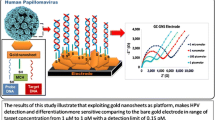
Abstract
A gene sensor for rapid detection of the Human Papillomavirus 16 (HPV 16) which is associated with the appearance of cervical cancer was developed. The assay is based on voltammetric determination of HPV 16 DNA by using interdigitated electrodes modified with titanium dioxide nanoparticles. Titanium dioxide nanoparticles (NPs) were used to modify a semiconductor-based interdigitated electrode (IDE). The surface of the NPs was then functionalized with a commercial 24-mer oligomer DNA probe for HPV 16 that was modified at the 5′ end with a carboxyl group. If the probe interacts with the HPV 16 ssDNA, the current, best measured at a working voltage of 1.0 V, increases. The gene sensor has has a ∼ 0.1 fM limit of detection which is comparable to other sensors. The dielectric voltammetry analysis was carried out from 0 V to 1 V. The electrochemical sensitivity of the IDE is 2.5 × 10−5 μA·μM−1·cm−2.

Schematic of an interdigitated electrode (IDE) modified with titanium dioxide nanoparticles for voltammetric determination of HPV 16 DNA by using an appropriate DNA probe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
'Cervical cancer is the second biggest cause of malignancy cancer-related death in women around the world, and it happens following the constant infection, with a particular subset of Human Papillomavirus (HPV) type [1,2,3]. HPV contains double-stranded DNA as a genetic material that belongs to the family Papillomaviridiae. HPV strains contain more than 100 types, including high and low-risk types, have been identified so far. Fifteen strains of HPV have been identified to keep women at high risk for cervical cancer [4]. Cervical cancer infection is stimulated by HPV high-risk type strains 16, 18, 31, 33 and 35 [5, 6]. HPV 16 is the worst strain for the progression of cervical cancer followed by 18, 31, 33 and 35. Cervical cancer infections start when the HPV virus is in the cervix then entered cells through microabrasions and infect the cells. Several weeks after infection, the virus replicates and spread through the cells. Cervical cancer can be treated if it can be detected at an early stage. HPV associated cervical cancer has symptoms such as vaginal and contact bleeding and vaginal mass may show the existence of malignancy [7].
Serological methods were used for recognition of HPV IgG and IgM antibodies. However, sufficient production of antibodies needs 5 days after the existence of sickness symptoms. Besides that, serological test suffered from cross contamination reaction with other HPV strains due to antigenic determinants on polyclonal antibodies. Supplementary Table S1 showed comparison of the performance of detection of HPV and cervical cancer by using various methods.
Characterization of disease based on their unique hereditary genetic profiles is expanding and being studied as compared to more conventional strategies of disease characterization. Currently, virological techniques combined Polymerase Chain Reaction (PCR) for the amplification of specific genes are chosen as they are sensitive, low-risk cross-contamination and less assay time compared to serological procedures [8]. However, the identification methods including stained agarose gel electrophoresis for visualization of PCR product. With arising of nanotechnology today, the advance of nanobiosensor is replacing such traditional methodology. Nanobiosensor based on nucleic acid hybridization has been produced for economical and fast detection of DNA.
Nanoparticles and electrode materials which are biocompatible and had good conductivity have been chosen for biosensor studies [9,10,11,12,13,14]. The nanobiosensor as electronic systems for cancer detecting has to gain wide enormous application in biomedical and biosensor applications. Electrode materials which are biocompatible and had good conductivity have been chosen for biosensor studies [11,12,13,14]. The detection of HPV by using TiO2 as a semiconductor in IDE biosensor was required by consuming low fluid volume, high response, better process control, high surface-volume ratio, reusable, compact system and fast diagnose steps. In the present study, microfabricated TiO2 based interdigitated electrode (IDE) sensor was utilized as a solution for all these constraints that have been faced by the currently available techniques [15]. This electrical based nanobiosensor integrated with biomolecule probes allowed to detect the changes in charge when HPV DNA target binds to the immobilized probe. TiO2 based IDE proved to be sensitive, direct and will be suitable for the specific detection of a large species are suffering from cancer. It has a quick response time, low detection limitation and label-free method, make this device to be different from previous detections. This study explores the attainability of HPV DNA detection on TiO2 based IDE nanobiosensor offering a low fluid volume consumption, high-throughput analysis and response, fast detection, better process control, high surface to volume ratio, sensitive and selective, reusable, compact system, cost-effective and fast diagnosis.
Materials and methods
Chemicals and reagents
3-Aminopropyltriethoxysilane (APTES) was bought from Sigma–Aldrich, USA. It was stored in a dry, ventilated place and firmly sealed before use. Single-stranded carboxylated 24mer (Genbank Accession No.: A18875.1) oligonucleotide probe composed from Human Papillomavirus (HPV) for the recognition of target DNA HPV 16 was synthesized commercially [16]. Table 1 shows the list of sequences for 24-mer probe, complementary, and non-complementary target oligonucleotides. The 24mer probe was utilized as a model framework for immobilization, while other 24mer strands were utilized for target analysis. The supplied lyophilized DNA samples were diluted in deionized distilled water to reconstitute before use.
Interdigitated electrode (IDE) fabrication
IDE nanosensor was fabricated according to Nadzirah et al. [15]. The IDE fabrication process involved nanobiosensor was explained details in Supplementary Method S1: Interdigitated Electrode (IDE) Fabrication. Supplementary Fig. S1 showed the schematic diagram of the fabrication process involved eight steps. Schematic diagram of recognition of biomolecules on synthetically treated silicon substrates utilizing APTES is shown in Supplementary Fig. S2. The strategy includes: (a) silicon substrates cleaning by piranha solution; (b) silane layer surface modification using APTES (24%, v/v) solution; (c) titanium dioxide (TiO2) deposition [15]; (d) HPV DNA Probe immobilization; and (e) HPV DNA target hybridization. Preparation of TiO2 solution and deposition was followed according to Nadzirah et al. [15]. Very thin aluminum metal was deposited on the pads area so that it can cover that area from TiO2 film deposition [15, 18]. Deposition of TiO2 methods was explained detail in Supplementary Method S2.
Surface modification on TiO2 based IDE
TiO2 based IDE was functionalized using APTES as a linker and also facilitator to immobilize biomolecules on the inorganic surface. 1.5 μl APTES (24% in deionized distilled water) was dropped on an active site of TiO2 based IDE device. TiO2 based IDE device was drying in a dry cabinet for 15 min until it completely dry. After that, TiO2 based IDE device was ready for electrical measurement.
Immobilization of human papillomavirus (HPV) DNA probe
A modified of the 5’end probe with a carboxyl group (COOH) was needed onto the surface since APTES has amine terminated functional group toward the end. HPV Probe DNA (1 μM) which was diluted in deionized distilled water was dropped in the active area. The device was incubated for 2 h in a moist environment at an ambient temperature. Excess DNA probe was rinsed free from the surface by washing the device 3 times with the intervals of 5 min using deionized distilled water. After the immobilization process, TiO2 based IDE was ready for electrical measurement.
Hybridization of HPV 16 DNA target
The DNA of HPV 16 was initially denatured into by heating in thermoshaker at 95 °C for 5 min after that stop the reaction by keeping on ice for 5 min. One microliter of this single-stranded DNA was then dropped on the active area of IDE and incubated for 1 h. Unbound DNA was removed from the surface by washing the chip.
Testing different concentrations of HPV DNA target
In this study, different concentrations of HPV DNA target in the buffer were prepared to test the detection limit of TiO2 based IDE. Different concentrations of target DNA (10−1, 10−2, 10−3, 10−4, 10−5, 10−6 and 10−7 μM) were prepared from 1 μM HPV DNA target stock solution, by serial dilution with an appropriate volume of deionized distilled water. 1 μl sample was dropped on the active area of IDE by using micropipette. After complementation and washing, the IDE device was ready for electrical measurement.
Electrical measurement
Specifically, after the immobilization of the HPV 16 DNA probe, TiO2 based IDE current was measured by probing two terminal probes. TiO2 based IDE substrate device was being able to be monitored through the current flowing to the channel. It also flew to the substrate when the source electrode voltage was swept. A single range of voltage was supplied to the source of the terminal in interims of 0.05 V with a consistent set at 100 mA. At the same time, the current was measured and showed as a current-voltage (I-V graph). Hybridization measurements are taken using current-voltage (I-V) (KEITHLEY, 6487) to determine the change in current flow for TiO2 based IDE active area before and after DNA hybridization.
High-performance analytical tests on TiO2 based IDE sensing surface
To test the reproducibility on the TiO2 based IDE sensor surfaces, 4 different devices of nanogaps were modified with APTES and then HPV DNA probe was immobilized. On the surfaces, 1 μM of complementary HPV DNA target was hybridized and measured in the electric flow. The repeatability test was done for four TiO2 based IDE for four different concentrations of single-stranded HPV DNA target (10 μM, 10 nM, 10 pM, 10 fM).
Validation assay using signal amplification
Validation assay using signal amplification was referred according to HC2 High-Risk HPV DNA Test® [19]. There were 8 patient clinical specimens had been tested to validate the effective of fabricated IDE biosensor. Digene Microplate Luminometer 2000 (DML 2000™) Instrument was switched on at least 60 mins before the signal detection. The DR2 (75 μl) was pipetted into a new falcon tube by using multichannel pipette with reverse pipetting technique into each wells of micro plate. The micro plate was incubated for 15 min in dark places to avoid direct sunlight for 15 mins. The microplate was read on the Digene Microplate Luminometer 2000 in 15 min and not exceeds 30 min.
Results and discussion
Titanium dioxide (TiO2) was the commonly researched single-crystalline framework in the surface science investigation of metal oxides [20,21,22,23]. Semiconductor TiO2 has adequately positive valence band edge to oxidize water to oxygen [24]. It is also an addition stable material in the presence of aqueous electrolyte solutions. TiO2 is moderately cheap, exceedingly stable chemically, and the photo-generated holes are very oxidizing. TiO2 which was inorganic nanoparticles has a decent potential to be utilized as a base component and a part of the biosensor to distinguish the occurrence of biomolecules. This may permit TiO2 nanoparticles to be utilized as a part of our innovation of biomedical and clinical applications [25, 26]. To make a genuine application conceivable with clinical examples, the target DNA sequence would be longer as genome sequences after being restricted by restriction enzyme [27]. HPV contains virion that has a double-stranded, circular DNA genome of around 7900 bp, and is separated into 3 regions, region of the non-coding long control (LCR, ~1 kb), and the regions of protein-coding early (E, ~4 kb) and late (L, ~3 kb). The viral genome encodes 6 early (E1, E2, E4, E5, E6, and E7) and two late (L1 and L2) proteins. By synthesizing appropriate sequence from this genome this study demonstrated the detection of Human Papillomavirus (HPV) 16, as it associated with cervical cancer cells.
TiO2 based IDE sensor
Initially, the wafer was cleaned and covered with the insulating material. In this research, we utilized SiO2 as the insulator because it is cheap, broadly utilized, and a good barrier layer for moisture and mobile ions. The band gap (G) between the terminal electrodes was the most important geometric parameter in deciding the distribution and quality strength of the electric field and the current density compared to the width (W) and height (H) of the IDEs. A flimsy layer (400 nm) of positive photoresist (þPR) was deposited, and the undesirable silicon on the wafer surface was etched to form silicon nanogap with sizes of 1 μm utilizing the chrome mask. They were trimmed to the desired nanoscale sizes utilizing plasma processes. The oxides were developed on the surface of the micro-sized electrodes, which consumes the silicon, and is later etched away by the buffered oxide etcher. The amount of silicon used relies on the aggregate infiltration of the oxide, which is constrained by the development of oxygen through the oxide-silicon interface.
The mask design of IDE was performed using AUTOCAD software, and a single IDE is zoomed-in to identify its full specs. The design of interdigitated electrodes is shown below. Supplementary Fig. S1(a) shows mask design of IDE using AUTOCAD software, and a single IDE is zoom-in to identify its full specs. While Supplementary Fig. S1(b) shows the real chrome mask. The size of electrode have been utilized was 5.5 × 0.25 mm, pad size 2 × 2 mm, the gap between end electrode terminal to the pad is 3 mm and a number of electrodes is 20 with the gap size between two electrodes is 10 nm. IDE is a device which comprises two interlocking comb-shaped metallic coatings in the fashion of a zippier. It’s made out of two electrodes with two connection tracks, on a silicon substrate. These IDEs offer a few points of interest, for example, working with a low volume of sample and avoiding from repetitive cleaning of solid electrodes. Sensor surface purities due to cleaning process were important to stabilize the uniformity of 3-aminopropyltriethoxysilane (APTES) as Self Assemble Monolayer (SAM) layers [28]. The interdigitated setup ordinarily improves sensitivity and detection limits. They are appropriate for decentralized assays, to create particular (bio) sensors and other electrochemical studies.
Electrical detection of HPV 16 DNA using TiO2 based IDE sensor
TiO2 based IDE was functionalized with APTES and 24 mer HPV 16 DNA probe (Table 1). A fragment of HPV 16 identified in the E6 region was selected as the target, which is complementary to the immobilized HPV 16 DNA probe. After hybridization, HPV 16 DNA target was bound to the DNA probe functionalized TiO2 based IDE, achieving negative charges to the sensor surface because of resistance change in TiO2 based IDE. The fundamental detecting mechanism for this study included the current change prior to and after the hybridization of DNA target. DNA conveys a net negative charge when hybridization happens, there would be a change in negative charge on the surface of TiO2 based IDE. The reaction change prior to and after the hybridization corresponds to the attachment of the HPV DNA target on the TiO2 based IDE surface. I-V curve was obtained in the measuring solution after HPV DNA probe attachment onto the TiO2 based IDE sensor surface. The measurement was directed again in the same condition after HPV DNA target was hybridized to the immobilized HPV DNA probe. Figure 1a illustrates the TiO2 based IDE working I-V curve before and after 1 μM concentration of HPV 16 DNA synthetic target was hybridized to the complementary HPV 16 DNA functionalized TiO2 based IDE. The TiO2 based IDE current was dropped, thus the resistance of TiO2 based IDE expanded after the negative charges were acquainted with TiO2 based IDE surface because of DNA/DNA hybridization.
a Typical TiO2 based IDE working I-V curves before and after 1 μM of the HPV 16 DNA synthetic target were hybridized to the complementary HPV DNA probe functionalized TiO2 based IDE surface. b The impedance of bare IDE, TiO2 based IDE 2, TiO2 based IDE/APTES, TiO2 based IDE/APTES/HPV 16 DNA probe (Immobilization), and TiO2 based IDE/APTES/HPV 16 DNA probe/ HPV 16 DNA target (Hybridization)
Electrochemical impedance spectroscopy (EIS) was added besides voltammetry determination in Fig. 1b to demonstrate the successfully of assemble the biosensor interface. Figure 1b showed the impedance of bare IDE, TiO2 based IDE, TiO2 based IDE/APTES, TiO2 based IDE/APTES/HPV 16 DNA probe (Immobilization), and TiO2 based IDE/APTES/HPV 16 DNA probe/ HPV 16 DNA target (Hybridization). Impedance measurements can provide valuable information about the kinetics of monolayer formation, and a rough estimate of the dielectric constant. Comparing to voltammetric capacitive biosensors, biosensors based on EIS have been widely explored for their ability to capture complex resistance changes due to binding events at biosensor’s electrode sites [29].
To further confirm the current decrease was brought by the hybridization of complementary HPV 16 DNA target to the immobilized DNA probe, 1 μM scrambled DNA sequences were hybridized to the DNA-functionalized TiO2 based IDE surface. As shown in Fig. 2, TiO2 based IDE working I-V curves prior to and after hybridization demonstrated the hard change in current, showing no HPV 16 DNA scrambled DNA sequences were hybridized to the TiO2 based IDE surface.
The synthetic DNA had a lower infectivity than natural DNA, indicating approximately one lethal error per 500 bp. Most of the experiments were performed at pH 7.4 which was near to neutral regardless of pH which has an adverse effect for DNA adsorption. The lack of a good understanding of the sensing mechanism hampers the further exploitation of these promising nano sensors.
Hybridization of HPV DNA target at various concentrations
I-V curves of hybridization of HPV DNA target at various concentrations were shown in Fig. 3. The sensor was tested with the initial trial with a low concentration of HPV DNA and which is able to detect early detection of the disease effectively. TiO2 based IDE device can detect HPV-associated cervical cancer at different concentrations in order to validate the device capabilities for detecting pre-cancer development. Using this method, HPV 16 DNA target ha detected in the 1 to 10−7 μM concentration range without apparent effort to upgrade the system for sensitivity. The concentration 1 μM of HPV DNA probe can detect 1 × 10−4 μM HPV target DNA equivalent to 100 pM (Fig. 3). By further change of DNA hybridization effectiveness, e.g. ionic strength of the electrolyte and ssDNA probe get together, the performance and sensitivity were improved.
Analytical performance of TiO2 based IDE
We further tested the analytical performance of TiO2 based IDE, as shown in Fig. 4. Figure 4a showed the result of I-V characteristic s showed significant differences in the measured current (A) values for each of DNA hybridization. It was observed, that upon hybridization with a complementary target, a decrease in current was recorded. There was no change in the current (A) for non-complementary and single mismatch compared with the current of immobilized DNA probe at 1 V. The decrease in current (A) was associated with DNA hybridization and indicated that DNA is negatively charged because they have phosphate backbone. The use of a DNA probe due to phosphate backbone, it can be immobilized with p-type semiconductor based transistor surface. Hybridization of a DNA strand from solution was converted to a current response within the Debye length and depends on the analyte concentration, composition, and pH [49]. Stronger binding between DNA duplex due to hydrogen binding resulted in a higher number of negatively charged DNA bound to surface, so a larger current change happened in this study.
TiO2 based IDE biosensor; a I-V characteristics by different steps of surface functionalization. b Hybridization specificity demonstrated by the conductance to the complementary, one-base mismatched, non-complementary DNA sequences at 1 V. c Current response curve of TiO2 based IDE biosensor with different concentrations of HPV DNA. d Calibration graph of the relative change in current, display limit of detection (LOD)
To further verify the device specificity, the relative change in conductance of the device was plotted (Fig. 4b). The change in conductance was observed when 10 μM of the non-complementary target was applied to immobilized DNA probes. The sensitivity and Limit of Detection (LOD) of the TiO2 based IDE where shown in Fig. 4c and d. The effect of different concentration of complementary target HPV DNA, ranging from 10fM to 10 μM was studied. The I-V characteristics in Fig. 4c indicated the current increased with increment of target HPV DNA concentration. By adding more negative charge on the surface, p-type of TiO2 based IDE accumulated of charge carrier around TiO2 based IDE. The sensitivity of TiO2 based IDE device was the slope of the calibration plot at Fig. 4c. The electrochemical sensitivity of.
2.5 × 10–5 μA·μM−1·cm−2 and it became saturated after that. LOD can be used to evaluate the ability of the device to detect and predict the lowest concentration of an analyte in the clinical target of HPV DNA. A calibration graph was plotted in Fig. 4d showed the relative change in current proportional to the HPV DNA concentration. To the best of our knowledge, the sensitivity and LOD required are among the best in the detection of HPV DNA compared to previously reported as shown in Supplementary Table S1.
Analytical performance of repeatability, reproducibility, and stability of the biosensor
The capability of the TiO2 based IDE for repeated HPV DNA detection which was considered as repeatability performance was investigated. The sensor was treated with 0.01 M NaOH for 2 min to dehybridize DNA duplex on the TiO2 based IDE, which was back to the condition with the probe. The repeatability test was done on the different TiO2 based IDE device as shown in Fig. 5. The current values for 5 cycles showed no significant changes with slightly differences in hybridization and dehybridization. Relative standard deviation (R.S.D.) of hybridized and dehybridized cycles for regeneration cycles was found less than 5% for the same TiO2 based IDE. Good repeatability of the TiO2 based IDE sensor was shown from this result. A further test was examined to analyze the reproducibility of the sensor by comparing 5 different TiO2 based IDEs as in Supplementary Fig. S2. The results showed a satisfactory reproducibility performance of the sensor with R.S.D. larger at 25% compared to repeatability performance. This result due to minor variation in the TiO2 based IDE dimension and also the purity of the nanomaterial that being used in the experimental procedures. The TiO2 based IDE has promising values in the monitoring of HPV DNA with stable and excellent repeatability and reproducibility for stability performances of biosensors.
Validation experiments were carried out using commercializes methods, Hybrid Capture II (HCII) to confirm the effective of fabricated IDE biosensor. Figure 6 showed analytical performance of the hybrid capture assay for HPV. E6 dependence of chemiluminescent signal output for 8 cervical scrapes specimen. Specimen 2 was identified as positive while analytical performance of chemiluminescent signal output zoomed out for negative cervical scrapes specimen.
Conclusion
As a proof of concept in the study, TiO2 based IDE nanobiosensor utilizing nucleic acid hybridization and electrical detection has been produced with specific and sensitive detection of HPV 16. It is shown that this electrical biosensor was able to detect as low as 0.1 fM concentration (LOD) with a greatly enhanced sensitivity of 40 × 10−4 AM−1 with high specificity, repeatability, and reproducibility. Compared to the traditional lab-based methodologies including serological and virological test, this assay has a few points of interest. To begin with, TiO2 based IDE sensor is sensitive compared to another technique. Second, the TiO2 based IDE sensor permits quick detection than conventional techniques. Third, TiO2 based IDE is label-free, dispensing additional procedures for labeling. Fourth, TiO2 based IDE sensor can be made as portable detection by coordinating the sensor with electrical circuits that measure I-V for the development for HPV-associated cervical cancer early detection.
References
Schiffman M (2017) Cervical cancer screening: epidemiology as the necessary but not sufficient basis of public health practice. Prev Med 98:3–4. https://doi.org/10.1016/j.ypmed.2016.12.028
Reynoso-Noverón N, Peña-Nieves A, Rodríguez MO, Mohar-Betancourt A (2017) Cervical Cancer epidemiology. Cerv Cancer. https://doi.org/10.1007/978-3-319-45231-9_2
Flores-Pulido JJ, Martínez-Correa M (2015) [Cervical cancer and human papillomavirus. A glance from a family medical viewpoint]. Rev. Med. Inst. Mex. Seguro Soc
Souho T, Bennani B (2014) Oncogenic human papillomavirus genotyping by multiplex PCR and fragment analysis. J Virol Methods 196:45–49. https://doi.org/10.1016/j.jviromet.2013.10.024
de Sanjosé S, Brotons M, Pavón MA (2018) The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol 47:2–13. https://doi.org/10.1016/j.bpobgyn.2017.08.015
Joura EA, Ault KA, Bosch FX, Brown D, Cuzick J, Ferris D, Garland SM, Giuliano AR, Hernandez-Avila M, Huh W, Iversen OE, Kjaer SK, Luna J, Miller D, Monsonego J, Munoz N, Myers E, Paavonen J, Pitisuttithum P, Steben M, Wheeler CM, Perez G, Saah A, Luxembourg A, Sings HL, Velicer C (2014) Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomark Prev 23:1997–2008. https://doi.org/10.1158/1055-9965.EPI-14-0410
Cirilli AR, Cipot SJ (2012) Emergency evaluation and Management of Vaginal Bleeding in the nonpregnant patient. Emerg Med Clin North Am 30:991–1006. https://doi.org/10.1016/j.emc.2012.08.010
Parmin NA, Hashim U, Gopinath SCB, Nadzirah S, Rejali Z, Afzan A, Uda MNA (2019) Human papillomavirus E6 biosensing: current progression on early detection strategies for cervical Cancer. Int J Biol Macromol 126:877–890. https://doi.org/10.1016/j.ijbiomac.2018.12.235
Piao J, Zhou X, Wu X (2018) Colorimetric human papillomavirus DNA assay based on the retardation of avidin-induced aggregation of gold nanoparticles. Microchim Acta 185:3–9. https://doi.org/10.1007/s00604-018-3065-2
Azizah N, Hashim U, Gopinath SCB, Nadzirah S (2016) Gold nanoparticle mediated method for spatially resolved deposition of DNA on nano-gapped interdigitated electrodes, and its application to the detection of the human Papillomavirus. https://doi.org/10.1007/s00604-016-1954-9
Zhang Q, Qiao Y, Hao F, Zhang L, Wu S, Li Y, Li J, Song XM (2010) Fabrication of a biocompatible and conductive platform based on a singlestranded DNA/graphene nanocomposite for direct electrochemistry and electrocatalysis. Chem Eur J 16:8133–8139. https://doi.org/10.1002/chem.201000684
Sarma AK, Vatsyayan P, Goswami P, Minteer SD (2009) Recent advances in material science for developing enzyme electrodes. Biosens Bioelectron 24:2313–2322. https://doi.org/10.1016/j.bios.2008.09.026
Perumal V, Hashim U, Gopinath SCB, Rajintra Prasad H, Wei-Wen L, Balakrishnan SR, Vijayakumar T, Rahim RA (2016) Characterization of gold-sputtered zinc oxide Nanorods—a potential hybrid material. Nanoscale Res Lett 11:31. https://doi.org/10.1186/s11671-016-1245-8
Perumal V, Hashim U, Gopinath SCB, Haarindraprasad R, Foo KL, Balakrishnan SR, Poopalan P (2015) ‘Spotted Nanoflowers’: gold-seeded zinc oxide Nanohybrid for selective bio-capture. Sci Rep 5:12231. https://doi.org/10.1038/srep12231
Nadzirah S, Azizah N, Hashim U, Gopinath SCB, Kashif M (2015) Titanium dioxide nanoparticle-based interdigitated electrodes: a novel current to voltage DNA biosensor Recognizes E. coli O157:H7. PLoS One 10:e0139766. https://doi.org/10.1371/journal.pone.0139766
Parmin NA, Hashim U, Gopinath SCB (2017) Designing probe from E6 genome region of human papillomavirus 16 for sensing applications. Int J Biol Macromol 107:1738–1746. https://doi.org/10.1016/j.ijbiomac.2017.10.051
Azizah N, Hashim U, Gopinath SCB, Nadzirah S (2017) A direct detection of human papillomavirus 16 genomic DNA using gold nanoprobes. Int J Biol Macromol 94:571–575. https://doi.org/10.1016/j.ijbiomac.2016.10.060
Dhahi TS, Hashim U, Ahmed NM (2011) Fabrication and characterization of 50 nm silicon Nano-gap structures. Sci Adv Mater 3:233–238. https://doi.org/10.1166/sam.2011.1155
Qiagen (2008) hc2 high-risk HPV DNA test ®
Diebold U (2003) The surface science of titanium dioxide. Surf Sci Rep 48:53–229. https://doi.org/10.1016/S0167-5729(02)00100-0
Pabón BM, Beltrán JI, Sánchez-Santolino G, Palacio I, López-Sánchez J, Rubio-Zuazo J, Rojo JM, Ferrer P, Mascaraque A, Muñoz MC, Varela M, Castro GR, de la Fuente OR (2015) Formation of titanium monoxide (001) single-crystalline thin film induced by ion bombardment of titanium dioxide (110). Nat Commun 6:6147. https://doi.org/10.1038/ncomms7147
Pan X, Yang M-Q, Fu X, Zhang N, Xu YJ (2013) Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale 5:3601–3614. https://doi.org/10.1039/c3nr00476g
Jiang HB, Cuan Q, Wen CZ, Xing J, Wu D, Gong XQ, Li C, Yang HG (2011) Anatase TiO2 crystals with exposed high-index facets. Angew Chem Int Ed 50:3764–3768. https://doi.org/10.1002/anie.201007771
Scanlon DO, Dunnill CW, Buckeridge J, Shevlin SA, Logsdail AJ, Woodley SM, Catlow CRA, Powell MJ, Palgrave RG, Parkin IP, Watson GW, Keal TW, Sherwood P, Walsh A, Sokol AA (2013) Band alignment of rutile and anatase TiO2. Nat Mater 12:798–801. https://doi.org/10.1038/nmat3697
Liu L, Miao P, Xu Y, Tian Z, Zou Z, Li G (2010) Study of Pt/TiO2 nanocomposite for cancer-cell treatment. J Photochem Photobiol B Biol 98:207–210. https://doi.org/10.1016/j.jphotobiol.2010.01.005
Huo K, Gao B, Fu J, Zhao L, Chu PK (2014) Fabrication, modification, and biomedical applications of anodized TiO2 nanotube arrays. RSC Adv 4:17300. https://doi.org/10.1039/c4ra01458h
Behrendorff JBYH, Johnston WA, Gillam EMJ (2014) Restriction enzyme-mediated DNA family shuffling. Methods Mol Biol 1179:175–187. https://doi.org/10.1007/978-1-4939-1053-3_12
Gong P, Levicky R (2008) DNA surface hybridization regimes. Proc Natl Acad Sci 105:5301–5306. https://doi.org/10.1073/pnas.0709416105
Wang L, Veselinovic M, Yang L, Geiss BJ, Dandy DS, Chen T (2017) A sensitive DNA capacitive biosensor using interdigitated electrodes. Biosens Bioelectron 87:646–653. https://doi.org/10.1016/j.bios.2016.09.006
Acknowledgements
This research was supported by the Ministry of Higher Education (MOHE) under Grant Nos. FRGS/2/2014/TK03/UNIMAP/01/1. Author would like to thank all staff members of the Institute of Nanoelectronic Engineering in Universiti Malaysia Perlis for their technical advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Ethical approval
All samples were obtained with the permission from Universiti Malaysia Perlis (UniMAP) Health Centre and acquired with consent from patients who were informed about the research purposes, and this consent procedure was approved by the Medical Ethics Committee for the University Health Centre, UniMAP. The above-mentioned ethics committee specifically approved this study, which was conducted in accordance with the International Conference on Harmonisation–Good Clinical Practice (ICH–GCP) guidelines and the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3.87 MB)
Rights and permissions
About this article
Cite this article
Parmin, N.A., Hashim, U., Gopinath, S.C.B. et al. Voltammetric determination of human papillomavirus 16 DNA by using interdigitated electrodes modified with titanium dioxide nanoparticles. Microchim Acta 186, 336 (2019). https://doi.org/10.1007/s00604-019-3445-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3445-2