1, biomarker used in the diagnosis of non-small cell lung cancer) as a model analyte. The platform allows rapid and sensitive CYFRA 21-1 detection and offered the benefits of good sensitivity, selectivity, wide linear range, and acceptable precision, and is therefore concluded to hold great promise for biological applications. Importantly, the insights gained in this work are expected to facilitate the construction of other high-performance ECL detection systems based on MoOx QDs or their nanocomposites.
Materials and methods
Reagents and chemicals
MoS2 powder was purchased from Sigma Aldrich (average diameter 90 nm; Shanghai, China) (https://www.sigmaaldrich.com/china-mainland.html), and HAuCl4 was sourced from Aladdin Reagent Co., Ltd. (Shanghai, China) (https://www.aladdin-e.com/). Sodium citrate, tannic acid, H2O2, K2S2O8, NaH2PO4·2H2O, Na2HPO4·12H2O, KCl, NaOH, K3[Fe(CN)6], and K4[Fe(CN)6]·3H2O were procured from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) (https://www.reagent.com.cn/). CYFRA 21-1 and anti-CYFRA 21-1, carcino-embryonic antigen (CEA), fibronectin (FN), and α-fetoprotein (AFP) were obtained from Xiamen Wan Tai Kerry Biotechnology Co., Ltd. (Xiamen, China) (http://www.innodx.com/about/?19.html). Bovine serum albumin (BSA) was purchased from BBI Life Sciences Corporation (Shanghai, China) (https://www.sangon.com/). MoOx QDs were synthesized by previous reported procedures with some modification [15]. Gold nanoparticles (Au NPs) were synthesized following the previously reported procedures (see details in Supporting Information) [18]. All chemicals and solvents were of analytical grade and were used without further purification.
Instruments and measurements
Transmission electron microscopy (TEM) imaging and energy-dispersive X-ray spectroscopy (EDS) elemental analysis of the MoOx QDs were performed using a JEM-2100 instrument (JEOL, Japan). Fluorescence spectra were recorded on an Eclipse spectrofluorometer (Varian). Fourier transform infrared (FT-IR) spectra were recorded on a NicoletiS50 spectrometer (Thermo Fisher Scientific, China). Electrochemical impedance spectroscopy (EIS) measurements were conducted on a RST electrochemical work station (Suzhou Risetest Instrument Co., Ltd., China), and other electrochemical measurements were conducted on a CHI660b electrochemical workstation (Chenhua Instrument Company of Shanghai, China). ECL behavior was studied using an MPI-A ECL analyzer (Xi’An Remax Electronic Science & Technology, China) equipped with a photomultiplier tube (PMT) biased at 700 V. ECL experiments were performed in a conventional three-electrode setup comprising a modified/non-modified glassy carbon electrode (GCE, 3 mm in diameter) as a working electrode, an Ag/AgCl electrode as a reference electrode, and a Pt wire as a counter electrode.
Fabrication of the ECL immunosensor
The fabrication is illustrated in Scheme 1. The glassy carbon electrode (GCE) was successively polished with 0.3- and 0.05-μm alumina powder on fine chamois, cleaned by sequential ultrasonication in ethanol and water (3 min each), and blown dry with nitrogen. The surface of the pretreated electrode was coated with MoOx QDs solution (8 μL, 0.035 g mL−1) and dried under vacuum. Then, 5 μL of Au NP-chitosan (Au NP-CS) (5:1) was drop-cast on the electrode surface and allowed to dry under vacuum. Anti-CYFRA21-1 (5 μL, 50 μg mL−1) was drop-cast on the sensing interface and left overnight at 4 °C, which was followed by 1 h incubation with BSA (5 μL, 1 wt%) to block nonspecific adsorption. Finally, the GCE was washed with deionized water to afford the label-free ECL immunosensor, which was stored at 4 °C when not in use.
To optimize ECL response conditions, we investigated factors affecting the performance of the immunosensor, e.g., the amount of immobilized MoOx QDs, concentration of K2S2O8, and detection solution pH (Fig. S1).
Determination of CYFRA21-1
The immunosensor was incubated with CYFRA21-1 solutions of different concentrations for 1 h at 37 °C and thoroughly washed with phosphate buffer (0.1 M, pH 7.4). Measurements were performed in PBS containing 0.1 M KCl and 0.1 M K2S2O8 at scanning potentials range from −2.0 to 0 V, a scanning rate of 100 mV s−1, and a PMT voltage of 800 V. The sensing electrode was placed into the ECL cell, and ECL signals were recorded at different CYFRA21-1 concentrations. In the presence of CYFRA21-1, signal intensity decreases because of the formation of a non-conductive immunocomplex in the immunoreaction of CYFRA21-1 with anti-CYFRA21-1, which allows the quantitation of the former.
Results and discussion
Choice of materials
The MoOx QDs were chosen because they exhibit a stable and strong cathodic ECL signal when using K2S2O8 as the coreactant in aqueous solution of pH 7.4. Furthermore, Au NPs not only provides an effective matrix to capture a great deal of antibody, but also accelerates the electron transportation rate to enhance the ECL intensity. Combining with the excellent adhesive ability of chirosan (chit), a highly sensitive ECL immunoassay platform based on MoOx QDs/Au NPs-chit nanocompsite film has been successfully constructed.
Characterization of the QDs
The MoOx QDs were characterized by TEM, EDS, photoluminescence (PL), and FT-IR spectroscopy. As shown in Fig. 1a and b, the QDs are relatively uniform spherical particles (average diameter = 2–5 nm) and contain Mo and O (Fig. 1b). The FT-IR spectrum of MoOx QDs (Fig. 1c) features three main absorption peaks at 986, 835, and 553 cm−1, which are ascribed to Mo = O, doubly coordinated oxygen (Mo2–O), and triply coordinated oxygen (Mo3–O) stretching vibrations, respectively [19]. In addition, the fluorescence emission spectrum (λex = 315 nm) of MoOx QDs shows an emission maximum at 430 nm (Fig. 1d).
Performance of the assay
As already mentioned, MoOx QDs were chosen as a novel ECL probe to develop a bioassay platform. As the inherent ECL behavior of MoOx QDs has not been reported yet, we investigated the ECL properties of MoOx QDs immobilized on GCE in the presence of K2S2O8 as a co-reactant to probe the feasibility of this system. Figure 2a shows the ECL response curves of bare (curve a) and MoOx QD-modified (curve b) GCEs recorded at a scan rate of 100 mV s−1 within the potential range of −2.0 to 0 V. In contrast to the case of the bare GCE, strong cathodic ECL is observed for the MoOx QDs/GCE in the presence of externally added K2S2O8. The intensity of the latter signal does not significantly change during 10 continuous cyclic voltammetry scans (inset of Fig. 2a), i.e., the ECL emission of the MoOx QDs/GCE is fairly strong, stable, and hence suitable for the fabrication of ECL sensors.
The ECL spectrum of the MoOx QDs/S2O82− system was also investigated using a series of optical filters. As shown in Fig. 2b, the ECL emission maximum of this system is red-shifted by 105 nm relative to the PL emission maximum of MoOx QDs at 430 nm (Fig. 1d), as has also been observed for other ECL-active nanomaterials such as methionine-stabilized Au nanoclusters [20], Si nanocrystals [21], and CdSe nanocrystals [22]. This phenomenon is ascribed to the strong effect of surface states on electrochemical and ECL processes [23]. Taken together, the above results imply that MoOx QDs and S2O82− can form an excellent ECL system (MoOx QDs/S2O82−) in which QDs act as the luminophore and S2O82− acts as the co-reactant [5, 24, 25]:
During initial potential scanning in the negative direction, MoOx captures electrons (e−) and is reduced to MoOx•−, while S2O82− is reduced to SO4•− and SO42−. The reaction between MoOx•− and SO4•− affords excited MoOx* that emits light upon returning to the ground state.
ECL efficiency (ΦECL) is defined as the number of photons emitted per unit charge transferred during the chemiluminescence reaction and depends on the efficiency of excited state population and the quantum yield of emission from this state. The ΦECL of MoOx QDs in solution was calculated using [Ru(bpy)3]2+ as a standard according to the following equation [26].
Here, Φ°ECL = 5.0% is the ECL efficiency of [Ru(bpy)3]2+ obtained via an annihilation mechanism in 0.1 M ACN with 1 mM (TBA)BF4 [27], Qf and Q°f are the transferred faradaic charges for the MoOx QD film and [Ru(bpy)3]2+, respectively, and I and Io are the respective integrated PMT responses. We estimated ΦECL of MoOx QDs as 4.23%, revealing the excellent ECL properties of these QDs.
Characterization of the immunosensor
The operation of the ECL immunosensor relies on the specificity of antigen-antibody recognition. MoOx QDs/Au NPs-chit were used as antibody carriers and sensing platform for GCE modification, and the formation of a non-conductive antigen-antibody complex was expected to hinder electron transfer and thus decrease ECL response. The ECL behavior of the immunosensor was investigated in 0.1 M PBS (pH 7.4) containing of 0.1 M KCl and 0.1 M K2S2O8 using CYFRA21-1 as a model analyte. As shown in Fig. 3a, MoOx QDs/GCE exhibits strong ECL emission (curve a), which slightly decreases upon the introduction of Au NPs-chit (curve b). This decrease is attributed to the presence of CS (that is not conducive to ECL) or resonance energy transfer between Au NPs and MoOx QDs. The introduction of anti-CYFRA21-1 (curve c) and BSA (curve d) results in a further decrease of ECL signal intensity due to the blockage of electron transfer by inert protein molecules. Finally, the immunoreaction of CYFRA21-1 with the immobilized anti-CYFRA21-1 to afford a non-conductive immunocomplex results in an additional intensity decrease (curve e), as this complex hinders electron and mass transfer between ECL reagents and the electrode surface. This suggests that the intensity decrease can be utilized to quantify CYFRA21-1.
a ECL responses of (a) MoOx QDs, (b) MoOx QDs/Au NPs-chit, (c) MoOx QDs/Au NPs-chit/Ab, (d) MoOx QDs/Au NPs-chit/Ab/BSA, and (e) MoOx QDs /Au NPs-chit/Ab/BSA/CYFRA21-1. b Cyclic voltammograms and (C) EIS spectra of working electrodes modified with (a) nothing (bare electrode), (b) MoOx QDs, (c) MoOx QDs/Au NPs-chit, (d) MoOx QDs/Au NPs-chit/Ab, (e) MoOx QDs/Au NPs-chit/BSA/Ab, and (f) MoOx QDs/Au NPs-chit/BSA/Ab/CYFRA21-1
To better understand the process of immunosensor fabrication, individual fabrication stages were investigated by cyclic voltammetry (CV) and EIS measurements, which were performed in 5.0 mM K3[Fe(CN)6]/K4[Fe(CN)6] solution containing 0.10 M KCl. Figure 3b shows the CV curves of the stepwise modified electrode, showing that a pair of well-defined redox peaks (curve a) is observed for the bare GCE. After modification with MoOx QDs (curve b), the peak current decreases, while peak potential separation (ΔEp) increases, which suggests that the immobilized MoOx QDs attenuated electron transfer. However, the subsequent introduction of Au NPs-chit results in an increase of peak current and a decrease of ΔEp (curve c), i.e., the excellent electrical conductivity of Au NPs and the excellent film forming ability and adhesiveness of CS facilitates electron transfer. As the electrode was further incubated with anti-CYFRA21-1, BSA, and CYFRA21-1, the peak current (curves d, e, and f, respectively) progressively decreases, while ΔEp increases, since the non-conductive protein molecules hinder electron transfer [28, 29]. Figure 3c shows the electrochemical impedance spectra of bare and modified electrodes, demonstrating that the bare GCE (curve a) has a lower impedance than the MoOx QD-modified GCE (curve b), as MoOx QDs hinder surface electron transfer. The immobilization of Au NPs-chit results in an obvious decrease of impedance (curve c) due to the excellent electrical conductivity of Au NPs. Finally, the introduction of anti-CYFRA21-1, BSA, and CYFRA21-1 successively increases electron transfer resistance (curves d, e, and f, respectively), as the non-conductive protein layer acts a barrier for electron transfer. Taken together, the above findings are indicative of successful immunosensor fabrication.
Performance of the immunoassay
The immunosensor was used for CYFRA21-1 detection under optimized conditions (Fig. 4). Figure 4a shows that the ECL response (IECL) decreases with increasing CYFRA21-1 concentration, while Fig. 4b reveals that this decrease linearly depends on the logarithm of CYFRA21-1 concentration (log c) within the range of 0.001–350 ng mL−1. The linear relation can be described by the equation IECL = 759.5 log c + 2520, with a correlation coefficient of 0.9890. The detection limit (0.3 pg mL−1) was calculated as three times the standard deviation of the blank signal. Compared with the previous reports for detection of CYFRA21-1 [30,31,32,33,34], the constructed label-free ECL immunosensor has a good performance in terms of a wider linear range and a lower limit of detection (Table 1).
a ECL responses of the immunosensor in 0.1 M phosphate buffer (pH 7.4) containing 0.1 M KCl and 0.1 M K2S2O8 to CYFRA21-1 (from a to l: 0.001, 0.01, 0.05, 0.1, 0.2, 0.5, 1, 10, 50, 100, 200, 350 ng mL−1). b Dependence of ECL intensity on the concentration of CYFRA21-1. c Stability of ECL emission from GCE/MoOx QDs/Au NPs-chit/Ab/BSA under continuous scanning for 12 cycles at 1 pg mL−1 CYFRA21-1 in 0.1 M phosphate buffer (pH 7.4) containing 0.1 M KCl and 0.1 M K2S2O8. d Responses of the fabricated ECL sensor to different proteins. ∆ECL intensity I0-I, where I0 and I are the ECL intensities of GCE/MoOx QDs/Au NPs-chit/Ab/BSA in K2S2O8 solution in the absence and presence of targets, respectively
The operational stability of our sensing platform was investigated under the conditions of continuous cyclic potential scanning (Fig. 4c, 12 cycles at 1 pg mL−1 CYFRA21-1 standard solutions) in 0.1 M phosphate buffer (pH 7.4) containing 0.1 M KCl and 0.1 M K2S2O8, strong and stable ECL signals are observed (relative standard deviation (RSD) = 0.54%). Furthermore, we demonstrates that >90% of ECL intensity is retained after four-week storage at 4 °C and thus shows that the fabricated immunosensor exhibits excellent stability. Finally, sensor reproducibility was examined by determining intra- and inter-assay precisions. The former parameter was estimated by measuring the response of the sensor to 1 pg mL−1 CYFRA21-1 for six times, while the latter was determined by assaying the response of 10 modified electrodes to 1 pg mL−1 CYFRA21-1. As a result, intra- and inter-assay RSDs were obtained as 3.18 and 4.39%, respectively, which indicates that the fabricated immunosensor provides well-reproducible results. Besides, after the antigen of CYFRA21-1 was incubated onto the GCE/MoOx QDs/Au NPs-chit/BSA/Ab for 1 h, the electrode surface was regenerated by treatment with Gly − HCl (pH 2.2) buffer containing 0.05% Tween 20 to remove the coupled CYFRA21-1 on the electrode surface [35]. After each acid treatment, the surface was again incubated with the CYFRA21-1. The results displays that the immunosensor is stable up to 7 recycles without obvious loss of affinity and residual of CYFRA21-1 after elusion.
To further investigate the specificity of our immunosensor, we examined its responses to several interfering proteins, namely CEA, FN, AFP, and CA125 (Fig. 4d). Notably, responses to these proteins (0.2 μg mL−1) are much weaker than that to a 2000-fold smaller concentration of CYFRA21-1 (0.1 ng mL−1), which demonstrates the excellent specificity of our sensor.
Real sample application
To test the analytical reliability and application potential of the immunosensor, we employed it for the standard addition method-based analysis of human serum samples spiked with CYFRA21-1 standard solution to different final concentrations (2.00, 5.00, and 10.00 ng mL−1). The human serum samples were collected from the Second Hospital of Fuzhou. Prior to measurement, the serum samples were diluted 10 times (1:10 diluted with 0.1 M phosphate buffer solution, pH 7.4). Following that, 5 μL of the above sample was dropped onto the immunosensor and then thoroughly washed with phosphate buffer (0.1 M, pH 7.4) after the immunoreaction with 30 min of incubation at 37 °C. The concentration of CYFRA21-1 in the serum sample was calculated according to its degree of ECL inhibition. With the addition of different concentrations of CYFRA21-1 standard into the serum sample, the recoveries were calculated. As shown in Table 2, the observed recoveries range from 98.6 to 103.1%, while the corresponding RSDs range from 4.53 to 7.21%, which indicated that the resulting sensor has potential application in complicated real samples.
Conclusions
An ECL sensing platform based on MoOx QDs/Au NPs-chitosan film has been successfully fabricated. MoOx QDs were used as the ECL probe due to their excellent ECL performance with K2S2O8 as co-reactant. Under the optimized experimental conditions, CYFRA21-1 can be effectively assayed by monitoring the decreased ECL response upon the immunoreaction on the sensor surface. The immunoassay displays good performances with a wide linear range, high sensitivity, acceptable stability and reproducibility, indicating its potential applications for the detection of other biomolecules, such as other biomarkers, DNA, and cell. However, the relatively complex preparation process and low detection potential applied in this system might cause some side reactions for real samples detection, which limiting the application of MoOx QDs in other ECL sensors.
References
Lv XH, Pang XH, Li YY, Yan T, Cao W, Du B, Wei Q (2015) Electrochemiluminescent immune-modified electrodes based on Ag2Se@CdSe Nanoneedles loaded with Polypyrrole intercalated Graphene for detection of CA72-4. ACS Appl Mater Interfaces 7:867–872
Yao X, Yan PP, Tang QH, Deng AP, Li JG (2013) Quantum dots based electrochemiluminescent immunosensor by coupling enzymatic amplification for ultrasensitive detection of clenbuterol. Anal Chim Acta 798:82–88
Yan PP, Zhang J, Tang QH, Deng AP, Li JG (2014) A quantum dot based electrochemiluminescent immunosensor for the detection of pg level phenylethanolamine a using gold nanoparticles as substrates and electron transfer accelerators. Analyst 139:4365–4372
Liu X, Jiang H, Fang Y, Zhao W, Wang NY, Zang GZ (2015) Quantum dots based potential-resolution dual-targets Electrochemiluminescent Immunosensor for subtype of tumor marker and its serological evaluation. Anal Chem 87:9163–9169
Pang XH, Li JX, Zhao YB, Wu D, Zhang Y, Du B, Ma HM, Wei Q (2015) Label-free Electrochemiluminescent Immunosensor for detection of Carcinoembryonic antigen based on Nanocomposites of GO/MWCNTs-COOH/au@CeO2. ACS Appl Mater Interfaces 7:19260–19267
Geng J, Jia XD, Zhu JJ (2011) Sonochemical selective synthesis of ZnO/CdS core/shell nanostructures and their optical properties. CrystEngComm 13:193–198
Zhou J, Han TQ, Ma HM, Yan T, Pang XH, Li YY, Wei Q (2015) A novel electrochemiluminescent immunosensor based on the quenching effect of aminated graphene on nitrogen-doped carbon quantum dots. Anal Chim Acta 889:82–89
Hu LY, Zheng J, Zhao K, Deng AP, Li JG (2018) An ultrasensitive electrochemiluminescent immunosensor based on graphene oxide coupled graphite-like carbon nitride and multiwalled carbon nanotubes-gold for the detection of diclofenac. Biosens Bioelectron 101:260–267
Peng HP, Jian ML, Deng HH, Wang WJ, Huang ZN, Huang KY, Liu AL, Chen W (2017) Valence states effect on Electrogenerated Chemiluminescence of gold Nanocluster. ACS Appl Mater Interfaces 9:14929–14934
Zhang L, Hong MF, Chu ZJ, Xu H, Wang SP, Zhao XJ, Xiao SJ (2018) A new copper mediated on-off assay for alkaline phosphatase detection based on MoOx quantum dots. Microchem J 141:170–175
Xiao SJ, Zhao XJ, Zuo J, Huang HQ, Zhang L (2016) Highly photoluminescent MoOx quantum dots: facile synthesis and application in off-on pi sensing in lake water samples. Anal Chim Acta 906:148–155
Xiao SJ, Chu ZJ, Zhao XJ, Zhang ZB, Liu YH (2017) Off-on-off detection of the activity of acetylcholine esterase and its inhibitors using MoOx quantum dots as a photoluminescent probe. Microchim Acta 184:4853–4860
Yuan LL, Niu YS, Li RG, Zheng LH, Wang Y, Liu ML, Xu GF, Huang L, Xu YH (2018) Molybdenum oxide quantum dots prepared via a one-step stirring strategy and their application as fluorescent probes for pyrophosphate sensing and efficient antibacterial materials. J Mater Chem B 6:3240–3245
Wang N, Tang DY, Zou HY, Jia SY, Sun ZJ, Yang X, Peng J (2018) Synthesis of molybdenum oxide quantum dots with better dispersity and bio-imaging ability by reduction method. Opt Mater 83:19–27
Xiao SJ, Zhao XJ, Hu PP, Chu ZJ, Huang CZ, Zhang L (2016) Highly Photoluminescent molybdenum oxide quantum dots: one-pot synthesis and application in 2,4,6-trinitrotoluene determination. ACS Appl Mater Interfaces 8:8184–8191
Ding DD, Guo W, Guo CS, Sun JZ, Zheng NN, Wang F, Yan M, Liu SQ (2017) MoO3-x quantum dots for photoacoustic imaging guided photothermal/photodynamic cancer treatment. Nanoscale 9:2020–2029
Ding DD, Huang WC, Song CQ, Yan M, Guo CS, Liu SQ (2017) Non-stoichiometric MoO3-x quantum dots as a light-harvesting material for interfacial water evaporation. Chem Commun 53:6744–6747
Qiu JD, Peng HZ, Liang RP, Li J, Xia XH (2007) Synthesis, characterization, and immobilization of Prussian blue-modified au nanoparticles: application to Electrocatalytic reduction of H2O2. Langmuir 23:2133–2137
Sui LL, Xu YM, Zhang XF, Cheng XL, Gao S, Zhao H, Cai Z, Huo LH (2015) Construction of three-dimensional flower-like α-MoO3 with hierarchical structure for highly selective triethylamine sensor. Sensors Actuators B Chem 208:406–414
Peng HP, Deng HH, Jian ML, Liu AL, Bai FQ, Lin XH, Chen W (2017) Electrochemiluminescence sensor based on methionine-modified gold nanoclusters for highly sensitive determination of dopamine released by cells. Microchim Acta 184:735–743
Ding Z, Quinn BM, Haram SK, Pell LE, Korgel BA, Bard AJ (2002) Electrochemistry and electrogenerated chemiluminescence from silicon nanocrystal quantum dots. Science 296:1293–1297
Myung N, Ding Z, Bard AJ (2002) Electrogenerated Chemiluminescence of CdSe Nanocrystals. Nano Lett 2:1315–1319
Zhang XL, Tang ZR, Dong YP, Wang CM (2018) Electrogenerated chemiluminescence of ZnO nanorods and its sensitive detection of cytochrome C. Talanta 179:139–144
Wu D, Liu Y, Wang Y, Hu L, Ma H, Wang G, Wei Q (2016) Label-free Electrochemiluminescent Immunosensor for detection of prostate specific antigen based on Aminated Graphene quantum dots and carboxyl Graphene quantum dots. Sci Rep 6:20511
Lu Q, Wei W, Zhou ZX, Zhou ZX, Zhang YJ, Liu SQ (2014) Electrochemiluminescence resonance energy transfer between graphene quantum dots and gold nanoparticles for DNA damage detection. Analyst 139:2404–2410
Dennany L, Hogan CF, Keyes TE, Forster RJ (2006) Effect of surface immobilization on the Electrochemiluminescence of ruthenium-containing Metallopolymers. Anal Chem 78:1412–1417
Van Houten J, Watts RJ (1976) Temperature dependence of the photophysical and photochemical properties of the tris(2,2′-bipyridyl)ruthenium(II) ion in aqueous solution. J Am Chem Soc 98:4853–4858
Tang M, Zhou ZX, Li SG, Zhao F, Liu SQ (2018) Electrochemiluminescent detection of cardiac troponin I by using soybean peroxidase labeled-antibody as signal amplifier. Talanta 180:47–53
Zhou Y, Zhuo Y, Liao N, Chai YQ, Yuan R (2014) Ultrasensitive immunoassay based on a pseudobienzyme amplifying system of choline oxidase and luminol-reduced Pt@au hybrid nanoflowers. Chem Commun 50:14627–14630
Zeng Y, Bao J, Zhao YN, Huo DQ, Chen M, Qi YL, Yang M, Fa HB, Hou CJ (2018) A sandwich-type electrochemical immunoassay for ultrasensitive detection of non-small cell lung cancer biomarker CYFRA21-1. Bioelectrochemistry 120:183–189
Wang HQ, Ma ZF (2019) “Off-on” signal amplification strategy amperometric immunosensor for ultrasensitive detection of tumour marker. Biosens Bioelectron 132:265–270
Zeng Y, Bao J, Zhao YN, Huo DQ, Chen M, Yang M, Fa HB, Hou CJ (2018) A sensitive label-free electrochemical immunosensor for detection of cytokeratin 19 fragment antigen 21-1 based on 3D graphene with gold nanopaticle modified electrode. Talanta 178:122–128
Kumar S, Sharma JG, Maji S, Malhotra BD (2016) Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens Bioelectron 78:497–504
Shan J, Ma ZF (2016) Simultaneous detection of five biomarkers of lung cancer by electrochemical immunoassay. Microchim Acta 183:2889–2897
Han L, Wang D, Yan L, Petrenko VA, Liu AH (2019) Specific phages-based electrochemical impedimetric immunosensors for label-free and ultrasensitive detection of dual prostate-specific antigens. Sensors Actuators B Chem 297:126727
Acknowledgments
We sincerely acknowledge the financial support of the National Natural Science Foundation of China (21874020, 21405015), Fujian Province Health Commission Young and Middle-Aged Talent training project (2018-ZQN-62), Fujian Province Young Talent Supporting Project (2019B016), Joint Funds for the Innovation of Science and Technology, Fujian Province (2016Y9056, 2016Y9054), the Science and Technology Project of Fujian Province (2018 L3008), the Natural Science Foundation of Shandong Province (ZR2016BL19), and Program for Fujian Top-notch Innovative Personnel.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 245 kb)
Rights and permissions
About this article
Cite this article
Meng, X., Chen, X., Wu, W. et al. Electrochemiluminescent immunoassay for the lung cancer biomarker CYFRA21-1 using MoOx quantum dots. Microchim Acta 186, 855 (2019). https://doi.org/10.1007/s00604-019-3917-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3917-4