Abstract
Prominent electrochemiluminescence (ECL) in Ti-Fe–O nanotube arrays (Ti-Fe–O NTs) with K2S2O8 as the cathode coreactant is reported for the first time. Compared with pure titanium dioxide nanotubes (TiO2 NTs), this heterojunction could effectively reduce the band gap, facilitate electronic transitions, and move the ECL potential to a positive direction. The ECL performance motivated the development of an ultrasensitive ECL immunosensor for detecting cytokeratin fragment 21–1 (CYFRA21-1). Magnetic beads loaded with conductive carbon black (CCB/MNTs) were used to efficiently quench the ECL signal of a Ti-Fe–O NTs electrode and were combined with an ECL immunoassay to realize sensitive detection of CYFRA21-1. Over a CYFRA21-1 concentration range of 1.0 pg·mL−1 ~ 100 ng·mL−1, the change in the ECL signal was highly linear with the logarithm of the CYFRA21-1 concentration, and the limit of detection (LOD) was 0.114 pg·mL−1. This ECL immunosensor was used to successfully determine the CYFRA21-1 content in serum. The recovery of CYFRA21-1 in actual serum was 88.6 – 104.4%, and the RSD was 1.4 – 3.0%. The coreaction solution used in this work was PBS (0.1 M, pH = 7.4) containing 0.05 M K2S2O8, the scanning range was −1.0 – 0 V, the photomultiplier tube (PMT) was set to 750 V, and the scanning rate was 100 mV·s−1.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium dioxide nanotubes (TiO2 NTs) have been widely used in solar cells, photocatalysis, biosensors, etc., due to their large specific surface area, environmental protection, and relatively low cost [1,2,3,4,5]. Recently, Oriol’s group [6] reported the photoelectrocatalytic treatment of indigo carmine in an acidic aqueous solution using TiO2 NTs as photoanodes with good results. Although the application of TiO2 NTs to photoelectrochemical (PEC) and electrochemical (EC) fields has been extensively studied, the application of TiO2 NTs to the field of electrochemiluminescence (ECL) has rarely been studied. The main reason for this lack of research is that the wide band gap (3.2 eV) of TiO2 NTs makes it difficult to excite electrons, resulting in poor ECL performance. Generally, the band gap can be modified by methods such as dye sensitization [7], ion doping [8], metal deposition [9,10,11], and loaded nanomaterials [12,13,14].
The heterojunction of TiO2 and Fe2O3 has attracted increasing attention because of good performance. This heterojunction has effectively reduced the band gap (2.2 eV), promotes the electronic transitions, and enhanced ECL property compared to TiO2 NTs [15, 16]. The doping of Fe2O3 onto TiO2 has been implemented in a sol–gel system [17]. However, Fe2O3 has not been able to be atomically doped onto TiO2 in most studies [18]. Ti-Fe–O nanotubes (Ti-Fe–O NTs) have considerably extended the utilization of visible light owing to the unique tubular structure and uniform atom doping of these NTs. To the best of our knowledge, Ti-Fe–O NTs are a mature application in the field of lithium-ion batteries and PECs [18,19,20] but have rarely been reported for applications in the ECL field.
ECL has been widely used in the fields of biological sciences, immunoassays, and food analysis because of a high speed, low background noise, high sensitivity, and simple equipment [21,22,23]. Lung cancer has become one of the deadliest malignant tumors due to its high fatality rate [24]. Cytokeratin fragment 21–1 (CYFRA 21–1) can be used to detect non-small lung cancer cell markers. Due to its high specificity, it has been widely used in immunoassays and has become an important tool for the quantitative analysis of tumor markers [25, 26]. Therefore, it is of great significance to use Ti-Fe–O NTs as electrodes to target CYFRA21-1.
This article is the first report of ECL in Ti-Fe–O NTs with K2S2O8 as the cathode coreactant; an immunosensor for detecting CYFRA21-1 was designed with a Ti-Fe–O NTs electrode as an ECL emitter, and the principle is shown in Scheme 1. Magnetic beads loaded with conductive carbon black (CCB/MNPs) were used as amplifying quenchers and combined with Ab2 to detect CYFRA21-1 in serum. Experimental results showed that a Ti-Fe–O NTs electrode in a K2S2O8 coreactant produced ECL signals with high stability and reproducibility.
Experimental
Reagents and chemicals
CYFRA21-1 and cytokeratin 19 fragment antigen 21–1 antibodies (antiCYFRA21-1) were obtained from Shanghai Linc-Bio Science Co., Ltd. Prostate protein antigen (PSA) and carcinoembryonic antigen (CEA) were obtained from the Huayang Zhenglong Biochemical Products Research Office. Bovine serum albumin (BSA) and tetracycline-BSA (TC-BSA) were purchased from Shenzhen Kejie Bio Co., Ltd. N-(3-dimethylaminopropyl)-N’-ethyl-carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were purchased from Shanghai Sinopharm Chemical Reagent Co., Ltd. HIV-1 Tat protein was purchased from Shanghai Sangon Biotech Co., Ltd. Ulinastatin (UTI) was obtained from Eddie Pharmaceutical Co., Ltd. CCB was purchased from Nanjing Xianfeng Nano Co., Ltd. Affimag magnetic microspheres (MNPS) were obtained from the Tianjin Beisle Chromatography Technology Development Center. Hydrofluoric acid (HF) was purchased from Yantai Sanhe Chemical Reagent Co., Ltd. Oxalic acid (OA) was purchased from Tianjin Guangcheng Chemical Reagent Co., Ltd. Absolute ethanol (C2H5OH) was obtained from Sinopharm Chemical Reagent Co., Ltd. Ti-Fe alloy plates were purchased from Yinuo Metal Materials Co., Ltd. A Ti-Fe alloy plate was cut into 6 × 0.7 cm pieces (Fe ≤ 0.3).
Instrumentation
The ECL and other electrochemical properties were measured on an MPI-E ECL analysis system produced by Xi’an Raymine Analytical Instruments Co., Ltd. Scanning electron microscopy (SEM) was performed on an S-4800 electron microscope (Hitachi, Ltd., Japan). Energy dispersive spectroscopy (EDS) was carried out using an OXFORD Ultim Extreme. X-ray photoelectron spectroscopy (XPS) was carried out using a Thermo ESCALAB-250 (USA).
Preparation of an ECL biosensor
Ti-Fe–O NTs were successfully synthesized according to the literature [27]. The Ti-Fe–O NTs electrode was placed in 1 mL of PBS containing 3 mM OA, reacted for 5 h at 4 °C, and rinsed with ultrapure water and PBS. The OA-Ti-Fe–O NTs electrode was immersed in 1.0 mL of a solution containing 20 mg of EDC and 10 mg of NHS methyl imidazole to activate the carboxyl groups of OA at 25 °C for 1 h. Washed with PBS to remove excess EDC and NHS on the electrode. Placed the electrode in Ab1 at 4 °C and assembled for 15 h. The Ti-Fe–O NTs electrode assembled with Ab1 was placed in a 3% BSA solution at 4 ºC for 2 h to seal the non-specific active adsorption sites on the array electrode. The obtained electrode was labeled Ab1/Ti-Fe–O NTs.
The Ab1/Ti-Fe–O NTs were immersed in CYFRA21-1 at concentrations of 100 ng·mL−1, 10 ng·mL−1, 1 ng·mL−1, 500 pg·mL−1, 100 pg·mL−1, 10 pg·mL−1, and 1 pg·mL−1 and incubated at 37 °C for 1 h. The Ab1/Ti-Fe–O NTs bound to the antigen were labeled CYFRA21-1/Ab1/Ti-Fe–O NTs. The cells were further incubated with 1 mL of the Ab2/CCB/MNPs solution for 1 h at 25 °C. Finally, the cells were washed with ultrapure water and PBS and stored at 4 °C. The other conditions were as follows: the scanning potential was − 1.0 ~ 0 V, the scan speed was 100 mV·s−1, and the strength of the photomultiplier tube was 750 V.
Results and discussion
Characterization of nanocomposites
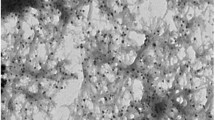
The morphologies of the Ti-Fe–O NTs were first investigated using SEM (Fig. 1a). The results showed the material had a continuous porous nanotube structure. The inner diameter of the nanotubes was approximately 60 ~ 70 nm. The EDS results demonstrated the presence of Ti, O, and Fe (Fig. 1b). The atomic ratios of Ti, Fe, and O were 46.64%, 0.1%, and 53.26%, respectively.
The elemental composition and valence state of the synthesized Ti-Fe–O NTs were analyzed by XPS. The results showed the presence of elemental Ti, Fe, O, and C in the material. Figure S1A shows 4 distinct peaks at approximately 284.94, 459.42, 531.57, and 711.75 eV that were attributed to C 1 s, Ti 2p, O 1 s, and Fe 2p, respectively. We analyzed the Fe elemental peak, which is shown in Fig. S1B. The XPS Fe 2p spectrum exhibits two main peaks at approximately 711.84 and 723.72 eV, corresponding to Fe 2p3/2 and Fe 2p1/2, respectively, and confirming the presence of Fe3+ [18]. The satellite vibration peak of 715.98 eV further confirmed that the nanotubes contained Fe3+ [19, 28]. Given the multiple confirmations of the presence of Fe2O3, the obtained nanotubes were considered to be Ti-Fe–O NTs during the whole experiment.
Figure 2a shows the ECL performance of the pure TiO2 NTs and Ti-Fe–O NTs electrodes in the K2S2O8 coreactant solution. Compared with the electrode modified with pure TiO2 NTs, the ECL intensity of the Ti-Fe–O NTs electrode was 5.7-fold higher, and the onset ECL potential was positively shifted to approximately 200 mV. The ECL intensity of the electrode modified with TiO2 NTs and Ti-Fe–O NTs was measured under the action of a filter with a wavelength of 420 ~ 640 nm, and the results are shown in Fig. 2b. The maximum emission wavelength of the ECL emission peak was approximately 540 nm. Compared with the TiO2 NTs electrode with a wavelength of 500 nm, the Ti-Fe–O NTs electrode exhibited a clear redshift, and the luminescent potential was positively shifted [27]. Compared to the TiO2 NTs, the Ti-Fe–O NTs had a narrower band gap, which is consistent with the results in the literature [18], such that electrons in the Ti-Fe–O NTs were more easily excited to produce the ECL phenomenon.
In order to further explore the performance of CCB/MNPs as quenching agents, the UV–vis absorption is shown in Fig. S2. It can be seen from the figure that there is a broad absorption peak in the wavelength range of 280 ~ 800 nm. This proved that CCB/MNPs were similar to blackbody materials. In addition, CCB was more environmentally friendly than common CdTe nanoparticles. Therefore, CCB/MNPs can be considered as a good quencher.
Optimization of experimental conditions
The pH of the coreactant significantly influenced the ECL of the Ti-Fe–O NTs electrode. Therefore, the pH of the K2S2O8 coreactant was optimized, and the result is shown in Fig. S3. As the pH of the K2S2O8 coreactant increased from 4 to 11, the ECL intensity first increased and then decreased. The ECL intensity had a maximum value at a K2S2O8 coreactant pH of 7.4, which was hence chosen as the optimal detection condition in this study.
ECL behavior of Ti-Fe–O NTs
In a previous report on TiO2 NTs [29], the ECL behavior of Ti-Fe–O NTs with K2S2O8 as a coreactant was studied, and the following mechanism for inducing ECL was proposed.
First, during a negative scan, S2O82− is reduced at the Ti-Fe–O NTs electrode surface, and holes (h+) are generated, as shown in Eqs. (1) and (2). The resulting h+ is injected into the valence band of Ti-Fe–O (Eq. (3)). As the scanning potential develops in the negative direction, electrons fill the conduction band and generate the excited species Ti-Fe–O* (Eq. (4)). Finally, when Ti-Fe–O* returns from the excited state to the ground state, an ECL signal is emitted (Eq. (5)).
In this study, Ti-Fe–O NTs were selected to modify electrodes, resulting in excellent ECL performance. CCB/MNPs were used as quenchers to suppress the Ti-Fe–O NTs signal. Therefore, an ultrasensitive ECL biosensor was fabricated to detect a CYFRA21-1 cell marker.
ECL detection of CYFRA21-1
In this paper, we selected CYFRA21-1 as an antigen for analysis and investigated the potential of using Ti-Fe–O NTs as ECL electrode materials and CCB/MNPs as quenchers. Figure S4 shows the change in the ECL signal when detecting 100 ng·mL−1 CYFRA21-1 with different quenchers. Curves (a) and (b) show that the ECL strength decreased by 20% after CYFRA21-1 was assembled on the Ti-Fe–O NTs electrode. The main reason for this result was that CYFRA21-1 partially hindered electron transfer during the ECL reaction process. When the CYFRA21-1/Ab1/Ti-Fe–O NTs electrode was immersed in the Ab2/CCB/MNPs solution, the ECL signal was quenched by 89% (curve c). However, when the electrode assembled with CYFRA21-1 only combined with Ab2/MNPs, the ECL signal was only quenched by 42% (curve d). A comparative analysis of the results shows that only the combination of CCB and MNPs achieved effective quenching. Therefore, CCB/MNPs were selected as quenchers for subsequent experiments.
In this paper, the obtained biosensor uses the ECL method to detect different concentrations of CYFRA21-1, and the results are shown in Fig. 3. It can be clearly seen from Fig. 3a that as the concentration of CYFRA21-1 increases from 1 pg·mL−1 to 100 ng·mL−1, the intensity of ECL gradually increases. Figure 3b is showing the relationship between the logarithm of the concentration of CYFRA21-1 and ΔI. The linear regression equation ΔI = 1978 logc + 2812 (R2 = 0.996), where ΔI is I0-I, I0 is the ECL intensity of the electrode after incubation in CYFRA21-1 solution, and I is the ECL intensity of CYFRA21-1/Ab1/Ti-Fe–O NTs electrode. In addition, the detection limit of detection (LOD) was 0.114 pg·mL−1.
Stability, repeatability, and selectivity
The prepared biosensor was maintained at 4 °C for 5 days. The result is shown in Fig. S5A, that is, the ECL curve of Ti-Fe–O NTs hardly changes in intensity over 5 days. This result indicates that the prepared ECL sensor had high stability. Figure S5B shows the results of a repeatability test performed on 5 different Ti-Fe–O NTs electrodes. The ECL strength of the 5 electrodes was almost stable, proving that the titanium ferrite electrode had good repeatability.
Figure 4a shows that Ti-Fe–O NTs generated strong and stable ECL signals. We investigated the selectivity of the CYFRA21-1 ECL biosensor. Under fixed detection conditions, we selected interfering proteins, including PSA, CEA, UTI, TC-BSA, and HIV-1 (at a 100 ng·mL−1 concentration), for detection. The results are shown in Fig. 4b: the interfering substances had almost no effect on the ECL biosensor, indicating that the biosensor had particularly good selectivity. Finally, we compared the performance of ECL methods that have been used to detect CYFRA21-1 over the past few years, and the results are shown in Table 1. The results show that our prepared biosensor for detecting CYFRA21-1 has a larger detection range and lower LOD than other detection methods.
Detection of real samples
In order to verify the practicability of real samples, the standard addition method is used to complete the relevant detection. The Affiliated Hospital of Yantai University provides serum with 0.5 ng·mL−1 of CYFRA21-1 in healthy students. Then, different concentrations of CYFRA21-1 (0.1 ng·mL−1, 1 ng·mL−1, 10 ng·mL−1) were added to the serum, and the mixture was used as the target for detection. Table 2 shows that the recoveries of CYFRA21-1 in actual samples ranged from 88.6 to 104.4%, and the RSD was below 3.0%. The results showed that the biosensor can be effectively applied to detect CYFRA21-1 in serum.
Conclusions
In this paper, the properties and applications of Ti-Fe–O-NTs electrodes were investigated using the ECL method. The Ti-Fe–O NTs electrode has high sensitivity and stable ECL signal. However, compared with the common glassy carbon electrode, the preparation process of this electrode is more complicated, takes longer, and needs to be stored at 4 °C. The Ti-Fe–O NTs electrode was used to generate the ECL signal, and a CCB/MNP complex was quenched with high efficiency to design a sandwich immunosensor for CYFRA21-1 detection, with the LOD being 0.114 pg·mL−1. The fabricated biosensor was applied to the detection of CYFRA21-1 in human serum, and satisfactory results were obtained. This result indicated the good potential of the biosensor prepared in this study for practical applications.
References
Bai J, Zhou B-X (2014) Titanium dioxide nanomaterials for sensor applications. Chem Rev 114:10131–10176
Nozik A-J (1983) Photoelectrochemical cells. Nature 414:338–344
Konstantinou I-K, Albanis T-A (2004) TiO2-Assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl Catal B-Environ 49:1–14
Meng N, Leung M, Leung D, Sumathy K (2007) A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew Sust Energ Rev 11:401–425
Mills A, Hunte S-L (1997) An overview of semiconductor photocatalysis. J Atmos Chem 108:1–35
Oriol R, Sirés I, Brillas E, Andrade A-D (2019) A hybrid photoelectrocatalytic/photoelectro-Fenton treatment of Indigo Carmine in acidic aqueous solution using TiO2 nanotube arrays as photoanode. J Electroanal Chem 847:113088
Yogi C, Kojima K, Takai T, Wada N (2009) Photocatalytic degradation of methylene blue by Au-deposited TiO2 film under UV irradiation. J Mater Sci 44:821–827
Park J-Y, Hwang K-J, Lee J-W, Lee I-H (2011) Fabrication and characterization of electrospun Ag doped TiO2 nanofibers for photocatalytic reaction. J Mater Sci 97:64–66
Yang L, Luo S, Liu R, Cai Q, Xiao Y, Liu S, Su F, Wen L (2010) Fabrication of CdSe nanoparticles sensitized long TiO2 nanotube arrays for photocatalytic degradation of anthracene-9-carbonxylic acid under green monochromatic light. J Phys Chem Lett 114:4783–4789
Guo J, Han Q, Zhong S, Zhu B, Huang W, Zhang S (2018) Au/M-TiO2 nanotube catalysts (M=Ce, Ga Co, Y): preparation, characterization and their catalytic activity for CO oxidation. J Sol-gel Sci Technol 86:699–710
Chen Y, Tang W, Ma J, Ge B, Wang X, Wang Y, Ren P, Liu R (2020) Nickel-decorated TiO2 nanotube arrays as a self-supporting cathode for lithium–sulfur batteries. Front Mater Sci 14:266–274
Qin X, Geng L, Wang Q, Wang Y (2019) Photoelectrochemical aptasensing of ofloxacin based on the use of a TiO2 nanotube array co-sensitized with a nanocomposite prepared from polydopamine and Ag2S nanoparticles. Microchim Acta 186:430
Su Y, Yang S, Liu W, Qiao L, Yan J, Liu Y, Zhang S, Fang Y (2017) Visible light photoelectrochemical sulfide sensor based the use of TiO2 nanotube arrays loaded with Cu2O. Microchim Acta 186:4065–4072
Wang W, Xie Y, Xia C, Du H, Tian F (2014) Titanium dioxide nanotube arrays modified with a nanocomposite of silver nanoparticles and reduced graphene oxide for electrochemical sensing. Microchim Acta 181:1325–1331
Akhavan O, Azimirad R (2009) Photocatalytic property of Fe2O3 nanograin chains coated by TiO2 nanolayer in visible light irradiation. Appl Catal A-Gen 369:77–82
Mei Q, Zhang F, Wang N, Yang Y, Wu R, Wang W (2019) TiO2/Fe2O3 heterostructures with enhanced photocatalytic reduction of Cr (VI) under visible light irradiation. RSC Adv 9:22764–22771
Sadeghi-Niaraki S, Ghasemi B, Habibolahzadeh A, Ghasemi E, Ghahari M (2019) Nanostructured Fe2O3@TiO2 pigments with improved NIR reflectance and photocatalytic ability. Mater Chem Phys 235:121769
Zhang Y-N, Huang W-N, Zhang Y-J, Tang B, Xiao H-S, Zhao G-H (2016) Fabrication and enhanced visible-light photoelectrochemical performance of periodic hierarchical 3D Ti-Fe-O structure. Mater Lett 168:24–27
Lu H-X, Dai G-H, Dai R-H, Ding X, Liu M-C, Sun H-H, Sun C-Q, Zhao G-H (2019) Visible-light-driven photoelectrochemical aptasensor based on reduced graphene oxide/Ti-Fe-O nanotube arrays for highly sensitive and selective determination of microcystin-LR. Electrochim Acta 324:134820
Madian M, Wang Z, Gonzalez-Martinez I, Oswald S, Mikhailova D (2020) Ordered Ti-Fe-O nanotubes as additive-free anodes for lithium ion batteries. Appl Mater Today 20:100676
Gui G, Zhuo Y, Chai Y-Q, Liao N, Zhao M, Han J, Zhu Q, Yuan R, Xiang Y (2013) Supersandwich-type electrochemiluminescenct aptasensor based on Ru(phen)32+ functionalized hollow gold nanoparticles as signal-amplifying tags. Biosens Bioelectron 47:524–529
Ml A, Cw A, Lc A, Dla B (2019) A novel electrochemiluminescence sensor based on resonance energy transfer system between nitrogen doped graphene quantum dots and boron nitride quantum dots for sensitive detection of folic acid. Anal Chim Acta 1090:57–63
Zhai Q-F, Li J, Wang E-K (2017) Recent advances based on nanomaterials as electrochemiluminescence probes for the fabrication of sensors. ChemElectroChem 4:1639–1650
Siegel R-L, Miller K-D, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30
Wieskopf B, Demangeat C, Purohit A, Stenger R, Gries P (1995) Cyfra 21–1 as a biologic marker of non-small cell lung cancer: evaluation of sensitivity, specificity, and prognostic role. Chest 108:163–169
Rui H-X, Chang Z-L, Yi L, Wen L-X, Wen B-Y (2015) Optimal cut-off values for CYFRA 21–1 expression in NSCLC patients depend on the presence of benign pulmonary diseases. Clin Chim Acta 440:188–192
Tian C-Y, Xu J-J, Chen H-Y (2012) A novel aptasensor for the detection of adenosine in cancer cells by electrochemiluminescence of nitrogen doped TiO2 nanotubes. Chem Commun 48:8234–8236
Zhang Q, Yu L, Xu C, Zhang W, Diao G (2020) A novel method for facile preparation of recoverable Fe3O4@TiO2 core-shell nanospheres and their advanced photocatalytic application. Chem Phys Lett 761:138073
Dai P, Ke J, Xie C, Wei L, Jin J (2020) An off–on electrochemiluminescence detection for microRNAs based on TiO2 nanotubes sensitized with gold nanoparticles as enhanced emitters. Anal Bioanal Chem 412:5779–5787
Zeng Y, Bao J, Zhao Y-N, Huo D-Q, Chen M (2018) A sensitive label-free electrochemical immunosensor for detection of cytokeratin 19 fragment antigen 21–1 based on 3D graphene with gold nanopaticle modified electrode. Talanta 178:122–128
Wang X, Zhang Y, Zhao L, Zhao D, Wang H, Yang J (2020) Polysaccharide-enhanced ARGET ATRP signal amplification for ultrasensitive fluorescent detection of lung cancer CYFRA 21–1 DNA. Anal Bioanal Chem 412:2413–2421
Wang Y-X, Guo X-Y, Liu J-W, Weng Z-X, Chen Y-X (2018) Development of chemiluminescent microparticle immunoassay for cytokeratin 19 fragment 21–1 content. Chin J Biologic 31:523–527
Yang H, Bao J, Huo D, Zeng Y, Hou C (2020) Au doped poly-thionine and poly-m-Cresol purple: Synthesis and their application in simultaneously electrochemical detection of two lung cancer markers CEA and CYFRA21–1. Talanta 224:121816
Funding
This work was financially supported by the National Natural Science Foundation of China (21778047), and the Natural Science Foundation of Shandong Province (grant no. ZR2021MB024).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hua, Q., Tang, F., Wang, X. et al. Electrochemiluminescent determination of CYFRA21-1 serum levels using Ti-Fe–O nanotubes immunoassay. Microchim Acta 189, 136 (2022). https://doi.org/10.1007/s00604-022-05239-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05239-z