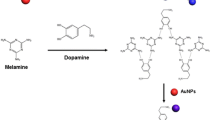
Abstract
Hemoglobin–stabilized gold nanoclusters (Hb–AuNCs) were prepared by using hemoglobin as both reducing and stabilizing agents. The Hb–AuNCs display a strong catalytic effect on the chemiluminescence (CL) reaction of luminol with NaIO4. The CL mechanism is discussed and the experimental variables are examined. It is found that dopamine strongly inhibits CL. This finding is exploited in a CL–based dopamine assay that works in the range between 0.3 and 9.0 nM and has a 0.1 nM detection limit. The relative standard deviation is 3.1% at a 5 nM dopamine level (for n = 11). The method was applied to the determination of dopamine in spiked human plasma with satisfactory results.

Hemoglobin (Hb) coated gold nanoclusters (AuNCs) display a strong catalytic effect on the luminol-periodate chemiluminescence (CL) system. It is found that the presence of dopamine strongly reduces CL, and this finding is exploited in the highly sensitive dopamine assay presented here.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemiluminescence (CL) has been extensively explored to analyze different trace substances in a veracity of samples [1,2,3]. The study of CL systems has been diverting from molecular systems to nanomaterial–assisted systems with the purpose to improve the properties of sensitivity, selectivity, and stability owing to the rapid development of nanoscience [4,5,6]. The catalytic behavior of nanomaterials in CL reactions is strongly dependent on their sizes [7], surface charge [8] and morphology [9,10,11].
Metal nanoclusters (NCs) are composed of several to tens of atoms with sizes between atoms and small nanoparticles that have attracted enormous attention since their discovered [12]. This can be witnessed by an explosive growth in publications concerning the application of metal NCs as fluorescent probes in biosensing and bioimaging [13, 14]. Besides of their famous fluorescent property, their catalytic activity has also attracted analytical scientist′s interest. For example, Chen et al. used metal NCs to catalyze the luminol CL reaction with H2O2 [15, 16] or KMnO4 [17] as the oxidants. Periodate (IO4 −) is colorless, thus avoiding emission absorption problems. Additionally, periodate is more stability than H2O2 and hypohalites and related oxidants. Periodate can oxidize luminol in an alkaline solution to produce CL [18, 19]. Up to date, there is no report with regard to the periodate–luminol CL system catalyzed by metal NCs.
Dopamine (3,4-dihydroxyphenethylamine) is one of the most important neurotransmitter and plays significant roles in the function of human metabolism, central nervous, renal and hormonal system. The level of dopamine in human body is an important biomarker for the diseases of schizophrenia and Parkinson. Therefore, it is of very urgent to develop sensitive and selective method for the determination of dopamine in biological fluid [20]. A variety of techniques have been reported for the detection of dopamine, including spectrophotometry [21, 22], fluorescence [23, 24], electrochemical methods [25,26,27], and chemiluminescence [28,29,30,31,32]. Among them, CL methods have the advantages of simple and inexpensive instrumentation, high sensitivity, and wide linear dynamic range.
In this work, hemoglobin–stabilized gold nanoclusters (Hb–AuNCs) were prepared through a biomineralization process by revisiting the previous work of Shamsipur et al. [33]. The reaction time is shortened from 30 days to 1.5 days (36 h) by increasing the reaction temperature from 37 °C to 60 °C. The prepared Hb–AuNCs exhibits a significant catalytic effect on the luminol–periodate CL reaction. This system is developed as a sensitive CL method for the determination of dopamine which strongly inhibits the CL signal. The practicability of the method is evaluated by the determination of dopamine in spiked human plasma samples.
Experimental
Apparatus
All CL measurements were conducted on an IFFM–D flow–injection CL analyzer (http://www.chinaremex.com, Xi′an Remax, China) equipped with a CR105 photomultiplier tube (http://www.hamamatsu.com.cn, Hamamatsu Photonics (China) Co., Ltd). Fluorescence spectra and CL spectra were obtained on a F–2700 fluorescence spectrophotometer (http://www.hitachi-hightech.com, Hitachi, Japan). Absorption spectra were recorded with a U–3900 UV–Visible spectrophotometer (http://www.hitachi-hightech.com, Hitachi, Japan). Transmission electron microscopy (TEM) was performed on a JEM–2100 transmission electron microscope (http://www.jeol.co.jp/cn, Japan Electronic Company, Japan) at an accelerating voltage of 200 kV. The X–ray photo electron spectrum (XPS) was measured on UltraDLD X–ray photoelectron spectrometer (http://www.kratos.com, Kratos, Britain) using Al–Kα as the exciting source (1486.6 eV) and C1s at 284.8 eV for binding energy calibration.
Chemicals
All chemicals used were of analytical–grade; water was produced from Milli–Q reference ultra–pure water system (http://www.astk.com.cn, Beijing ASTK Technology Development Co., Ltd., China). Dopamine was purchased from Shanghai Aladdin Bio–Chem Technology Co. Ltd. (http://www.aladdin-e.bioon.com.cn, Shanghai, China). Chloroauric acid (HAuCl4•4H2O) was obtained from Shanghai Chemical Reagent Factory (http://www.shiyicr.com.cn, Shanghai, China). Hemoglobin (Hb) was offered by Beijing Solarbio Science & Technology Co., Ltd. (https://solarbio.en.alibaba.com, Beijing, China). Luminol was purchased from Tianjin Fuchen Chemical Reagents Factory (http://www.tjfch.com/en, Tianjing, China). Sodium periodate (NaIO4) was bought from Tianjing Kemiou Chemical Reagent Co., Ltd. (http://www.tjkermel.com, Tianjing, China). Other chemicals were obtained from Xi’an Chemical Reagent Factory (http://www.crc-xa.com, Xi’an, China).
A 10.0 mM dopamine stock solution was prepared by dissolving 94.8 mg dopamine in 50 mL water. More dilution solutions of dopamine were prepared by diluting this stock solution before use. All dopamine solutions were protected from the light and stored in a refrigerator. Luminol stock solution (25 mM) was prepared by dissolving 0.2214 g luminol solid in 50 mL of 0.1 M NaOH solution. Luminol working solutions were prepared by diluting the luminol stock solution with a suitable concentration of NaOH solution. HAuCl4 solution (10.0 mM) and NaIO4 solution (10.0 mM) were prepared in water.
Synthesis of Hb–AuNCs
All glassware were soaked in concentrated HNO3 solution for 12 h, rinsed thoroughly with water, and then dried for use. The preparation of Hb–AuNCs was similar to the previous procedure of Shamsipur et al. [33] with some modification. In brief, aqueous HAuCl4 solution (2.8 mM, 5 mL) was mixed with Hb solution (50 mg/mL, 5 mL) at 60 °C with vigorously stirring. Ten min later, 1 mL of 1 M NaOH solution was added and the reaction was performed at 60 °C for 36 h. The resultant solution was centrifuged at 12000 rpm for 10 min to remove any large size of particles. The blackish green solution (Hb–AuNCs) was collected and stored in a refrigerator for further use.
CL measurement
Figure 1 shows the schematic diagram of CL flow system used. Hb–AuNCs solution (50 μL) was injected into the carrier H2O via a six–way injection valve, which was then merged with the combined stream of luminol solution and NaIO4 solution just prior to the flow cell. The CL signal produced in the flow cell was monitored by the photomultiplier tube biased a high voltage at 800 V and recorded as I0. In the case of dopamine, the carrier H2O was replaced by dopamine standard/sample solution and the responding CL signal was taken as I. The relative CL signal (ΔI), calculated by subtracting I from I0, was used to optimization, calibration, and quantification of dopamine.
Results and discussion
Revisiting the synthetic conditions of Hb–AuNCs
In the work of Shamsipur et al. [33] for the synthesis of Hb–AuNCs, 30 days was needed to complete the reaction. To shorten the reaction time, the synthesis conditions, including reaction temperature and reaction time, was revisited.
The effect of reaction temperature was examined in the range of 37 °C–80 °C. The reaction efficiency increased with increasing the reaction temperature as indicated by the rapid increase in the fluorescence intensity of Hb–AuNCs at 450 nm (upon excitation at 365 nm) (Fig. S1). When the reaction temperatures were above 60 °C, the fluorescence intensity remained a platform indicating the complete of the reaction.
The effect of reaction time was studied from 4 h to 48 h by fixing the reaction temperatures at 60 °C. As shown in Fig. S2, the fluorescence intensity increased as the reaction time was increasing from 4 h to 36 h. When the reaction time was longer than 36 h, the fluorescence intensity remained essentially constant, indicating the complete of the reaction. Therefore, the reaction temperatures of 60 °C and the reaction time of 36 h were employed for the preparation of the Hb–AuNCs.
The prepared Hb–AuNCs were characterized with UV–vis, fluorescence, TEM, and XPS. The Hb–AuNCs have a wide absorption band centering at 350–400 nm and lack typical surface plasmon resonance peak at 520 nm (Fig. 2a). The TEM image confirms the Hb–AuNCs are spherical in shape with a diameter about 3 nm (Fig. 2b). The XPS suggests the presence of Au(0) and Au(I) in the Hb–AuNCs (Fig. 2c). Upon excitation at 365 nm, the Hb–AuNCs emits blue emission with a maximal emission at about 450 nm (Fig. 2d), which was consistent with that of Shamsipur et al. [33]. All of these confirmed the successful preparation of the Hb–AuNCs.
Enhancement of the luminol–NaIO4 CL system by Hb–AuNCs
The effect of the Hb–AuNCs on luminol–NaIO4 CL system was investigated by a batch method. Into reaction cell, 0.5 mL of H2O, 0.5 mL of 0.5 mM luminol solution, and 0.5 mL of 1:10 dilution Hb–AuNCs solution were added successively. After homogeneous, 0.5 mL of 0.1 mM NaIO4 solution was introduced to initiate the CL reaction. As shown in Fig. 3a, the CL signal of luminol–NaIO4 reaction is obviously enhanced when the Hb–AuNCs is present in the system (curve a and b). To rule out possible catalytic activity from Hb, a control experiment was conducted by replacing Hb–AuNCs with Hb. Hb displays weak catalytic effect on the luminol–NaIO4 system (curve c), but it is much lower that of Hb–AuNCs (curve b). Therefore, the catalytic activity originates from the Hb–AuNCs not from Hb. Further investigation indicates dopamine significantly inhibits the CL signal of luminol–NaIO4–Hb–AuNCs reaction (curve d).
To elucidate the enhancing mechanism of the Hb–AuNCs on luminol–NaIO4 reaction, the CL spectra were investigated. To obtain the CL spectra, the CL flow cell was placed before the emission window of F–2700 fluorescence spectrophotometer with the excitation source turned off. The CL reagents were continuously driven into the flow cell by peristaltic pumps. The CL signal was recorded by the photomultiplier tube biased the high voltage at 700 V. As shown in Fig. 3b, the CL spectra of two reactions have the same spectrum profile and maximum emission wavelength, suggesting that they share the same CL emitter. The maximum emission wavelength locates at 425 nm, which is the typical emission of luminol reaction. Therefore, the CL emitter is still the excited sate 3–aminophthalate anions, the oxidation product of luminol [34]. The presence of the Hb–AuNCs does not alter the CL emitter, just enhances the CL intensity. Therefore, the enhancing mechanism is therefore attributed the catalytic effect of the Hb–AuNCs on the luminol–NaIO4 reaction.
Superoxide anion was reported to be generated from the reaction between periodate and dissolved O2 in alkaline solution [35]. The superoxide anion can oxidize luminol to produce CL emission [36]. In the presence of the Hb–AuNCs, the reaction between periodate and dissolved O2 is accelerated and more amounts of superoxide anion is produced. As a result, stronger CL emission is recorded. Dopamine belongs to polyhydroxy compounds. Such kind compounds have strong reducibility and can be used as scavenging agents of reactive oxygen species. Thus, in case of dopamine, the produced superoxide anion is eliminated and the CL signal is inhibited.
Optimization of CL conditions
Hb–AuNCs concentration has a critical influence on the CL reaction. The Hb–AuNCs concentration is expressed as the dilution ratio of the Hb–AuNCs with water. Figure 4a shows the relationship between the dilution ratio and the relative CL intensity. The relative CL intensity continues to decrease with the increase in the dilution ratio of the Hb–AuNCs. Considering the sensitivity and reagent consumption, the Hb–AuNCs in 1:20 dilution ratio is employed.
As the CL reagent, the concentration of luminol has important influence on the sensitivity. The effect of luminol concentration on the CL reaction was studied in the range of 10–750 μM and the results are shown in Fig. 4b. A maximum relative CL intensity is observed at 50 μM luminol. Higher or lower concentrations of luminol cause a decrease in the relative CL intensity. Thus, 50 μM luminol is selected as the optimum.
Luminol CL reaction occurs in an alkaline condition. The alkalinity of the reaction is controlled by NaOH added into luminol solution. The relative CL intensity increases as the concentration of NaOH was increasing from 0.01 M to 0.25 M, (Fig. 4c). The relative CL intensity decreases with the increase in the concentration of NaOH above 0.25 M. The relative CL intensity has a maximum at 0.25 M NaOH and this concentration of NaOH is employed.
Figure 4d shows the influence of the concentration of NaIO4 on the relative CL intensity. The relative CL intensity increases with increasing the concentration of NaIO4 up to 0.1 mM. Further increase in the concentration of NaIO4 results in a decrease in the relative CL intensity. Therefore, 0.1 mM NaIO4 is selected.
Response of the system to dopamine
Under above selected conditions, the response of the system for different concentrations of dopamine was investigated. The logarithm of the relative CL intensity (log ΔI) was found to be linear related with the logarithm of concentrations of dopamine (log c) from 0.3 to 9.0 nM (Fig. S3). The linear equation can be expressed log ΔI = 2.96 + 0.69 log c (nM) (r2 = 0.9921). The limit of detection is 0.1 nM. In comparison with other previously reported methods for the detection of dopamine, this method has higher sensitivity (Table 1). The 11 consecutive measurements of 5.0 nM dopamine solution produced a relative standard deviation of 3.12%, indicating the precision of the method is acceptable.
Interference study
In order to apply the method to the determination of dopamine in human plasma, the effect of some potential interfering species was investigated on the determination of 5.0 nM dopamine. The interfering species include 5.0 μM K+, Ca2+, Na+, glucose, uric acid, ascorbic acid, 0.5 μM lysine, lactate, oxalate, cysteine, ascorbic acid, Zn2+, 10.0 nM adrenaline and norepinephrine. Figure 5 shows the relative CL intensities of dopamine alone and of the mixture of dopamine with interfering species. In contrast to the CL intensity of dopamine alone, the CL intensities changed negligibly in the presence of interfering species, indicating the present CL system has good selectivity for dopamine. It should be indicated that 1000–fold is the highest tolerable ratio that we examined. The actual tolerable ratios for some interfering species are possible higher than this tolerable ratio.
Determination of dopamine in spiked human plasma samples
In order to verify the practicality, the method was applied to the determination of dopamine in spiked human plasma samples. Human plasma samples from three health persons were obtained from Xi′an Community Hospital. The protein in plasma samples was removed by centrifuging at 12000 rpm for 10 min. The supernatant was transferred into a test tube and used as sample. Into 0.5 mL of sample, a known amount of dopamine standard was added and diluted to 10.0 mL with water for detection. The content of dopamine in the sample was determined by standard addition method. As shown in Table S1, the method gave rise to good recoveries and acceptable precision, thus demonstrating the practicality of this method in the determination of dopamine in practical samples.
Conclusions
In summary, Hb–AuNCs were prepared by incubating HAuCl4 and Hb at 60 °C for 36 h through a biomineralization process. The Hb–AuNCs significantly catalyze the CL reaction of luminol with periodate and dopamine strongly inhibits the signal. The method allows to the measurement of dopamine in the concentration range of 0.3–9.0 nM. The method is very sensitive and can analyze dopamine in spiked human plasma. Beside adrenaline and norepinephrine also inhibit the CL signal. Further work is in progress aiming at on the determination of other catecholamine with this CL system.
References
Su YY, Deng DY, Zhang LC, Song HJ, Lv Y (2016) Strategies in liquid–phase chemiluminescence and theirs applications in bioassay. TRAC–Trend Anal Chem 82:394–411
Ocana–Gonzalez JA, Ramos–Payan M, Fernandez–Torres R, Navarro MV, Bello–Lopez MA Application of chemiluminescence in the analysis of wastewaters–A review. Talanta 122:214–222.
Seidel M, Niessner R (2014) Chemiluminescence microarrays in analytical chemistry: a critical review. Anal Bioanal Chem 406(3):5589–5612
Zhang ZF, Cui H, Lai CZ, Liu LJ (2005) Gold nanoparticle–catalyzed luminol chemiluminescence and its analytical applications. Anal Chem 77(10):3324–3329
Zhong JP, Yuan ZQ, Lu C (2016) Layered–nanomaterial–amplified chemiluminescence systems and theirs analytical applications. Anal Bioanal Chem 408(30):8721–8746
Shah SNA, Lin JM (2017) Recent advances in chemiluminescence based on carbonaceous dots. Adv Colloid Interfac 241:24–36
Duan CF, Cui H, Zhang ZF, Liu B, Guo JZ, Wang W (2007) Size–dependent inhibition and enhancement by gold nanoparticles of luminol–ferricyanide chemiluminescence. J Phys Chem C 111(12):4561–4566
Qi Y, Xiu FR (2016) Sensitive and rapid chemiluminescence detection of propranolol based on effect of surface charge of gold nanoparticles. J Lumin 171:238–245
Li J, Quan J, Du JX, Liu M (2013) Chemiluminescence determination of trimetazidine via inducing the aggregation of gold nanoparticles. Spectrochim Acta A 114:33–37
Qi YY, Li BX (2013) Enhanced effect of aggregated gold nanoparticles on luminol chemiluminescence system and its analytical application. Spectrochim Acta A 111:1–6.
Chen Q, Bai S, Lu C (2012) The new approach for captopril detection employing triangular gold nanoparticles–catalyzed luminol chemiluminescence. Talanta 89(2):142–148
Wilcoxon JP, Abrams BL (2006) Synthesis, structure and properties of metal nanoclusters. Chem Soc Rev 35(11):1162–1194
Song XR, Goswami N, Yang HH, Xie JP (2016) Functionalization of metal nanoclusters for biomedical applications. Analyst 141(11):3126–3140
Shang L, Dong SJ, Nienhaus GU (2011) Ultra–small fluorescent metal nanoclusters: synthesis and biological applications. Nano Today 6(4):401–418
Deng M, Xu SJ, Chen FN (2014) Enhanced chemiluminescence of the luminol–hydrogen peroxide system by BSA–stabilized au nanoclusters as a peroxidase mimic and its application. Anal Methods–UK 6(9):3117–3123
Xu SJ, Chen FN, Deng M, Sui YY (2014) Luminol chemiluminescence enhanced by copper nanoclusters and its analytical application. RSC Adv 4(30):15664–15670
Xu SJ, Deng M, Sui YY, Zhang YX, Chen FN (2014) Ultrasensitive determination of bisphenol a in water by inhibition of copper nanoclusters–enhanced chemiluminescence from the luminol–KMnO4 system. RSC Adv 4(84):44644–44649
Li GH, Wang NN, Li XH, Liu P (2015) Potassium periodate–luminol–silver nanoparticles as a new chemiluminescence system and its application to detect fenoterol and orciprenaline. Anal Lett 48(4):682–696
Li SF, Li XZ, Xu J, Wei XW (2008) Flow–injection chemiluminescence determination of polyphenols using luminol–NaIO4–gold nanopartices system. Talanta 75(1):32–37
Rasheed PA, Lee JS (2017) Recent advances in optical detection of dopamine using nanomaterials. Microchim Acta 184(5):1239–1266
Fang X, Ren HX, Zhao H, Li ZX (2017) Ultrasensitive visual and colorimetric determination of dopamine based on the prevention of etching of silver nanoprism by chloride. Microchim Acta 184(2):415–421
Chen ZB, Zhang CM, Zhou TH, Ma H (2015) Gold nanoparticle based colorimetric probe for dopamine detection based on the interaction between dopamine and melamine. Microchim Acta 182(5–6):1003–1008
Zhang XL, Zhu YG, Guo XH, Zhang B, Jia X, Dai B (2016) A simple, fast and low–cost turn–on fluorescence method for dopamine detection using in situ reaction. Anal Chim Acta 944:51–56
Diaz–Diestra D, Thapa B, Beltran–Huarac J, Weiner BR, Morell G (2017) L–cysteine capped ZnS:Mn quantum dots for room–temperature detection of dopamine with high sensitivity and selectivity. Biosens Bioelectron 87:693–700.
Alvare–Martos I, Ferapontova EE (2016) Electrochemical label–free aptasensor for specific analysis of dopamine in serum in the presence of structurally related neurotransmitters. Anal Chem 88(7):3608–3616.
Arulraj AD, Arunkumar A, Vijayan M, Viswanath KB, Vasantha VS (2016) A simple route to develop highly porous nano polypyrrol/reduced grapheme oxide composite film for selective determination of dopamine. Electrochim Acta 206:77–85
Li J, Zhang N, Sun QQ, Bai ZM, Zheng JB (2016) Electrochemical sensor for dopamine based on imprinted silica matrix–poly(anilineboronic acid) hybrid as recognition element. Talanta 159:379–386
Wabaidur SM, Alothman ZA, Alam SM, Lee SH (2012) Flow injection–chemiluminescence determination of dopamine using potassium permanganate and formaldehyde system. Spectrochim Acta A 96:221–225
Amjadi M, Manzoori JL, Hallaj T, Sorouraddin MH (2014) Strong enhancement of the chemiluminescence of the cerium(IV)–thiosulfate reaction by carbon dots, and its application to the sensitive determination of dopamine. Microchim Acta 181(5):71–677
Zhu Q, Chen YL, Wang WF, Zhang HG, Chen CL, Chen XG (2015) A sensitive biosensor for dopamine determination based on the unique catalytic chemiluminescence of metal–organic framework HKUST–1. Sensor Actuat B 210(3):500–507
Fan XQ, Feng Y, Su YY, Zhang LC, Lv Y (2015) A green solid–phase method for preparation of carbon nitride quantum dots and their applications in chemiluminescence dopamine sensing. RSC Adv 5(68):55158–55164
Gao WY, Qi LM, Liu ZY, Majeed S, Kitte SA, Xu GB (2017) Efficient lucigenin/thiourea dioxide chemiluminescence system and its application for selective and sensitive dopamine detection. Sensor Actuat B 238:468–472
Shamsipur M, Molaabasi F, Shanehsaz M, Moosav–Movahedi AA (2015) Novel blue–emitting gold nanoclusters confined in human hemoglobin, and their use as fluorescent probes for copper(II) and histidine. Microchim Acta 182(5):1131–1141.
Merenyi G, Lind J, Ericksen TE (1990) Luminol chemiluminescence: chemistry, excitation, emitter. J Biolumin Chemilumin 5(1):53–56
Lin JM, Yamada M (1999) Oxidation reaction between periodate and polyhydroxyl compounds and its application to chemiluminescence. Anal Chem 71(9):1760–1766
Du JX, Quan J, Wang YD (2012) Chemiluminescence determination of timolol maleate by gold nanoparticles–catalyzed luminol–N-bromosuccinimide system. Talanta 90:117–122
Acknowledgments
The authors gratefully acknowledge financial support from Fundamental Research Funds for the Central Universities (Grant No. xjj2015121).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 522 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Peng, W. & You, X. Determination of dopamine by exploiting the catalytic effect of hemoglobin–stabilized gold nanoclusters on the luminol–NaIO4 chemiluminescence system. Microchim Acta 184, 3539–3545 (2017). https://doi.org/10.1007/s00604-017-2374-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2374-1