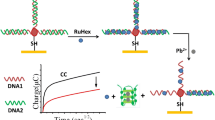
Abstract
We describe a sensitive and selective biosensor for the environmental metabolite 2-hydroxyfluorene (2-HOFlu). It is based on electrochemical impedance spectroscopy and was obtained by assembling a thiolated single-stranded DNA on a gold electrode via S-Au covalent bonding. It is then transformed to a K+-stabilized G-quadruplex-hemin complex which exhibits peroxidase-like activity to catalyze the oxidation of 2-HOFlu by H2O2. This results in the formation of insoluble products that are precipitated on the gold electrode. As a result, the charge transfer resistance (R CT) between the solution and the electrode surface is strongly increased within 10 min as demonstrated by using the ferro/ferricyanide system as a redox probe. The difference in the charge transfer resistances (ΔR CT) before and after incubation of the DNA film with 2-HOFlu and H2O2 serves as the signal for the quantitation of 2-HOFlu with a 1.2. nM detection limit in water of pH 7.4. The assay is highly selective over other selected fluorene derivatives. It was exploited to determine 2-HOFlu in spiked lake water samples where it displayed a detection limit of 3.6 nM. Conceivably, this method has a wide scope in that it may be applied to other analytes for which respective G-quadruplexes are available.

A G-quadruplex DNAzyme based impedimetric biosensor for sensitive detection of 2-hydroxyfluorene using hemin as a peroxidase enzyme mimic was constructed with a detection limit of 1.2 nM in water and 3.6 nM in spiked lake water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluorene is a ubiquitously persistent environmental contaminant with high mutagenicity, teratogenicity and carcinogenicity [1, 2], and it has been listed in the priority pollutants of the United States Environmental Protection Agency (US EPA). In environment, the main source of fluorene may come from production and use of fluorene as it is an important chemical material, which has been widely used as raw material of some pharmaceuticals (such as analgesic, sedative, anticonvulsant medication and anti hypertension medication), insecticide, herbicide, dyes, organic glass and accessory ingredients etc. [3], which is also the main pathway that the human body exposed to fluorene. Fluorene is metabolized in the human body and biodegraded by microbial consortia in the environment [4–6], and the major metabolite is 2-hydroxyfluorene (2-HOFlu) [7], which also has metabolism-dependent cytotoxicity due to the bioactivation of 2-HOFlu. In addition, it has been confirmed that the exposure to fluorene was correlated with the corresponding metabolite of 2-HOFlu in human body [8]. Thus, 2-HOFlu has been considered as a biological marker to evaluate fluorene exposure levels [7], which can provide evidence for environmental health risk warnings, and occupational safety evaluation. Therefore, it is of significance to develop efficient assays for the determination of 2-HOFlu.
Commonly, the traditional methods applied for the quantification of 2-HOFlu in the laboratory are high-performance liquid chromatography (HPLC) systems with fluorescence detection [7, 8], liquid chromatography-mass spectrometry (LC-MS) [9] and gas chromatography–mass spectrometry (GC-MS) [10]. However, there are still some disadvantages, such as time-consuming pretreatment of the sample prior to chromatography analysis [11, 12]. What’s more, the expensive equipment, highly qualified technicians and a laboratory environment also limit the scope of their applications in field monitoring [13]. Under this situation, an achievement of sensitive, simple and economical approach for the detection of 2-HOFlu is highly imperative. In contrast, electrochemical technique is an interesting and convenient alternative that has allowed one to increase the sensitivity and selectivity, simplify the detection process [14], and is ease of miniaturization [15]. Electrochemical DNA sensors are of particular interest and have been widely employed for the task of on-site detection contaminations for environmental monitoring [16–19], analysis of food, point-of-care diagnostics, fast detection of bioterrorism agents [20] as well as many other important applications due to its convenience, high selectivity and sensitivity. Especially, electrochemical impedance spectroscopy (EIS) has been proven to be a most powerful and sensitive tool for probing the features of surface-modified electrodes [21]. And EIS biosensors ([Fe(CN)6]3-/4- as redox probe couple) possess unique advantages, such as the ability to separate the surface binding events from the solution impedance, ease of signal quantification, less damage to the biological interactions being measured, and most importantly, label-free on the DNA strand [22–24]. However, to the best of our knowledge, little efforts have been given to the development of biosensors for exploring the 2-HOFlu, and no fluorescent, colorimetric and electrochemical DNA sensors have been reported for 2-HOFlu detection. So developing EIS DNA sensor with high specificity, sensitivity is still of a great challenge.
The catalytic DNA is a single strand guanine-rich (G-rich) deoxyribonucleic acid that upon binding with hemin in a G-quadruplex structure reveals peroxidase-like activity [25]. Such complexes have extensively been exploited in medicine, biology and material sciences owing to the advantages of thermal stability, simpler preparation and purification, low production cost and less nonspecific adsorption over conventional protein enzymes [26–29]. To warrant catalytic activity, the G-rich DNA has to adopt a G-quadruplex structure stabilized by cations (such as K+), which then bind hemin to form a K+-stabilized G-quadruplex/hemin complex (DNAzyme) [27]. The DNAzyme catalyzes the redox reaction between the target molecules and hydrogen peroxide, producing oxidized products [30]. In addition, it has been reported that DNAzyme catalyzes the H2O2-mediated oxidation of some substrates (such as chloronaphthol), forming an insoluble precipitation layer on the electrode surface [31, 32]. However, with DNA/DNAzyme as a sensing probe, electrochemical detection of 2-HOFlu has not been reported. Therefore, the G-rich DNA modified films were prepared with the aim of detecting 2-HOFlu based on the DNAzyme catalytic oxidation of 2-HOFlu.
In the present study, an efficient and cost-effective assay for the in situ amplification determination of 2-HOFlu was reported based on the DNAzyme sensor by electrochemical impedance spectroscopy (EIS). Simply, a G-rich DNA was immobilized on the gold electrodes, and then transformed into G-quadruplex in the presence of K+. The formed K+-stabilized G-quadruplex acted as DNAzyme (with hemin as cofactor) to catalyze H2O2-mediated oxidation of 2-HOFlu, producing insoluble oxidative product precipitated on the DNA films. The mechanism was characterized by UV–vis spectroscopy, fluorescence spectra, differential pulse voltammetry (DPV) and electrochemical impedance spectroscopy (EIS). Depending on the difference of charge transfer resistance change (ΔR CT) before and after reaction with 2-HOFlu, 2-HOFlu can be sensitively and selectively detected with a detection limit of 1.2 nM in Tris-NaClO4 buffer solution. Finally, the DNA sensor was also challenged in natural lake water sample with a detection limit of 3.6 nM.
Material and methods
Materials
K3[Fe(CN)6], K4[Fe(CN)6], NaClO4, KClO4, ABTS (2,2′-azino-bis(3-ethylbenzothiazoline)-6-sulfonate), methanol, H2O2, DMSO (dimethyl sulfoxide), hemin, HClO4, Tris [tris-(hydroxymethyl)-aminomethane], 2-hydroxyfluorene (2-HOFlu), 9-hydroxyfluorene (9-HOFlu), 9-fluorenone and fluorene were purchased from Sigma-Aldrich (http://www.sigmaaldrich.com/china-mainland.html) and used without further purification. The gold electrodes, 99.99 % (w/w) polycrystalline with a diameter of 1 mm, were obtained from Aida Instrument Inc. in Tianjin (China) (http://www.tjaida.cn). Lake water sample was collected from Baiyangdian Lake in Hebei province (China) and filtered with the 0.45 μm fiber membrane prior to use. The stock solutions of 2-HOFlu, 9-HOFlu, 9-fluorenone and fluorene were first prepared with methanol and stored at 4 °C in the dark, then diluted to different concentrations with Tris-HClO4 buffer (20 mM, pH = 7.4) until used for electrochemical experiment. The stock solution of hemin was prepared with DMSO and kept at −20 °C in the refrigerator. The stock solution of 10 μM thiolated DNA was prepared in Tris-HClO4 buffer (20 mM, pH = 7.4) and then heated at 80 °C for 5 min to remove DNA aggregates and to dissociate any intermolecular interaction, obtaining uniform single-standed DNA solution and gradually cooled to room temperature prior to use. All other solutions were prepared with Milli-Q water (18.2 MΩ cm resistivity) from a Millipore Milli-Q system (Thermo Scientific EASYpure II).
The following DNA sequences: thiolated DNA (1)/(2) with poly-(T) tail at the 5′-end were purchased from Shanghai Sangon Biological Engineering Technology & Service Co. Ltd (http://www.sangon.com). The DNA (2) contains the G-rich hemin-binding aptamer named PW 17 (the underlined bold region).
-
5′-HO-(CH2)6-S-S-(CH2)6-TTTTTTTTT-ATGAC-ATTG-AAATC-ATA-3′ (1)
-
5′-HO-(CH2)6-S-S-(CH2)6-TTTTTTTTT-GGGTA-GGGC-GGGTT-GGG-3′ (2)
Monolayer preparation
The gold electrodes were cleaned prior to DNA modification as reported before [19]. Briefly, the gold electrodes were first mechanically polished on a microcloth (Buehler) with Gamma alumina (0.05 μm) slurries, followed by successive sonication in ethanol and Milli-Q water for 5 min. Then, the electrodes were electrochemically cleaned in 1 mol L−1 H2SO4 by cyclic voltammetry (CV) (0–1.5 V vs Ag/AgCl) cycling to remove any remaining surface impurities. After thoroughly rinsed with Milli-Q water, the gold electrodes were blown-dry with a nitrogen gun, and ready for DNA modification. The thiolated DNA solution was diluted in 20 mM Tris-HClO4 buffer (pH = 7.4) with a final concentration of 1 μM. Then, the DNA films on the gold electrodes were prepared by incubating the freshly cleaned gold electrodes in the prepared DNA solutions, followed by backfilling potential pinholes and defects by soaking the DNA films in 1 mM 6-mercaptohexanol for 1 h. After rinsing with 20 mM Tris-HClO4 buffer, the electrodes were then sequentially incubated in the Tris-HClO4 buffer (10 mM K+, pH = 7.4) for 1 h, hemin/Tris-HClO4 solution (1 μM hemin, 10 mM K+, pH = 7.4) for 1 h and different concentration of 2-HOFlu/Tris-HClO4 solution (1 mM H2O2, pH = 7.4) for 10 min. All experiments were conducted at 25 °C in Tris-HClO4 buffer solution (20 mM, pH = 7.4).
Electrochemical measurements
Electrochemical impedance spectroscopy (EIS) was carried out to measure the modification process of the electrode surface. Impedance spectra were measured using a potentiostat frequency analyzer (EG&G 2273). The ac voltage amplitude was 5 mV, and the voltage frequencies used for EIS measurements ranged from 100 kHz to 100 mHz. The applied potential was 250 mV vs. Ag/AgCl (formal potential of the redox probe [Fe(CN)6]3-/4- in the Tris-HClO4 buffer solution). As reported before [19], a conventional three-electrode system was used, and all the measurements were carried out in an enclosed and grounded Faraday cage. The reference electrode was constructed by sealing an Ag/AgCl wire into a glass tube with a solution of saturated KCl that was capped with a Vycor tip. The counter electrode was a platinum wire. All measurements were repeated for a minimum of three times with separate electrode to obtain the statistically meaningful result.
UV–vis spectroscopic analysis
Typically, the peroxidase-mimicking DNAzyme activity assay was carried out in the ABTS-H2O2 reaction system. Firstly, the K+-stabilized G-quadruplex complex was prepared by was diluting DNA strand (2) in Tris-HClO4 buffer solution (20 mM, pH = 7.4) containing 10 mM K+. Then, hemin was added after 1 h, obtaining the hemin/K+-stabilized G-quadruplex complex (DNAzyme (2)). The same procedure was also done for the arbitrary DNA strand (1), obtaining non-DNAzyme (1). All experiments were conducted at room temperature.
For the DNAzyme activity assay experiment, the peroxidation reaction was initiated by the addition of 10 μL of H2O2 (200 mM) to 790 μL of Tris-HClO4 buffer solution (20 mM, pH = 7.4) containing 2 mM ABTS and 0.05 μM non-DNAzyme (1) /DNAzyme (2). The absorption spectra of the reaction mixture were then recorded within 4 min with a Cazy 50 Scan UV–vis Spectrophotometer with the wavelength (λ) ranged from 500 to 390 nm and scan rate of 600 nm min−1.
Fluorescence spectrometry measurement
The fluorescence measurements were performed on a Cary Eclipse fluorescence spectrophotometer (VARIAN, USA) with the following settings: λex = 252 nm, λem = 332 nm for 2-HOFlu, 5 nm slit and PMT detector voltage = 650 V. Briefly, 5 μM 2-HOFlu Tris-HClO4 solution (20 mM, pH = 7.4) containing 1 mM H2O2 in the absence and presence of 50 nM DNAzyme (2) were respectively achieved by stirring for 10 min at room temperature. Then the corresponding fluorescence emission spectra were measured.
Safety considerations
The fluorene and its derivatives are potentially carcinogenic agents. MSDS information for these chemicals should be consulted, and precautions should be taken for using them (i.e., wearing gloves and mask). All experiments should be done in the laboratory fume hood.
Results and discussion
Optimization of pH
K+-stabilized G-quadruplex/hemin complex (DNAzyme) is able to catalyze the H2O2-mediated oxidation of ABTS, producing the free-radical cation ABTS•+, showing a maximal absorption at 422 nm [29]. Therefore, the change in the absorption signal at 422 nm can be employed to monitor the formation and the activity of the DNAzyme. Actually, the activity of the DNAzyme is very important for the feasible application of the DNA sensor to detect 2-HOFlu. Therefore, the DNAzyme activity was first explored by the colorimetric assay with the pH of the Tris-HClO4 buffer solution (20 mM) ranged from 4 to 10 as shown in Fig. S1 (Electronic Supplementary Material). The results showed the DNAzyme exhibits lower catalytic activity when the buffer solution is acidic (<6) or alkaline (>8.5), and the optimum pH for the DNAzyme catalytic activity was about 7.4, suggesting the catalytic activity of the DNAzyme was pH dependent. Therefore, the pH of the Tris-HClO4 buffer was adjusted to 7.4 prior to 2-HOFlu detection.
Electrochemical detection of 2-hydroxyFluorene
As reported before [19], the DNA (2) films were prepared by incubating the freshly cleaned gold electrodes in 1 μM DNA (2) solution, followed by backfilling potential pinholes and defects through soaking the DNA (2) films in 1 mM 6-mercaptohexanol. Then the DNA (2) strands self-assembled on the gold electrodes surface were transformed into K+-stabilized G-quadruplex/hemin complex (DNAzyme). Subsequently, the formed DNAzyme on the gold electrodes was applied to catalyze H2O2-mediated oxidation of 2-HOFlu, immersing the electrodes in 10 μM 2-HOFlu Tris-HClO4 solution (20 mM, pH = 7.4) containing 1 mM H2O2. Electrochemical impedance spectroscopy (EIS) was carried out to characterize the 2-HOFlu oxidation on the DNAzyme film and the representative Nyquist plots before and after incubating with 10 μM 2-HOFlu were shown in Fig. 1. The impedance spectra were analyzed with the modified Randles’ equivalent circuit (inset of Fig. 1) [33], and the fitting results were listed in Table 1.
Representative Nyquist plots (−Zim vs. Zre) for DNA (2) films (white circle) before and after interaction with 10 μM 2-HOFlu for 10 min (Containing 1 mM H2O2) (black circle). Measured data were shown as symbols with calculated fit to the equivalent circuit as solid lines. Inset: the measured data were fit to the equivalent circuit; R s, solution resistance; R CT, charge-transfer resistance; C film, capacitance of the DNA (2) films; R x and CPE, resistance and nonlinear capacitor accounting for 6-mercaptohexanol film
The solution resistance, R s, is the resistance between the reference electrode and the films of DNA (2) on the gold electrodes, which ranged from 6.8 to 7.5 Ω·cm2. C film accounts for the capacitance of the DNA (2) films on the gold electrodes. As shown in Fig. 2, C film was slightly decreased after immersed in 10 μM 2-HOFlu solution (containing 1 mM H2O2), revealing that the oxidation interaction on the DNA (2) film might result in an increase in the film thickness that led to a decreased dielectric constant [31]. The combination of R x and the constant phase element (CPE) accounts for the behavior of the 6-mercaptohexanol groups on the electrode surfaces [34]. Mass transport is not a major contributor because of the absence of Warburg impedance as shown in Fig. 1.
a The absorption spectra in Tris-HClO4 buffer solution (20 mM, pH = 7.4): (a) buffer solution; (b) ABTS; (c) DNA(1)+hemin+ABTS+H2O2; (d) hemin+ABTS+H2O2; (e) DNA(2) + hemin + ABTS + H2O2. (DNA: 0.05 μM; hemin: 0.05 μM; ABTS: 2 mM; K+: 10 mM; H2O2: 2.5 mM); b Fluorescence spectra of 2-HOFlu (5 μM) in Tris-HClO4 buffer solution (20 mM, pH = 7.4) containing 10 mM K+ in the absence and presence of 50 nM DNAzyme (2) and 1 mM H2O2 for 10 min at 25 °C. (a: 5 μM 2-HOFlu; b: 5 μM 2-HOFlu +1 mM H2O2; c: 5 μM 2-HOFlu+50 nM DNAzyme (2) +1 mM H2O2)
The most important parameter is the charge-transfer resistance, R CT, which is the result of the resistance to charge transfer between the solution-based redox probe [Fe(CN)6]3-/4- and the electrode surface. As shown in Table 1, after incubating the DNA (2) films with 10 μM 2-HOFlu solution (containing 1 mM H2O2) for 10 min, the R CT increased from 1150 (30) Ω·cm2 (R CT(before)) to 2555 (53) Ω·cm2 (R CT(after)) and the R CT difference (∆R CT = R CT(after) - R CT(before)) was about 1405 (57) Ω·cm2. This can be explained by the H2O2-mediated oxidation of 2-HOFlu catalyzed by the transformed DNAzyme films from the G-DNA (2). An insoluble product is formed that hinders electron transfer [31]. Therefore, the ∆R CT was a very significant parameter to be applied for the detection of 2-HOFlu.
Demonstration of the mechanism
As for the mechanism, the K+-stabilized G-quadruplex/hemin complex (DNAzyme) is critical. For comparison, the G-rich DNA (2) and the arbitrary DNA (1) were selected to catalyze ABTS-H2O2 system in the presence of K+ and/or hemin as the DNAzyme is able to catalyze the H2O2-mediated oxidation of ABTS, producing the free-radical cation ABTS•+, showing a maximal absorption at 422 nm [29]. The results were shown in Fig. 2a. No absorption peak was observed in the presence of ABTS (curve b), the sensing system showed a very low absorption signal at 422 nm; and a slightly higher absorption signal appeared when hemin was added (curve d), which was due to the weak catalytic activity of hemin to the H2O2. However, no catalytic activity was observed when the arbitrary non-DNAzyme DNA (1) was used (curve c). As expected, the absorption intensity greatly increased in the presence of DNAzyme (2) (curve e), which was attributed to the formed DNAzyme (2) that significantly enhanced the catalytic activity, producing much more free-radical cation ABTS•+ [35]. The results demonstrated that in the presence of K+ and hemin, the G-rich DNA (2) is transformed into a K+-stabilized G-quadruplex/hemin and exhibit catalytic activity.
Next, the K+-stabilized G-quadruplex/hemin complex (DNAzyme (2) transformed from single strand DNA (2) was applied to catalyze H2O2-mediated oxidation of 2-HOFlu, which was characterized through the fluorescence spectrometry as shown in Fig. 2b. As shown in Fig. 2b, 5 μM 2-HOFlu exhibited strong fluorescence at 332 nm with an excitation at 253 nm (curve a). Upon addition of 1 mM H2O2 for 10 min, the intensity of the fluorescence was slightly decreased (curve b). Alternatively, upon adding 1 mM H2O2 and 50 nM DNAzyme (2) (curve c), the fluorescence intensity of 2-HOFlu was sharply decreased (about 84.1 % of the fluorescence intensity lost), indicating that 2-HOFlu was quickly oxidized by H2O2 to its products without fluorescence at 470 nm. The reason might be due to the destroyed structure of conjugated molecule 2-HOFlu, resulting in the fluorescence quenching. However, the positive results confirmed that the DNAzyme can significantly accelerate the H2O2-mediated oxidation of 2-HOFlu.
Furthermore, the formation of the K+-stabilized G-quadruplex/hemin complex (DNAzyme (2)) on the electrodes was confirmed by the differential pulse voltammetry (DPV). As shown in Fig. 3a, no reduction peak was observed for the DNA films (curve c), and a weak reduction peak of hemin appeared at −0.315 V for the DNA (1) modified film after incubation in the K+/hemin solution (curve b), which is attributed to the non-specific interaction of hemin with DNA. However, a very strong peak was observed at about −0.335 V after immersing the K+-stabilized G-quadruplex DNA (2) modified film in the hemin solution (curve a). This peak corresponds to the reduction of hemin that binding with the K+-stabilized G-quadruplex [36], and also indicated the formation of hemin/G-quadruplex complex on the electrode [37].
a Differential pulse voltammograms of the fabricated biosensor measured in Tris-NaClO4 buffer solution (20 mM, pH = 7.4): (a) G-DNA (2) + K+ +hemin; (b) DNA (1) + K+ +hemin; (c) DNA (1) (K+ 20 μM, hemin 2 μM); b The time-dependent ΔR CT changes of the DNAzyme (2) modified flims (a) and non-DNAzyme DNA (1) modified films (b) in the presence of 10 μM of 2-HOFlu containing 1 mM H2O2 in Tris-NaClO4 buffer solution (20 mM, pH = 7.4). Error bars are derived from a minimum of three electrodes
Subsequently, the ∆R CT as a function of reaction time at the DNA (2) films on the gold electrodes were further explored by using an arbitrary non-DNAzyme (1) and DNAzyme (2). As shown in Fig. 3b., a significant ∆R CT change was observed when the DNAzyme (2) was utilized until no change was observed at 10 min, while only a very small increase of ∆R CT for the non-DNAzyme (1) modified films, which was presumably caused by the absorbed hemin on the arbitrary DNA (1) in the pre-incubation procedure. These results were well in accordance with the UV–vis analysis and the DPV measurements. Therefore, the increased ∆R CT confirmed the precipitation layer on the DNA (2) films due to the effectively catalytic oxidation of 2-HOFlu by H2O2. In addition, the 10 min reaction time was chosen in the present study.
Based on the results discussed above, it can be concluded that: (1) the thiolated DNA (2) was modified on the gold electrodes via the strong covalent Au-S bond; (2) the immobilized single-strand DNA (2) folded into G-quadruplex structure in the presence of K+ and further forming K+-stabilized G-quadruplex/hemin complex by binding with hemin; (3) the formed K+-stabilized G-quadruplex/hemin complex can effectively catalyze the H2O2-mediated oxidation of 2-HOFlu, producing insoluble oxidative products precipitated on the DNA films.
Sensitivity and selectivity
Based on the mechanism, single strand DNA (2) was applied as a sensing probe to immobilize on the gold electrodes for electrochemical detection of 2-HOFlu by EIS. First, the G-DNA (2) was self-assembled on the gold electrodes. Then, in the presence of K+ and hemin, DNA (2) transformed into K+-stabilized G-quadruplex/hemin complex on the electrodes. Next, the prepared electrodes were incubated in the solutions of 2-HOFlu (containing 1 mM H2O2) with the concentration ranged from 0.1 nM to 100 μM. EIS was respectively recorded and analyzed with the help of the equivalent circuit shown inset of Fig. 1.
The relationship between the ∆R CT of the DNA (2) films and the concentrations of 2-HOFlu was shown in Fig. 4a. Upon decreasing the concentration of 2-HOFlu from 100 μM to 0.1 nM, the lower concentration of 2-HOFlu was used, the less oxidative product precipitated on the DNA films. The result directly led to a decreased ∆R CT until no distinct change in ∆R CT was observed with the 1 nM 2-HOFlu used. The inset of Fig. 4a showed the linear relationship between ∆R CT and the concentrations of 2-HOFlu ranged from 10−5 to 10−8 M, and the linear regression equation was Y (∆R CT) = 3271 + 358.7 lg [C2-HOFlu] with a correlation coefficient of 0.99. The detection limit was determined to be 1.2 nM (S/N = 3), which can be compared with that of HPLC, GC-MS and LC-MS assays as shown in Table S1.
Sensitivity for 2-HOFlu in Tris-NaClO4 buffer (a): relationship between ΔR CT and the concentrations of 2-HOFlu; Selectivity of the sensor in Tris-NaClO4 buffer for 100 nM 2-HOFlu and other selected compounds (such as 9-hydroxyfluorene (9-HOFlu) 9-fluorenone and fluorene) (b); Sensitivity for 2-HOFlu in lake water sample (c): relationship between ΔR CT and the concentrations of 2-HOFlu. Error bars were derived from a minimum of three electrodes
In addition, the selectivity of the sensor was also exploited through incubating the prepared gold electrodes with other possible fluorene derivatives (such as 100 nM 9-HOFlu, 9-fluorenone and fluorene), respectively. As shown in Fig. 4b, only 2-HOFlu can cause a considerable increase in ∆R CT while other selected fluorene derivatives made a small ∆R CT. Besides, the determination of 2-HOFlu was hardly not affected by the selected fluorene derivatives in the co-existing system, which indicated that the reported DNA biosensor was specifically sensitive for 2-HOFlu detection. It should be noted that, however, why the selected fluorene derivatives performed different impedance behavior from that of 2-HOFlu? As for this question, further studies are undergoing in our laboratory. Nevertheless, such ability of the reported DNA sensor to discriminate 2-HOFlu from selected fluorene derivatives by EIS added a new and important dimension of selectivity. Furthermore, it is of great significance to investigate whether DNAzyme can play roles in the H2O2-mediated oxidation of other environmental pollutants, such as chlorophenols, aminophenols nitrophenols and other toxic pollutants. And it is hopeful that the peroxidase-like DNAzyme can substitute the protein enzymes for prospective future application to other protein enzymes related researches.
Practical application in lake water
Finally, the potential performance of the DNA sensor for the 2-HOFlu analysis in natural water sample was also explored. Figure 4c showed the relationship of the ∆R CT and the concentrations of 2-HOFlu in the natural lake water. As shown in Fig. 4c, after incubating the DNA sensor in the lake water containing 2-HOFlu (1 mM H2O2) with the concentrations ranged from 100 μM to 0.1 nM, a continuously decreased R CT was respectively obtained as well. The inset of Fig. 4c showed the linear relationship between ∆R CT and the concentrations of 2-HOFlu ranged from 10−5 M to 10−8 M, and the linear regression equation was Y (∆R CT) =2695.8 + 303.8 lg [C2-HOFlu] with a correlation coefficient 0.99. The detection limit was calculated to be 3.6 nM (S/N = 3). The results showed that the assay exhibited excellent capacity of resisting disturbance and is efficiently applied to detect 2-HOFlu in natural water sample.
Conclusions
In this paper, we designed an in situ amplification sensing strategy to detect 2-HOFlu with high sensitivity and selectivity by electrochemical impedance spectroscopy. The G-rich DNA strand was self-assembled on the gold electrodes and transformed into K+-stabilized G-quadruplex/hemin complex in the presence of K+ and hemin, which can catalyze H2O2-mediated oxidation of 2-HOFlu to produce insoluble products precipitated on the DNA films, causing an increased charge transfer resistance. Depending on the difference in charge transfer resistance, 2-HOFlu was sensitively and sensitively detected with the detection limit of 1.2 nM in Tris-HClO4 buffer. Furthermore, the DNA sensor was successfully applied to detect 2-HOFlu in natural lake water sample with a detection limit of 3.6 nM. The sensing assay has the potential to be further extended to investigate the interaction with other contaminants to evaluate their environmental toxicity. And the peroxidase-like DNAzyme has a wide scope in that it may be applied to other protein enzymes related researches.
References
Ma L, Chu S, Wang X, Cheng H, Liu X, Xu X (2005) Polycyclic aromatic hydrocarbons in the surface soils from outskirts of Beijing, China. Chemosphere 58:1355
Yin C, Jiang X, Yang X, Bian Y, Wang F (2008) Polycyclic aromatic hydrocarbons in soils in the vicinity of Nanjing, China. Chemosphere 73:389
Wu H, Xue Y, You Q, Huang Q, Ou J (2009) Analysis of pyrolysis components of biomass tar by GC-MS. Chem Eng Oil Gas 38:72
Sepic E, Bricelj M, Leskovsek H (2003) Toxicity of fluoranthene and its biodegradation metabolites to aquatic organisms. Chemosphere 52:1125
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1
Luan TG, Yu KSH, Zhong Y, Zhou HW, Lan CY, Tam NFY (2006) Study of metabolites from the degradation of polycyclic aromatic hydrocarbons (PAHs) by bacterial consortium enriched from mangrove sediments. Chemosphere 65:2289
Toriba A, Chetiyanukornkul T, Kizu R, Hayakawa K (2003) Quantification of 2-hydroxyfluorene in human urine by column-switching high performance liquid chromatography with fluorescence detection. Analyst 128:605
Chetiyanukornkul T, Toriba A, Kizu R, Hayakawa K (2004) Urinary 2-hydroxyfluorene and 1-hydroxypyrene levels in smokers and nonsmokers in Japan and Thailand. Polycycl Aromat Compd 24:467
Kamiya M, Toriba A, Onoda Y, Kizu R, Hayakawa K (2005) Evaluation of estrogenic activities of hydroxylated polycyclic aromatic hydrocarbons in cigarette smoke condensate. Food Chem Toxicol 43:1017
Campo L, Rossella F, Fustinoni S (2008) Development of a gas chromatography/mass spectrometry method to quantify several urinary monohydroxy metabolites of polycyclic aromatic hydrocarbons in occupationally exposed subjects. J Chromatogr B 875:531
Ding YP, Liu WL, Wu QS, Wang XG (2005) Direct simultaneous determination of dihydroxybenzene isomers at C-nanotube-modified electrodes by derivative voltammetry. J Electroanal Chem 575:275
Zhu G, Gai P, Wu L, Zhang J, Zhang X, Chen J (2012) β-cyclodextrin-platinum nanoparticles/graphene nanohybrids: enhanced sensitivity for electrochemical detection of naphthol isomers. Chem Asian J 7:732
Wang Y, Wang J, Yang F, Yang X (2010) Spectrophotometric detection of lead (II) ion using unimolecular peroxidase-like deoxyribozyme. Microchim Acta 171:195
Drummond TG, Hill MG, Barton JK (2003) Electrochemical DNA sensors. Nat Biotechnol 21:1192
Wang P, Mai Z, Dai Z, Zou X (2010) Investigation of DNA methylation by direct electrocatalytic oxidation. Chem Commun 46:7781
Andreescu S, Sadik OA (2004) Trends and challenges in biochemical sensors for clinical and environmental monitoring. Pure Appl Chem 76:861
Vestergaard M, Kerman K, Tamiya E (2007) An overview of label-free electrochemical protein sensors. Sensors 7:3442
Wu LD, Lu X, Jin JB, Zhang HJ, Chen JP (2011) Electrochemical DNA biosensor for screening of chlorinated benzene pollutants. Biosens Bioelectron 26:4040
Liang G, Li T, Li XH, Liu XH (2013) Electrochemical detection of the amino-substituted naphthalene compounds based on intercalative interaction with hairpin DNA by electrochemical impedance spectroscopy. Biosens Bioelectron 48:238
Wang GL, Zhou LY, Luo HQ, Li NB (2013) Electrochemical strategy for sensing DNA methylation and DNA methyltransferase activity. Anal Chim Acta 768:76
Luo L, Zhang Z, Ding Y, Deng D, Zhu X, Wang Z (2013) Label-free electrochemical impedance genosensor based on 1-aminopyrene/graphene hybrids. Nanoscale 5:5833
Li X, Shen L, Zhang D, Qi H, Gao Q, Ma F, Zhang C (2008) Electrochemical impedance spectroscopy for study of aptamer-thrombin interfacial interactions. Biosens Bioelectron 23:1624
Bogomolova A, Komarova E, Reber K, Gerasimov T, Yavuz O, Bhatt S, Aldissi M (2009) Challenges of electrochemical impedance spectroscopy in protein biosensing. Anal Chem 81:3944
Chen Z, Li L, Zhao H, Guo L, Mu X (2011) Electrochemical impedance spectroscopy detection of lysozyme based on electrodeposited gold nanoparticles. Talanta 83:1501
Xiao Y, Pavlov V, Niazov T, Dishon A, Kotler M, Willner I (2004) Catalytic beacons for the detection of DNA and telomerase activity. J Am Chem Soc 126:7430
Pavlov V, Xiao Y, Gill R, Dishon A, Kotler M, Willner I (2004) Amplified chemiluminescence surface detection of DNA and telomerase activity using catalytic nucleic acid labels. Anal Chem 76:2152
Kosman J, Juskowiak B (2011) Peroxidase-mimicking DNAzymes for biosensing applications: a review. Anal Chim Acta 707:7
Willner I, Shlyahovsky B, Zayats M, Willner B (2008) DNAzymes for sensing, nanobiotechnology and logic gate applications. Chem Soc Rev 37:1153
Zhou XH, Kong DM, Shen HX (2010) G-quadruplex-hemin DNAzyme-amplified colorimetric detection of Ag+ ion. Anal Chim Acta 678:124
Liu X, Freeman R, Golub E, Willner I (2011) Chemiluminescence and chemiluminescence resonance energy transfer (CRET) aptamer sensors using catalytic hemin/G-quadruplexes. ACS Nano 5:7648
Won BY, Shin S, Fu RZ, Shin SC, Cho DY, Park HG (2011) A one-step electrochemical method for DNA detection that utilizes a peroxidase-mimicking DNAzyme amplified through PCR of target DNA. Biosens Bioelectron 30:73
Patolsky F, Lichtenstein A, Willner I (2003) Highly sensitive amplified electronic detection of DNA by biocatalyzed precipitation of an insoluble product onto electrodes. Chem Eur J 9:1137
Li XH, Zhou Y, Sutherland TC, Baker B, Lee JS, Kraatz HB (2005) Chip-based microelectrodes for detection of single-nucleotide mismatch. Anal Chem 77:5766
Liang G, Li XH, Liu XH (2013) Electrochemical detection of 9-hydroxyfluorene based on the direct interaction with hairpin DNA. Analyst 138:1032
Orbach R, Willner B, Willner I (2015) Catalytic nucleic acids (DNAzymes) as functional units for logic gates and computing circuits: from basic principles to practical applications. Chem Commun. doi:10.1039/C1034CC09874A
Liu L, Liang Z, Li Y (2012) Label free, highly sensitive and selective recognition of small molecule using gold surface confined aptamers. Solid State Sci 14:1060
Yin H (2012) Amplified electrochemical microRNA biosensor using hemin-G-quadruplex complex as the sensing element. Analyst 27:7140
Acknowledgments
We are grateful for financial support from the National Science Foundation for Innovative Research Group (51121003) and the National Natural Science Foundation of China (21377013), Major State Basic Research Development Program (2013CB430405) and Fundamental Research Funds for the Central Universities and the China Postdoctoral Science Foundation (2014 M550854).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 56 kb)
Rights and permissions
About this article
Cite this article
Liang, G., Liu, X. G-quadruplex based impedimetric 2-hydroxyfluorene biosensor using hemin as a peroxidase enzyme mimic. Microchim Acta 182, 2233–2240 (2015). https://doi.org/10.1007/s00604-015-1565-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1565-x