Abstract
We report on a bienzyme-channeling sensor for sensing glucose without the aid of mediator. It was fabricated by cross-linking horseradish peroxidase (HRP) and glucose oxidase (GOx) on a glassy carbon electrode modified with multiwalled carbon nanotubes (MWNTs). The bienzyme was cross-linked with the MWNTs by glutaraldehyde and bovine serum albumin. The MWNTs were employed to accelerate the electron transfer between immobilized HRP and electrode. Glucose was sensed by amperometric reduction of enzymatically generated H2O2 at an applied voltage of −50 mV (vs. Ag/AgCl). Factors influencing the preparation and performance of the bienzyme electrode were investigated in detail. The biosensor exhibited a fast and linear response to glucose in the concentration range from 0.4 to 15 mM, with a detection limit of 0.4 mM. The sensor exhibited good selectivity and durability, with a long-term relative standard deviation of <5 %. Analysis of glucose-spiked human serum samples yielded recoveries between 96 and 101 %.

A novel bienzyme-channeling sensor for glucose sensing has been constructed without the aid of mediator. This biosensor was fabricated by cross-linking horseradish peroxidase (HRP) and glucose oxidase (GOD) onto glass carbon electrode (GCE) modified with multiwall carbon nanotubes (MWNTs) which accelerated the electron transfer between the HRP and electrode.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the first report on glucose enzyme biosensors by Clark and Lyons [1], the analysis of glucose has attracted intense research interest. Glucose is a necessary substance directly involved in metabolic processes in the body [2]. The monitoring of glucose is essential in clinical diagnosis of kinds of diseases, such as Islet cell carcinoma and glucose metabolism disorders [3]. Among developed approaches, amperometric biospecific enzyme glucose biosensors have been generally considered in terms of the simple operation, sensitive determination, fast response, and low cost [4].
Usually, the glucose biosensors are based on monoenzyme-glucose oxidase (GOx) through the oxidation of glucose and the reduction of dissolved oxygen [5]. However, low sensitivity and serious interference problem arising from uric acid, ascorbic acid at high anodic potentials limited the development of monoenzyme-glucose biosensor. An alternative approach to improve the biosensor performance is construction of bienzymatic peroxidase/oxidase biosensors [6–8]. In this system, H2O2 generated in the process of GOx oxidizing glucose is subsequently reduced by the horseradish peroxidase (HRP). HRP is then electrically connected to the electrode surface at low applied potentials [9]. Compared with monoenzyme biosensor, this cascade schemes amplified the electrochemical responses and obviously enhanced the sensitivity of the biosensor [10]. Otherwise, the detection of glucose is under mild applied potentials, thus increasing the specificity of the biosensor and avoiding the accumulation of H2O2 which would inactivate GOx [11, 12].
Different electrode modifying materials would influence the biosensor performance such as the sensitivity, selectivity, stability, etc [13]. Carbon nanotubes (CNTs) has been widely used as modifying material for the construction of biosensors during the past decade because of their unique structures, large edge/basal plane ratio, enhanced electronic properties, and rapid electrode kinetics [14, 15]. CNTs could accelerate the electron-transfer between the electrode and redox centers of enzymes, and improve the conductive property, thus the performance of CNTs-based biosensor is improved largely [16, 17]. There have several reports about cooperating of two enzymes (peroxidase and oxidases) for the construction of bienzyme biosensors to detect glucose [18–21], cholesterol [22], acetylcholine [23], glucosinolate [24], and a-1-fetoprotein [25].
Thereinto, HPR/GOx was immobilized on CNTs modified electrode for glucose detection by various immobilization methods, such as adsorption [26], entrapment in gels, membranes or polymer matrices [20, 27–31] and layer-by-layer assembly [32–34] with/without redox mediator. As we known, reliable immobilization of enzyme on electrode is vital in biosensor fabrication concerning of simple immobilization process and high retention of its bioactivity and stability [17]. Adsorption is simple and easily carried out, but it suffers from stability problem. Entrapment or encapsulation results in a low enzymatic reaction rate because of diffusion-controlled process [34]. Layer-by-layer assembly is advantageous due to its simplicity and versatility, whereas stability may be lower compared with the chemical methods [35].
Cross-linking was a commonly used immobilization technique with strong interaction between enzymes and carriers, thus reducing the leakage of enzymes and improving the stability of the biosensor. Previously, we constructed an amperometric H2O2 monoenzyme biosensor by cross-linking HRP to CNTs-modified glass carbon electrode surface, which showed the advantages of good stability [36]. The aim of the current study is to fabricate a stable bienzyme biosensor for glucose detection using cross-linking technique. Glutaraldehyde was used as the cross-linked reagent to attach HRP and GOx onto multiwall carbon nanotubes (MWNTs) which were immobilized on glassy carbon electrode, and bovine serum albumin was added to provide suitable micro-circumstance for HRP and GOx. By combining the benefits of CNTs and cross-linking film, the biosensor offered an excellent amperometric response for glucose with high stability and quick response. The influencing factors on the bienzyme sensor response to glucose were examined in detail.
Experimental
Reagents
HRP (250 U mg−1) was purchased from Sino-American Biotechnology Company (Luoyang, China http://www.sabc.com.cn). GOx (145 U mg−1) was supplied by Wako pure Chemical Industies, Ltd. (Osaka, Japan http://www.wako-chem.co.jp). Glutaraldehyde and bovine serum albumin were purchased from Sigma (St. Louis, Mo, USA http://www.sigmaaldrich.com). MWNTs were purchased from Shenzhen Nanotech Port Co. Ltd. (Shenzhen, China http://www.nanotubes.com.cn). Phosphate buffer solutions (0.1 M) with various pH values were prepared by mixing stock standard solutions of K2HPO4 and KH2PO4 and adjusting the pH with HCl or NaOH. All double-distilled water was used in all experiments. H2O2 solutions were prepared daily from 35 % H2O2 solution. All other chemicals were of analytical grade and used without further purifications.
Apparatus and measurements
Electrochemistry measurements were performed with a Bioanalytical Systems BAS-100B/W electrochemical analyzer (BAS Co, U.S.A http://www.basinc.com). All electrochemical experiments employed a three-electrode cell (10 mL, single electrolyte compartment) with a glass carbon working electrode (GCE), a platinum wire auxiliary electrode and an Ag/AgCl (3 M NaCl solution) reference electrode. All potentials were referred to this reference electrode. All measurements were carried out in isothermal reactor at constant temperature (30 ± 0.2 °C) with 0.1 M phosphate buffer solution as background electrolyte. Cyclic voltammetric experiments were carried through in quiescent solutions with the scan rate of 50 mV s−1.
Fabrication of bienzyme biosensors
MWNTs were pretreated as described previously [36]. The GCE (3 mm in diameter) was polished with 0.3 μm alumina slurry followed by rinsing thoroughly with doubly distilled water. Then it was ultrasonicated in ethanol and double distilled water for several minutes, and allowed to dry at room temperature.
The biosensor was prepared by immobilizing the MWNTs and bienzyme on GCE in three steps. In the first step, the MWNTs were immobilized by casting 25 μL of treated MWNTs solution onto the GCE and then evaporating the N, N-dimethylformamide solvent in air to form MWNTs modified electrode (MWNTs-GCE). In the second step, HRP was immobilized on MWNTs-GCE based on the cross-linking reaction. 5 μL of the optimized enzyme solutions containing HRP (40 mg mL−1), bovine serum albumin (80 mg mL−1) and glutaraldehyde (0.10 %, 50 μL mL−1) were coated onto the MWNTs film, and was rotated with 5,000 rpm for 10 s and allowed to stay in air for dry. In the third step, 5 μL of the optimized enzyme solutions containing GOx (20 mg mL−1), bovine serum albumin (80 mg mL−1) and glutaraldehyde (0.10 %, 50 μL mL−1) were casted on HRP-MWNTs-GCE based on the cross-linking reaction and was rotated with 5,000 rpm and dried in ambient conditions. Then the GOx-HRP-MWNTs-GCE was obtained. When not in use, the resulted electrode was stored in 0.1 M phosphate buffer solution with pH 7.0 at 4 °C.
Result and discussion
Design of the electron transfer pathway
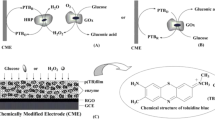
In preliminary experiment, two methods were used to immobilize bienzyme onto the electrode. One method was integrating the mixture of HRP and GOx within one layer, but it was found the current response was quite low. Although the co-immobilization of HRP and GOx made enzymatically generated H2O2 immediately reduced by HRP, the far distance of HRP to the electrode surface caused by the larger bulk of GOx molecule than HRP led to a steric hindrance effect, which did not facilitate electron transfer between MWNTs and HRP. Thus, we anticipated a bienzyme sensor architecture using two layers to have high current responses. A first layer contained cross-linked HRP onto a MWNTs-modified electrode enabled fast electron transfer between the oxidized active site of HRP and the electrode surface. On top of this layer, a second layer was precipitated by cross-linking GOx with glutaraldehyde and bovine serum albumin. The bienzyme sensor architecture and the presumed electron-transfer pathway are shown in Fig. 1. The reaction pathway can be approximately expressed as:
The amperometric response of bienzyme electrode were preliminarily tested with 0, 5 and 10 mM glucose in pH 7.0 phosphate buffer solution. As shown in Fig. 2, amperometric response increased with increasing concentration of glucose from 0 to 10 mM. To verify whether the current response was due to direct catalysis of GOx with glucose, a control experiment in the absence of HRP was performed. No current response to glucose could be observed at the GOx-MWNTs-GCE, which confirmed that the catalytic current is due to the catalysis react of HRP with enzymatically generated H2O2.
Optimization of the working electrode architecture
Since the different loading of HRP and GOx at the electrode might affect the performance of the biosensor, the enzyme compositions (HRP/GOx ratio) were optimized by measuring the amperometric responses of 5 mM glucose. From Fig. 3 it can be seen that higher current response was resulted for a higher HRP/GOx ratios because increasing of HRP activity improved the sensitivity of bienzyme sensor [7]. A largest current response was observed for a HRP/GOx ratio of 2/1 (40 mg HRP/20 mg GOx). This HRP/GOx ratio was taken for the subsequent construction of the bienzyme biosensor. However, when the ratio of HRP/GOx was larger than 2/1, much lower response currents were observed. As GOx is indispensable for the catalysis of glucose, an appropriate amount of GOx is necessary.
Effects of reduction potential
Studies to investigate the dependence of the biosensor response on the applied potential were performed. We explored the effects of applied potential for the amperometric detection of glucose at HRP-GOx-MWNTs-GCE over the potential range from −150 to 100 mV. The result was shown in Fig. 4. The current response increased as the applied potential shifted positively from −150 to −50 mV and arrived at a maximum value at −50 mV. It is preferable to control the lower working potential to avoid or decrease the interference from other electroactive substances potentially present in serum such as ascorbic acid, uric acid etc. Therefore, a detection potential of −50 mV was selected for further work.
Effect of pH and temperature
The study of the influence of pH on the amperometric response of the biosensor was investigated between 4.0 and 9.0 in 0.1 M phosphate buffer solution. The maximum current response was attained at pH 7.0 which is near to the optimum pH observed for free GOx molecule. Therefore, the suitable pH with the maximal performance of the bienzyme biosensor was set at pH 7.0.
The effect of temperature on the bienzyme biosensor response was studied by measuring the steady-state current of 5 mM glucose solutions at temperatures ranging from 20 to 60 °C. As the temperature ranged from 20 to 40 °C, the current response increased. With the temperature up to 50 °C, the current response decreases rapidly, probably due to the denaturation of the enzymes. So 30 °C was chosen for further experiments.
GOx-HRP-MWNTs-GCE biosensor performance
By using the optimized working parameters, the response of glucose to GOx-HRP-MWNTs film at GCE was studied using chronoamperometry mode. A linear calibration plot for glucose (R = 0.9983) was obtained in the 0.4–15 mM concentration range, and the linear regression equation was I = 8.6325 + 1.3287 C (unit of C is mM). The limit of detection was 0.4 mM at a signal-to-noise ratio of 3.
The apparent Michaelis-Menten constant (Km), an indication of the enzyme-substrate kinetics, is commonly used to evaluate the biological activity of immobilized enzymes. The calculated Km obtained from the Lineweaver-Burk equation [37] was estimated to be 7.7 μM, much lower than that the bienzyme immobilized on poly(toluidine blue O) [26], neutral red functionalized CNTs [27] and thionin functionalized CNTs [19].
Where, Iss is the steady state current after the addition of substrate, C is the bulk concentration of the substrate and Imax is the maximum current measured under saturated substrate conditions. The low Km value indicated that the immobilized HRP and GOx possessed high enzymatic activity, and the present electrodes exhibited high biologically affinity to glucose. This fact suggests that the Michaelis-Menten equilibrium was favored in our electrode design, probably as a consequence of the microenvironment provided by glutaraldehyde and bovine serum albumin.
Stability and repeatability
The stability of HRP-GOx-MWNTs-GCE was also tested. It was found that the current response to the glucose decreased about 10 % after storage in 0.1 M pH 7.0 phosphate buffer solution at 4 °C for 20 days, indicating good stability of HRP-MWNTs-GCE. Therefore, HRP-GOx molecules can be firmly immobilized on the surface of the MWNTs and not leak out of the biosensor. The response time of the sensor, calculated as the time elapsed between 5 and 95 % of response height, was fast (less than 5 s). The reproducibility was checked by monitoring the current response for 10 replicate injections of 5.0 mM glucose with an applied potential of −50 mV. The relative standard deviation was 4.5 %, indicating a good reproducibility of the biosensor. Furthermore, the fabrication reproducibility of six different electrodes, showed an acceptable reproducibility with a relative standard deviation of 6.4 % for the currents determination at a glucose concentration of 5 mM. The good stability and reproducibility was partially attributed to the strong interactions between enzymes and carriers via cross-linking immobilization, which resulted in little leakage of HRP- GOx molecules from the biosensor.
Selectivity
The effects of common interfering species on the amperometric responses at the GOx-HRP-MWNTs modified electrode at −50 mV were studied. Common interferences for glucose detection in real application include ascorbic acid, uric acid, dopamine and acetaminophen. The amperometric responses were obtained by adding interfering species of different concentration to the solution containing 5 mM glucose. No apparent change (<5 %) in the current response was found when the concentration of ascorbic acid, uric acid, dopamine and acetaminophen was less than 2 mM, 3 mM, 1 mM and 1 mM, respectively. Compared with nonenzyme biosensor, the anti-interferential ability is much higher than that glucose oxidase adsorbing on poly(methyl methacrylate)-bovine serum albumin core-shell nanoparticles with the tolerance concentration of only 0.1 mM for ascorbic acid and 0.5 mM for uric acid, respectively [34]. This result indicated good selectivity of the biosensor because lower detection potential could limit the oxidation of the easy-oxidized interfering substances. Table 1 showed the parameter of GOx-HRP-MWNTs in terms of analytical performance and stability are compared with earlier reported amperometric glucose biosensors.
Sample analysis
To demonstrate the straightforward real application of the glucose biosensor, two serum samples were analyzed. The sample was diluted to its half concentration with 0.1 M phosphate buffer solution at pH 7.0. The diluted samples were then determined by the as-prepared bienzyme system at a potential of −50 mV. Table 2 summarized the obtained results. As can be seen, the mean recoveries ranged between 96 and 101 %. These results indicated that the biosensor suffered from littlie interference from serum sample matrix and thus can be directly used to determine glucose in serum without sample pretreatment.
Conclusion
In this work, we presented a simple mediator-free amperometric bienzymatic glucose biosensor by cross-linking GOx and HRP on the MWNTs modified electrode. The special nano-structure of MWNTs resulted in a high catalytic activity of the immobilized enzymes and accelerates the direct electron transfer between the heme in HRP and the electrode. The designed bienzyme-channeling sensor provides a promising strategy to construct sensitive, stable and anti-interferential amperometric biosensors for glucose with fast response. It shows potential that the strategy developed in this work may be easily extended for the preparation of other multienzyme biosensors for detection of uric acid, cholesterol, etc.
References
Clark LC, Lyons C (1962) Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci 102:29–45
Chen X, Chen J, Wang FB, Xiang X, Luo M, Ji XH, He ZK (2012) Determination of glucose and uric acid with bienzyme colorimetry on microfluidic paper-based analysis devices. Biosens Bioelectron 35:363–368
Wang GF, He XP, Wang LL, Gu AX, Huang Y, Fang B, Geng BY, Zhang XJ (2013) Non-enzymatic electrochemical sensing of glucose. Microchim Acta 180:161–186
Yang SL, Lu ZZ, Luo SL, Liu CB, Tang YH (2013) Direct electrodeposition of a biocomposite consisting of reduced graphene oxide, chitosan and glucose oxidase on a glassy carbon electrode for direct sensing of glucose. Microchim Acta 180:127–135
Chen XJ, Zhu JW, Chen ZX, Xu CB, Wang Y, Yao C (2011) A novel bienzyme glucose biosensor based on three-layer Au–Fe3O4@SiO2 magnetic nanocomposite. Sensors Actuators B Chem 159:220–228
Hervagault JF, Joly G, Thomas D (1975) Kinetic studies dealing with an immobilized bienzyme system. Eur J Biochem 51:19–23
Matsumoto R, Mochizuki M, Kano K, Ikeda T (2002) Unusual response in mediated biosensors with an oxidase/peroxidase bienzyme system. Anal Chem 74:3297–3303
Rasmussen M, Ritzmann RE, Lee I, Pollack AJ, Scherson D (2012) An implantable biofuel cell for a live insect. J Am Chem Soc 134:1458–1460
Delvaux M, Walcarius A, Demoustier-Champagne S (2005) Bienzyme HRP-GOx-modified gold nanoelectrodes for the sensitive amperometric detection of glucose at low overpotentials. Biosens Bioelectron 20:1587–1594
Li F, Wang Z, Chen W, Zhang SS (2009) A simple strategy for one-step construction of bienzyme biosensor by in-situ formation of biocomposite film through electrodeposition. Biosens Bioelectron 24:3030–3035
Dai ZH, Bao JC, Yang XD, Ju HX (2008) A bienzyme channeling glucose sensor with a wide concentration range based on co-entrapment of enzymes in SBA-15 mesopores. Biosens Bioelectron 23:1070–1076
Sorochinskill VV, Kurganov BI (1996) Diffusion-kinetic theory of stationary behaviour of amperometric bienzyme electrodes. Biosens Bioelectron 8:709–718
Wang F, Hu SS (2009) Electrochemical sensors based on metal and semiconductor nanoparticles. Microchim Acta 165:1–22
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Merkoci A, Pumera M, Liopis X, Del Valle M, Alegret S (2005) New materials for electrochemical sensing VI: carbon nanotubes. TrAC Trends Anal Chem 24:826–838
Chen SH, Yuan R, Chai YQ, Zhang LY, Wang N, Li XL (2007) Amperometric third-generation hydrogen peroxide biosensor based on the immobilization of hemoglobin on multiwall carbon nanotubes and gold colloidal nanoparticles. Biosens Bioelectron 22:1268–1274
Wang J, Liu GD, Jan MR (2004) Ultrasensitive electrical biosensing of proteins and DNA: carbon-nanotube derived amplification of the recognition and transduction events. J Am Chem Soc 126:3010–3011
Castillo J, Gáspár S, Sakharov I, Csöregi E (2003) Bienzyme biosensors for glucose, ethanol and putrescine built on oxidase and sweet potato peroxidase. Biosens Bioelectron 18:705–714
Sheng QL, Zheng JB (2009) Bienzyme system for the biocatalyzed deposition of polyaniline templated by multiwalled carbon nanotubes: a biosensor design. Biosens Bioelectron 24:1621–1628
Jeykumari DRS, Narayanan SS (2008) Fabrication of bienzyme nanobiocomposite electrode using functionalized carbon nanotubes for biosensing applications. Biosens Bioelectron 23:1686–1693
Eguílaz M, Villalonga R, Yáñez-Sedeño P, PingarrÓn JM (2011) Designing electrochemical interfaces with functionalized magnetic nanoparticles and wrapped carbon nanotubes as platforms for the construction of high-performance bienzyme biosensors. Anal Chem 83:7807–7814
Cai XJ, Gao X, Wang LS, Wu Q, Lin XF (2013) A layer-by-layer assembled and carbon nanotubes/gold nanoparticles-based bienzyme biosensor for cholesterol detection. Sensors Actuators B Chem 181:575–583
Hou SH, Ou ZM, Chen Q, Wu BY (2012) Amperometric acetylcholine biosensor based on self-assembly of gold nanoparticles and acetylcholinesterase on the sol–gel/multi-walled carbon nanotubes/choline oxidase composite-modified platinum electrode. Biosens Bioelectron 33:44–49
Serafín V, Agüí L, Yáñez-Sedeño P, PingarrÓn JM (2009) Glucosinolate amperometric bienzyme biosensor based on carbon nanotubes-gold nanoparticles composite electrodes. Electroanalysis 21:1527–1532
Niu H, Yuan R, Chai YQ, Mao L, Liu HJ, Cao YL (2013) Highly amplified electrochemiluminescence of peroxydisulfate using bienzyme functionalized palladium nanoparticles as labels for ultrasensitive immunoassay. Biosens Bioelectron 39:296–299
Yao YL, Shiu KK (2008) A mediator-free bienzyme amperometric biosensor based on horseradish peroxidase and glucose oxidase immobilized on carbon nanotube modified electrode. Electroanalysis 20:2090–2095
Zhu LD, Yang RL, Zhai JL, Tian CY (2007) Bienzymatic glucose biosensor based on co-immobilization of peroxidase and glucose oxidase on a carbon nanotubes electrode. Biosens Bioelectron 23:528–535
Wang WJ, Wang F, Yao YL, Hu SS, Shiu KK (2010) Amperometric bienzyme glucose biosensor based on carbon nanotube modified electrode with electropolymerized poly(toluidine blue O) film. Electrochim Acta 55:7055–7060
Jeykumari DRS, Narayanan SS (2009) Fabrication of an amperometric bienzyme biosensing system with neutral red functionalized carbon nanotubes. Analyst 134:1618–1622
Jeykumari DRS, Narayanan SS (2009) Functionalized carbon nanotube-bienzyme biocomposite for amperometric sensing. Carbon 47:957–966
Chen H, Xi FN, Gao X, Chen ZC, Lin XF (2010) Bienzyme bionanomultilayer electrode for glucose biosensing based on functional carbon nanotubes and sugar–lectin biospecific interaction. Anal Biochem 403:36–42
Singh K, Singh BP, Chauhan R, Basu T (2012) Fabrication of amperometric bienzymatic glucose biosensor based on MWCNT tube and polypyrrole multilayered nanocomposite. J Appl Polym Sci 125:E235–E246
Lin JH, He CY, Zhao Y, Zhang SS (2009) One-step synthesis of silver nanoparticles/carbon nanotubes/chitosan film and its application in glucose biosensor. Sensors Actuators B Chem 137:768–773
He CX, Liu JH, Zhang QL, Wu C (2012) A novel stable amperometric glucose biosensor based on the adsorption of glucose oxidase on poly(methyl methacrylate)-bovine serum albumin core–shell nanoparticles. Sensors Actuators B Chem 166–167:802–808
de Oliveira RF, de Moraes ML, Oliveira ON Jr, Ferreira M (2011) Exploiting cascade reactions in bienzyme layer-by-layer films. J Phys Chem C 115:19136–19140
Xu SX, Zhang XF, Wan T, Zhang CX (2011) A third-generation hydrogen peroxide biosensor based on horseradish peroxidase cross-linked to multi-wall carbon nanotubes. Microchim Acta 172:199–205
Kamin RA, Wilson GS (1980) Catalysis of electrode processes by multiply-charged metal complexes electrostatically bound to polyelectrolyte coatings on graphite electrodes, and the use of polymer-coated rotating disk electrodes in diagnosing kinetic and conduction mechanisms. Anal Chem 52:1192–1198
Zhang YQ, Fan YJ, Cheng L, Fan LL, Wang ZY, Zhong JP, Wu LN, Shen XC, Shi ZJ (2013) A novel glucose biosensor based on the immobilization of glucose oxidase on layer-by-layer assembly film of copper phthalocyanine functionalized graphene. Electrochim Acta 104:178–184
Zhang SY, Tang S, Lei JP, Dong HF, Ju HX (2011) Functionalization of graphene nanoribbons with porphyrin for electrocatalysis and amperometric biosensing. J Electroanal Chem 656:285–288
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21305009 and 21005010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, S., Qi, H., Zhou, S. et al. Mediatorless amperometric bienzyme glucose biosensor based on horseradish peroxidase and glucose oxidase cross-linked to multiwall carbon nanotubes. Microchim Acta 181, 535–541 (2014). https://doi.org/10.1007/s00604-014-1175-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1175-z