Abstract
The authors describe an impedimetric method for the quantitation of the DNA of the human papilloma virus (HPV) type 16. A glassy carbon electrode (GCE) was modified with gold nanosheets and is shown to be superior to a common gold disk electrode. A single-stranded 25mer oligonucleotide (ssDNA) acting as the probe DNA was immobilized via its thiolated 5′ end on both electrodes. After hybridization with target (analyte) DNA, electrochemical impedance spectra were acquired in the presence of hexacyanoferrate as a redox marker. The sensor can distinguish between complementary, non-complementary and single base pair mismatches of HPV ssDNA. At a 1 mM hexacyanoferrate concentration, the biosensors respond to target DNA in the 1 μM to 1 pM concentration range, and the detection limit is 0.15 pM. The results illustrate that the use of gold nanosheets on a GCE distinctly improves the detection and differentiation of HPV compared to using bare gold.

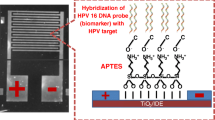
Schematic of exploiting gold nanosheets as a platform for HPV detection. The method works in the 1 μM to 1 pM HPV concentration range and has a 0.15 pM detection limit..
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human papilloma virus (HPV) infection is associated with cancers such as cervical cancer and sexually transmitted diseases (STDs), especially genital warts and condylomas. There are 230 papilloma virus classified as being of low, intermediate and high risk on the basis of their role in the incidence of cervical malignancy. High-risk genotypes of HPV potentially lead to the development of cervical cancer, the third most common cancer among women after endometrium and ovarian cancers in the USA. It is a major cause of morbidity and mortality worldwide and the second cause of cancer deaths in women in developing countries. Among 14 high risk types of HPV, two of them including subtypes 16 and 18, are the most important high-risk genotypes worldwide which are observed in 62% of cervical cancers [1].
Up to 93% of cervical cancers are preventable, therefore their identification are of great importance. HPV cannot be distinguished through cell culture methods and serologic tests due to the low sensitivity and specificity. In contrast, molecular cervical cancer screening technologies including hybrid capture assay and polymerase chain reaction (PCR) which are based on the detection of the DNA of viruses are effective methods to diagnose the HPV infection. However, sample preparation and purification in these methods are time consuming and labor intensive.
In general DNA-based sensors have found numerous critical applications such as clinical diagnosis, environmental pollution analysis and food safety. Different methods including mass spectroscopy [2], fluorescence imaging/spectroscopy [3] and electrochemical techniques [4] have been developed over the past decades. DNA-based sensors are basically divided into two main categories: employing tags for target DNA (labeling DNA targets) and with no tags (label free) [5]. Common labels utilized for DNA hybridization detection are fluorescent dyes, redox active enzymes, magnetic particles and different types of nanoparticles [6]. In a label-free approach, DNA sensors operate based on the detection of unlabeled DNA sequences. This can be performed for example by measuring the signal due to the direct oxidation of DNA bases or using techniques which are sensitive to changes in the electrical properties of bio-modified electrode surfaces, such as quartz crystal microbalance, surface plasmon resonance, sensors based on optical properties and electrochemical impedance spectroscopy (EIS) [7].
Among all these methods, electrochemical protocols offer many advantages over others due to their simplicity, high sensitivity, great selectivity and relative low cost for the detection of DNA hybridization [8]. As the electrochemical reactions produce a signal directly and do not need a separate transducer, electrochemical based methods can be applied as a straightforward technique for the detection of biological species [9]. Electrochemical DNA-based sensors detect the hybridization of two complementary pieces of DNA by monitoring of current through the electrode. Based on their great advantageous such as high sensitivity, fast response, simplicity, portability and lower cost, they have found lots of applications [10].
In this regard, the new horizons rely on the employing nanostructured electrodes with large surface areas and specific electronic characteristics. Nanostructures with different compositions, structures and geometries have been employed in various types of chemical and biological sensors such as identifying pathogens or DNA of viruses. For example, quantum dots (as fluorescent tags),silica nanoparticles, gold nanoparticles and carbon nano-onions have been utilized in DNA biosensors [11].
Employing nanostructures in electrochemical impedance DNA-based sensors have two main advantages, the first is improving the impedimetric response that can affect the sensitivity and reproducibility of sensor [5]. In this regards different types of nanostructures including carbon nanostructured diamond and nanotubes, silicon nanoparticles, gold nanoparticles, polymeric nanostructures and nanocomposites have been used as sensing platform, accordingly. The other advantage relates to the signal amplification via employing nanomaterials such as quantum dots [5]. In this regards nanostructures with high active surface area can be applied as electrode for electrochemical impedance sensors that enhance the performance of the sensor, significantly [12].
Wang et al. reported sensitive detection of human hepatitis B and papilloma viruses using EIS technique [13] SWCNTs/Au nanoparticles were used as a platform for the self-assembly of single-stranded probe DNA. We have compared the performance of two fundamental categories of electrodes employing bulk gold and TiO2 nanotube arrays. Also, in order to confirm the results, we have done numerical simulation on the mechanism of electrode performance. Results showed that geometrical and electrical parameters of electrodes can have a direct effect on the response of sensors [14].
Synthesis and application of gold nanostructures with various morphologies have attracted many researchers, due to their optical, electrical, and chemical properties [15]. For instance, gold nanostructures have been widely used in plasmonic applications [16], surface-enhanced Raman spectroscopy(SERS) [17], and chemical and biological sensing [18].
Among different morphologies of gold nanostructures, gold nanosheets have interesting properties. They have many edges and corners and therefore can serve as more active sites for catalysis compared to spherical nanoparticles [19].
Previously, the high electrocatalytic properties of gold nanosheet modified electrodes were reported toward oxidation of some important analytes [20].
Here in this paper, the nanostructured electrode based on gold nanosheets has been introduced as a platform for DNA-sensing. The electrochemical impedance response of various concentrations of complementary HPV target sequence DNA and non-complementary sequence upon immobilization and hybridization of HPV DNA on gold nanosheets has been measured and compared with traditional gold electrodes. The results show that nanostructured electrode based on gold nanosheets has the potential to consider as an electrode in bio-sensing applications.
Materials and method
Materials
Choline choride (ChCl), gallic acid (GaA), glyserol (Gly), gum arabic (GA), potassium chloride, K4Fe(CN)6, K3Fe(CN)6, ethanol, HCl, KCl, NaCl, EDTA and Na3C6H5O7 were purchased from Merck (www.Merckmillipore.Com).
Tetrachloroauric acid (HAuCl4) was prepared by dissolving 99.99% gold metal in aqua regia (1:3 ratio of conc. Nitric acid and conc. Hydrochloric acid by volume) at 80 °C under gentle stirring until the gold metal completely dissolved. The solution was further boiled until it almost dried and volume-adjusted to 25 mL and kept as the stock HAuCl4 solution.
The buffers used in this work are as follows: DNA immobilization buffer: buffer Tris-EDTA (TE, 10 mM Tris–HCl, 1 mM EDTA, pH 8.0), hybridization buffer: 2× saline-sodium citrate (SSC,300 mM NaCl, 20 mM Na3C6H5O7, pH 7.0). Electrolyte solution is 0.2 M KCl solution containing 2 mM K4Fe(CN)6/K3Fe(CN)6 (1:1). All solutions were prepared in deionized water (DI water).
25-mer ssDNA sequence with HS-(CH2)6-modification at the 5′-end with HPLC purification (as HPV 16 probe) and all target oligonucleotides with BIO-RP purification were purchased from Bioneer Corporation (South Korea) (www.bioneer.com) and were used as-received. Stock solutions (100 μM) of the DNA sequences were prepared with sterile distilled water (SD water) and stored at a refrigerator at −20 °C.
Specificity of the oligonucleotide sequences were investigated with Basic Local Alignment Search Tool (BLAST) [21], the ssDNA sequences designed were as follows:
-
25-merthiolated sequence of HPV type 16 ssDNA (probe):
-
5′-SH-AAAGCAAAGTCATATACCTCACGTC-3′.
-
25-mer sequence of HPV type 16ssDNA (complementary target):
-
5′-GACGTGAGGTATATGACTTTGCTTT -3′.
-
25-mer sequence of ssDNA (non complementary target):
-
5′-TTGCAAGACAGTATTGGAACTTACA-3′.
-
25-mer single mismatch sequence of HPV type 16ssDNA:
-
5′- AACGTGAGGTATATGACTTTGCTTT-3′.
Synthesis of gold nanosheets
Gold nanosheets were synthesized from HAuCl4 through reduction utilizing ChCl/GaA/Gly deep eutectic solvent (DES) as the reducing and directing, and GA as the stabilizing and shape-controlling agents, based on the method reported by Tohidiet al. [20].ChCl/GaA/Gly DES were formed by gently stirring the ChCl, GaA and Gly at 100 °C until a clear, homogenous liquid formed after 1 h. The ChCl:GaA:Gly ratio was 1:0.25:0.25 as reported in the literature [22].
Briefly, in a 100 mL vial, 100 mL ChCl/GaA/Gly DES solution (0.01% w/v) and GA (1.5 mg mL−1) were mixed under vigorous stirring for 15 min. 1.5 ml of HAuCl4 solution (0.1 M) were added to the above solution with continuous stirring (500 rpm) under ambient conditions. Large gold nanosheets were synthesized after 8 h. The gold nanosheets were purified by washing with ethanol and DI water through repeated steps of centrifugation and removal of supernatant. Finally, the gold nanosheets were redispersed in 1 mL of DI water.
Pretreatment of gold electrodes
Prior to probe DNA immobilization, a gold electrode (GE) with 2 mm in diameter was cleaned. The electrode was polished with 0.3 and 0.05 μm alumina slurry on a pad to a mirror like surface, rinsed with DI water and finally cleaned with ethanol and DI water in a sonicator bath for 2 min. The polished electrode was dipped into 0.1 M H2SO4solution and the potential was cycled between −500 and 1000 mV (versus Ag/AgCl) until the shape of cyclic voltammograms did not change any more.
Preparation of glassy carbon- gold nanosheet (GC-GNS) electrode
Glassy carbon (GC) electrode with 2 mm in diameter was cleaned. The electrode was polished with 0.3 and 0.05 μm alumina slurry on a pad to a mirror like surface, rinsed with DI water and finally cleaned with ethanol and DI water in a sonicator bath for 2 min. After that 2 μL of gold nanosheet solution was placed onto the electrode surface and allowed to dry at room temperature for 60 min.
Immobilization and hybridization of HPV DNA
For immobilization of the probe DNA, 2 μL of the thiol modified oligonucleotide (1 μM) in TE buffer (10 mM Tris–HCl, 1mM EDTA, pH 8.0) was placed on the surface of the electrode and after that the electrode were held upside-down in a beaker. The end of the beaker was sealed with a rubber cap to protect the solution from evaporation. After the electrode was kept in room temperature for 2 h, it was washed carefully and repeatedly with DI water. For hybridization with target DNA, 2 μL of DNA target solution (in various molarity from 1 × 10−6 to 1 × 10−12 M) in 2× saline sodium citrate buffer (SSC, pH 7.0) was contacted with the electrode surface (modified with the probe DNA) and the electrode were held upside-down in the beaker. After the electrode was kept in room temperature for 2h, it was thoroughly rinsed with the 2× SSC buffer and used for EIS measurement. The electrode for blank measurements was prepared in the same way as the other electrodes. The only difference lies in hybridization step, where the electrode was placed in SSC free of target DNA.
Electrochemical measurements
Electrochemical impedance responses were monitored using a frequency response analyzer and AUTOLAB PGSTAT302N potentiostat with a conventional three electrode test cell utilizing Ag/AgCl as a reference and a platinum wire as an auxiliary electrodes. We perform EIS measurements with the FRA32M module. The electrolyte was 0.2 M KCl buffered solution containing 2mM K4Fe(CN)6/K3Fe(CN)6 (1:1). All impedance spectra were taken using ac modulation of 10 mV over the frequency range from 0.05 Hz to 1 MHz without dc bias with respect to open circuit potential. All measurements were conducted at room temperature.
Results and discussion
In impedimetric DNA sensors, gold nanostructures were mainly used to achieve an amplification of the analytical signal. In fact, the presence of gold nanostructures on the electrode surface strongly influence the charge transfer process, thus increasing the variation of the charge transfer resistance, both for electrostatic repulsion and for sterical hindrance issues.
Gold nanosheets in comparison with other gold nanostructures have many edges and corners and therefore can serve as more active sites for catalysis compared to spherical nanoparticles [19]. Among different sizes of gold nanosheets, micrometer-sized gold nanosheets have interesting structure due to the combined properties of their size on a micrometer scale and thickness on a nanometer scale. Previously, the high electrocatalytic properties of gold nanosheet modified electrodes were reported toward oxidation of some important analytes. The high conductivity and sharp edges of the gold nanosheets are responsible for this electrocatalytic activity [20].
Figure 1a shows the top view scanning electron microscopy (by VEGA SEM | TESCAN) image of the synthesized gold nanosheets. Large nanosheets (lateral sizes up to a few micrometers) are the main product with low amounts of semi-spherical nanoparticles. As it has been illustrated in Fig. 1b, AFM profile line maps (by JPK NanoWizard II AFM) indicates that the thicknesses of the gold nanosheets are around ~15-20 nm. Fig. 1c shows the X-ray diffraction (XRD) patterns of gold nanosheets (by XRD STOE STADI P type (Germany)). The peaks in the XRD pattern are in excellent agreement with the standard values of the face-centered-cubic (fcc) lattice of Au [23]. Five peaks of (111), (200), (220), (311) and (222) planes of fcc Au can be observed (2θ = 35–100) in the XRD patterns [20].
Electrochemical characterization of GE and GC-GNS electrodes were investigated using cyclic voltammetry technique. Figure 1d shows the cyclic voltammograms of GE and GC-GNS in 0.1 M H2SO4 solution. The active surface area were obtained 8.09 × 10−2 cm2 and 1.27 × 10−1 cm2 for GE and GC-GNS electrodes, respectively (for details see ESM). It is clear that active surface of GC-GNS is larger than GE. The roughness factor of GE and GC-GNS modified electrode are 2.5 and 4.1, respectively (for details see ESM).
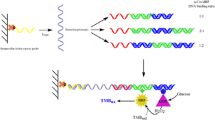
Figure 2a and b represent the electrochemical impedance spectra (Nyquist plots, Z’ versus Z”), using [Fe(CN)6]3−/4−as redox maker ions, for the bare, probe DNA modified, probe and MCH modified and the duplex-formed with target DNA of GE and GC-GNS electrodes, respectively.
Electrochemical impedance spectra of complementary sequence hybridization test for a GE and b GC-GNS under different condition consist of: bare electrodes ( ), electrodes modified with probe DNA(
), electrodes modified with probe DNA( ),DNA plus MCH modified electrode (
),DNA plus MCH modified electrode ( ) and those obtained after hybridization with various target oligonucleotides (
) and those obtained after hybridization with various target oligonucleotides ( )
)
As shown in Fig. 2a and b, the diameter of the semicircles were decreased by immobilization of the probe DNA. The corresponding charge transfer resistances (RCT) were dropped from 320 to 84.92 Ω for GE and from 770 to 1 Ω for GC-GNS electrode. These changes are basically attributed to the decreased repulsive interactions (electrostatic) between the redox maker ions and the surface of electrodes by the immobilized probe DNA; the repulsion impeded the charge transfer through the interface [10]. As it has been shown in these diagrams, the probe functionalized electrode surfaces exposed to the MCH solution have much larger RCT values, with values of 485.2 Ω for gold and 1816 Ω for GC-GNS electrode, respectively. MCH has sever prevention to charge transfer between electrolyte and electrode surface due to electrostatic and steric repulsion, therefore the diameter of semicircle was increased significantly [24].The hybridization with fully complementary DNA sequences increased the diameter of the semicircle, meaning that RCT values was increased by the formation of the complementary duplex (RCT = 1547Ω for GE and RCT = 3562Ω for GC-GNS electrode). The electrochemical behavior of the fully complementary duplex appearance is due to duplex formation would double the negative charge at the electrode surfaces, and the electrostatic repulsion of the redox marker ions would increase RCT compared to the probe DNA alone. The electrochemical behavior of the fully complementary double strand DNA (ds-DNA) in our study are consistent with previous reports [25].The effect of the immobilization and hybridization of probe DNA for two electrodes on charge transfer resistance is summarized in diagrams of Fig. S-1.
To check the specificity of DNA sensing, the measurements have been repeated by examining the EIS curves under employing non-complementary sequences (Fig. 3a and b). The amount changes in RCT values utilizing non-complementary sequence were much lower than complementary sequence (ds DNA formation). Non-specific adsorption had only a small effect on this sensing system. The calculated RCT values are summarized in Fig. S-2.
Considering an experiment with fewer mismatches, another phenomenon was observed with the single base mismatches. Fig. 4a and b show Nyquist plots of experiments with a mismatch at the first part of the ds DNA. RCT values increased a few larger than fully complementary one due to the hybridization of this sequence to the probe DNA. It is believed that the presence of single base mismatches at the beginning of the DNA sequences may induce a structural distortion of the surface bound ds DNA [10] and also, it is well known that water molecules make cluster around mismatched bases in ds DNA to compensate for the loss of hydrogen bonds [26].The distorted structure of the DNA duplex and the presence of lots of hydrations around them is matched bases may increase the steric repulsion and prevent approach of [Fe (CN)6]3−/4- ions to the electrode surfaces.
The changes in RCT values after DNA probe immobilization and hybridization with complementary, non-complementary and one base mismatch sequence in GC-GNS electrode are significantly more than GE. These results suggest that gold nanosheets can remarkably improve the amount of probe DNA attachment and the sensitivity of detection. The calculated RCT values are summarized in Fig. S-3.
The sensitivity of the DNA sensors were also investigated by testing the response of sensors to various concentrations of complementary oligonucleotides sequences (from 1 μM to 1 pM) with probe oligonucleotides-modified electrode (Fig. 5). The resulting RCT values increase with increasing concentration of complementary oligonucleotides sequence. These were seen directly from the semicircle part of the impedance spectrum in Fig. 5a and b.
a and b Electrochemical impedance spectra (Nyquist plots) of bare electrodes ( ), electrodes modified with probe DNA (
), electrodes modified with probe DNA ( ), DNA plus MCH modified electrode (
), DNA plus MCH modified electrode ( ) and those obtained after hybridization with various concentrations of oligonucleotides(
) and those obtained after hybridization with various concentrations of oligonucleotides( ). a GE and b GC-GNS electrode. c A linear relation between the ΔRCT and the logarithm of the target ssDNA concentration in both electrodes.
). a GE and b GC-GNS electrode. c A linear relation between the ΔRCT and the logarithm of the target ssDNA concentration in both electrodes.
Figure 5 c illustrates that the variations of RCT values have a linear relation with the logarithm of target HPV concentrations over the range from 1 μM to 1 pM in both electrode types (GC-GNS and GE). It is clearly observed that the sensitivity of gold nanosheet electrode is more than GE. Detection limits of 0.65 and 0.15 pM can be estimated for the GE and the GC-GNS, respectively. They were estimated via Eq. (1):
where Sb is the signal of blank and σb is the standard deviation of blank. The reproducibility of the GC-GNS electrode and DNA probe immobilization were investigated with three independently prepared electrodes and relative standard deviations (RSD) values were calculated to be 5.6% and 7.1%, respectively. RSD value of probe modified-electrode was less than 9% that was calculated by the successive detection of 10 pM of the HPV16 target over three independently probe modified-electrodes. Besides, the RSD values for GE were 4.2, 6.7 and 7.6% for bare GE, DNA probe immobilization and probe modified-electrode in the presence of 10 pM of HPV16, respectively. Slight difference in RSD values of GC-GNS electrode and GE is due to the electrode preparation procedure. In the case of GC-GNS electrode, GNSs may form random morphology on the substrate (GC), so in order to eliminate the variation of GNSs morphology for multiple detections with different electrodes, the resistance values were normalized with respect to the electrochemical surface area of the electrodes. The calculated RSD values are comparable to other reported values [13].
Also, the repeatability of modified electrodes were evaluated by the analysis of impedimetric responses three times using the same electrodes where shown as error bars in Fig. 5c.
Table 1 shows comparison of some electrochemical DNA sensors (based on label as well as label-free detection). As seen in Table 1, the suggested sensor has a low detection limit and wide linear range, comparable to other DNA sensors.
It is believed that high surface area of gold nanosheets and their specific structures with sharp edges are responsible for sensitive detection of HPV.
Conclusion
A sensitive electrochemical DNA biosensor was developed for specific detection of HPV DNA based on GC-GNS modified electrode. Voltammetry and EIS Responses of GC-GNS electrode was compared with GE. The studies show that gold nanosheets can increase the active area and surface roughness of the gold nanosheet modified electrode and as a result improve the sensitivity of DNA biosensor. The suggested biosensor demonstrated good selectivity with one base pair mismatch and remarkable detection limit. The biosensor has a great potential in HPV DNA diagnostics and clinical analysis.
References
Berek JS, Novak E (2011) Berek and Novak's gynecology. Wolters Kluwer Health/Lippincott Williams & Wilkins
Hsu IH, Chen W-H, Wu T-K, Sun Y-C (2011) Gold nanoparticle-based inductively coupled plasma mass spectrometry amplification and magnetic separation for the sensitive detection of a virus-specific RNA sequence. J Chromatogr A 1218(14):1795–1801. doi:10.1016/j.chroma.2011.02.005
Sivaraman D, Biswas P, Cella LN, Yates MV, Chen W (2011) Detecting RNA viruses in living mammalian cells by fluorescence microscopy. Trends Biotechnol 29(7):307–313. doi:10.1016/j.tibtech.2011.02.006
Ocaña C, Lukic S, del Valle M (2015) Aptamer-antibody sandwich assay for cytochrome c employing an MWCNT platform and electrochemical impedance. Microchim Acta 182(11–12):2045–2053
Bonanni A, del Valle M (2010) Use of nanomaterials for impedimetric DNA sensors: a review. Anal Chim Acta 678(1):7–17. doi:10.1016/j.aca.2010.08.022
Jia F, Duan N, Wu S, Dai R, Wang Z, Li X (2016) Impedimetric salmonella aptasensor using a glassy carbon electrode modified with an electrodeposited composite consisting of reduced graphene oxide and carbon nanotubes. Microchim Acta 183(1):337–344
Kafka J, Pänke O, Abendroth B, Lisdat F (2008) A label-free DNA sensor based on impedance spectroscopy. Electrochim Acta 53(25):7467–7474. doi:10.1016/j.electacta.2008.01.031
Yao W, Wang L, Wang H, Zhang X, Li L, Zhang N, Pan L, Xing N (2013) An electrochemiluminescent DNA sensor based on nano-gold enhancement and ferrocene quenching. Biosens Bioelectron 40(1):356–361. doi:10.1016/j.bios.2012.08.002
Ebtisam SW, Ravil AS (2006) Biosensors for virus detection. In: Smart biosensor technology. CRC Press, Optical Science and Engineering, pp 567–596. doi:10.1201/9781420019506.ch21
Ito T, Hosokawa K, Maeda M (2007) Detection of single-base mismatch at distal end of DNA duplex by electrochemical impedance spectroscopy. Biosens Bioelectron 22(8):1816–1819. doi:10.1016/j.bios.2006.08.008
Bartolome JP, Echegoyen L, Fragoso A (2015) Reactive carbon Nano-onion modified glassy carbon surfaces as DNA sensors for human papillomavirus oncogene detection with enhanced sensitivity. Anal Chem 87(13):6744–6751. doi:10.1021/acs.analchem.5b00924
Bonanni A, Pividori MI, del Valle M (2010) Impedimetric detection of influenza a (H1N1) DNA sequence using carbon nanotubes platform and gold nanoparticles amplification. Analyst 135(7):1765–1772. doi:10.1039/C000532K
Wang S, Li L, Jin H, Yang T, Bao W, Huang S, Wang J (2013) Electrochemical detection of hepatitis B and papilloma virus DNAs using SWCNT array coated with gold nanoparticles. Biosens Bioelectron 41:205–210. doi:10.1016/j.bios.2012.08.021
Karimizefreh A, Mahyari FA, Vaez Jalali M, Mohammadpour R, Sasanpour P (2014) Human papilloma virus detection using DNA biosensor with electrochemical impedance spectroscopy. Journal of Coupled Systems and Multiscale Dynamics 2(3):164–168
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA (2012) The golden age: gold nanoparticles for biomedicine. Chem Soc Rev 41(7):2740–2779. doi:10.1039/c1cs15237h
Lee YJ, Schade NB, Sun L, Fan JA, Bae DR, Mariscal MM, Lee G, Capasso F, Sacanna S, Manoharan VN, Yi GR (2013) Ultrasmooth, highly spherical monocrystalline gold particles for precision plasmonics. ACS Nano 7(12):11064–11070. doi:10.1021/nn404765w
Wu HL, Tsai HR, Hung YT, Lao KU, Liao CW, Chung PJ, Huang JS, Chen IC, Huang MH (2011) A comparative study of gold nanocubes, octahedra, and rhombic dodecahedra as highly sensitive SERS substrates. Inorg Chem 50(17):8106–8111. doi:10.1021/ic200504n
Campos-Ferreira DS, Nascimento GA, Souza EVM, Souto-Maior MA, Arruda MS, Zanforlin DML, Ekert MHF, Bruneska D, Lima-Filho JL (2013) Electrochemical DNA biosensor for human papillomavirus 16 detection in real samples. Anal Chim Acta 804:258–263. doi:10.1016/j.aca.2013.10.038
Hong X, Tan C, Chen J, Xu Z, Zhang H (2015) Synthesis, properties and applications of one- and two-dimensional gold nanostructures. Nano Res 8(1):40–55. doi:10.1007/s12274-014-0636-3
Tohidi M, Mahyari FA, Safavi A (2015) A seed-less method for synthesis of ultra-thin gold nanosheets by using a deep eutectic solvent and gum arabic and their electrocatalytic application. RSC Adv 5(41):32744–32754. doi:10.1039/C4RA17053A
Van Ranst M, Kaplan JB, Burk RD (1992) Phylogenetic classification of human papillomaviruses: correlation with clinical manifestations. J Gen Virol 73(10):2653–2660
Maugeri Z, Dominguez de Maria P (2012) Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: levulinic acid and sugar-based polyols. RSC Adv 2(2):421–425. doi:10.1039/C1RA00630D
Nootchanat S, Thammacharoen C, Lohwongwatana B and Ekgasit S (2013) Formation of large H2O2-reduced gold nanosheets via starch-induced two-dimensional oriented attachment. RSC Adv 3:3707–3716. doi:10.1039/C3RA22830D
Liu B, Xie J, Lee JY, Ting YP, Chen JP (2005) Optimization of high-yield biological synthesis of single-crystalline gold Nanoplates. J Phys Chem B 109(32):15256–15263. doi:10.1021/jp051449n
Civit L, Fragoso A, Hölters S, Dürst M, O'Sullivan CK (2012) Electrochemical genosensor array for the simultaneous detection of multiple high-risk human papillomavirus sequences in clinical samples. Anal Chim Acta 715:93–98. doi:10.1016/j.aca.2011.12.009
Peng H, Soeller C, Cannell MB, Bowmaker GA, Cooney RP, Travas-Sejdic J (2006) Electrochemical detection of DNA hybridization amplified by nanoparticles. Biosens Bioelectron 21(9):1727–1736. doi:10.1016/j.bios.2005.08.011
Jampasa S, Wonsawat W, Rodthongkum N, Siangproh W, Yanatatsaneejit P, Vilaivan T, Chailapakul O (2014) Electrochemical detection of human papillomavirus DNA type 16 using a pyrrolidinyl peptide nucleic acid probe immobilized on screen-printed carbon electrodes. Biosens Bioelectron 54:428–434. doi:10.1016/j.bios.2013.11.023
Civit L, Fragoso A, O'Sullivan CK (2010) Electrochemical biosensor for the multiplexed detection of human papillomavirus genes. Biosens Bioelectron 26(4):1684–1687. doi:10.1016/j.bios.2010.06.072
Zari N, Amine A, Ennaji MM (2009) Label-free DNA biosensor for electrochemical detection of short DNA sequences related to human papilloma virus. Anal Lett 42(3):519–535. doi:10.1080/00032710802421897
Nasirizadeh N, Zare HR, Pournaghi-Azar MH, Hejazi MS (2011) Introduction of hematoxylin as an electroactive label for DNA biosensors and its employment in detection of target DNA sequence and single-base mismatch in human papilloma virus corresponding to oligonucleotide. Biosens Bioelectron 26(5):2638–2644. doi:10.1016/j.bios.2010.11.026
Tran LD, Nguyen DT, Nguyen BH, Do QP, Le Nguyen H (2011) Development of interdigitated arrays coated with functional polyaniline/MWCNT for electrochemical biodetection: application for human papilloma virus. Talanta 85(3):1560–1565. doi:10.1016/j.talanta.2011.06.048
Campos-Ferreira DS, Souza EVM, Nascimento GA, Zanforlin DML, Arruda MS, Beltrão MFS, Melo AL, Bruneska D, Lima-Filho JL (2016) Electrochemical DNA biosensor for the detection of human papillomavirus E6 gene inserted in recombinant plasmid. Arab J Chem 9(3):443–450. doi:10.1016/j.arabjc.2014.05.023
Sabzi RE, Sehatnia B, Pournaghi-Azar MH, Hejazi MS (2008) Electrochemical detection of human papilloma virus (HPV) target DNA using MB on pencil graphite electrode. J Iran Chem Soc 5(3):476–483. doi:10.1007/bf03246005
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 116 kb)
Rights and permissions
About this article
Cite this article
Karimizefreh, A., Mahyari, F.A., VaezJalali, M. et al. Impedimetic biosensor for the DNA of the human papilloma virus based on the use of gold nanosheets. Microchim Acta 184, 1729–1737 (2017). https://doi.org/10.1007/s00604-017-2173-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2173-8






 ), electrodes modified with probe DNA (
), electrodes modified with probe DNA ( ), DNA plus MCH modified electrode (
), DNA plus MCH modified electrode ( ) and those obtained after hybridization with various target oligonucleotides (
) and those obtained after hybridization with various target oligonucleotides ( ). a GE and b GC-GNS electrode.
). a GE and b GC-GNS electrode.
 ), electrodes modified with probe DNA (
), electrodes modified with probe DNA ( ), DNA plus MCH modified electrode (
), DNA plus MCH modified electrode ( ) and those obtained after hybridization with various target oligonucleotides (
) and those obtained after hybridization with various target oligonucleotides ( ). a GE and b GC-GNS electrode.
). a GE and b GC-GNS electrode.