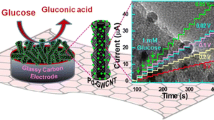
Abstract
We have electrodeposited a composite film consisting of graphene oxide, chitosan and glucose oxidase directly on a glassy carbon electrode (GCE) through electrochemical reduction of a solution of the 3 components under controlled direct electrical potential. The procedure takes only several minutes, and the thickness of the resulting film is uniform and controllable. The GOx has uncompromised bioactivity and exhibits reversible 2-proton and 2-electron transfer in presence of glucose. It therefore can be used amperometric sensing of glucose. The biosensor has a fast response (<3 s), a detection limit of 0.4 μM (which is 50-fold lower compared to the biosensor prepared by drop-casting solutions of the same materials onto an GCE), and a linear response in the 0.4 μM to 2 mM concentration range (which again is much better than that of the biosensor prepared by the drop-casting method). Other features include high reproducibility, long-time storage stability, and satisfactory selectivity. We presume that the direct single-step electrodeposition of this nanocomposite offers a promising approach towards novel types of highly sensitive and stable electrochemical biosensors.

We describe a fast and easy way for the fabrication of graphene-chitosan-GOx film by one-step electrodeposition under controlled potential. The direct electron transfer reaction of GOx immobilized on graphene-chitosan hybrids is observed, and therefore can be used for amperometric sensing of glucose. The biosensor shows a fast response (<3 s), a detection limit of 0.4 μM, and a linear response in the 0.4 μM to 2 mM concentration range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus, which is one of the most prevalent and costly diseases in the world, has become a worldwide public health problem [1]. Nearly 40 million people are suffering from diabetes in China [2]. The metabolic disorder, named as hyperglycemia and hypoglycemia, may result in numerous complications such as heart disease, kidney failure and blindness [3]. Therefore, monitoring of blood glucose levels is very important to the treatment and control of diabetes mellitus. Although many methods have been developed for the determination of glucose, amperometric biosensors based on glucose oxidase (GOx) have become the most convenient tools for their low detection limit, high selectivity and high sensitivity [3–5]. In recent years, the direct electron transfer between GOx and electrode surface has received widespread attention [4, 6–9]. Direct electron transfer between redox enzyme and the electrode surface can be used to investigate the enzyme-catalyzed reactions in biological systems, laying the electrochemical basis for the study of the structure of enzyme, kinetics and thermodynamics of redox transformations of enzyme molecule, and metabolic processes involving redox transformations [4, 10]. However, the direct electron transfer between the redox center of enzyme and the electrode surface is often shielded by the insulating outer protein shell, making the realizing of the direct electron transfer between enzyme and electrode surface extremely difficult. On the other hand, direct adsorption of enzyme onto the electrode surface may frequently result in enzyme denaturation and inactivation [11]. Therefore, it is important to discover appropriate materials capable of retaining the bioactivity of enzyme and connecting the active center of redox enzyme with the electrode surface.
Graphene, a flat monolayer of carbon atoms in a closely packed honeycomb two-dimensional lattice, has attracted much attention since its first discovery in 2004 [12–15]. Graphene possesses many novel thermal, mechanical and electrical properties, such as good biocompatibility, high surface area, excellent electrical conductivity, electron mobility at room temperature and flexibility. The excellent conductivity and small band gap are favorable for transferring electrons among the biomolecules, the high surface area is helpful to increase the loading of the enzyme molecules on the surface [4, 14]; moreover, graphene-based chemical sensors can also have a much higher sensitivity because of the low electronic noise from thermal effect [4, 16, 17]. Therefore, graphene can be an excellent electrode material to construct enzyme based biosensor, and several GR-based glucose biosensors have been reported. Kang et al. dispersed graphene in chitosan to prepare graphene–chitosan solution, and coated GOx on the graphene–chitosan film. They studied the electrochemical behavior of GOx at graphene–chitosan modified electrode and demonstrated the direct electron transfer reaction of GOx at the modified electrode. The results indicated that the graphene could provide a favorable microenvironment for the enzyme and promote the direct electron transfer at the electrode surface [4]. Shan et al. developed a glucose biosensor based on immobilization of GOx in thin film of chitosan containing graphene and gold nanoparticles (AuNPs) at a gold electrode, the graphene/AuNPs/GOx/chitosan composites film also realized direct electron transfer between GOx and the electrode surface and exhibited prominent amperometric response to glucose [6]. However, in these systems, there were three main issues. First, the graphene was obtained from chemical reduction of graphene oxide (GO) sheets, and the graphene solution was directly drop − casting onto the electrodes. In that way, the thickness of the resulting films was hardly uniform and controllable, the sensor fabrication was not so reproducible; Second, toxic chemicals are involved during the chemical reduction of graphene oxide (GO) sheets to graphene; Third, graphene are not readily soluble in usual solvent media, this make the dispersion and stabilization of graphene in solvent media very poor. Though polymer solvent can improve the solubility of graphene, the graphene sheets tend to be agglomeration in the solvent.
To resolve these problems, the obtention of graphene-polymer with electrochemical reduction of GO-polymer looks promising. Compared with chemical reduction method, the electrochemical reduction method is simple and is suitable for selective deposition of films with controllable thickness, which exhibited good stability, reproducibility and short response time [18–20]; And the electrochemical reduction of GO sheets in aqueous solutions results in their irreversible agglomerate [21], therefore, when the GO sheets in direct contact with an electrode accept electrons to suffer from electrochemical reduction, the resulted graphene sheets will also be insoluble, and thus directly attach to the electrode surface [22]. Moreover, the obtained graphene can be functionalized through the integration of graphene with conductive polymers and yield novel types of electrically conductive nanocomposites [14, 23–27]. Recently, our group has reported that graphene nanosheets can be electrodeposited onto glassy carbon electrode through cyclic voltammetric reduction of GO, and the electrochemical reduction potential of GO was about −1.0 V. We also discovered that the electrodeposition could occur on any conducting surfaces, and moreover, the graphene coating was very stable as a result of its poor insolubility in common solvents [22]. Meanwhile, from the previous researches of our group [22, 28], we found that graphene nanosheets which electrodeposited onto glassy carbon electrode through cyclic voltammetric reduction of GO exhibited layered nanostructures. Thus the graphene agglomeration could be prevented, and more enzymes could be effectively entrapped into both graphene and polymer during the electrodeposition.
Chitosan, a linear hydrophilic polymer obtained by deacetylation of nature chitin, is commonly used to disperse nanomaterials and immobilize enzymes for constructing biosensors due to its excellent capability for film-forming, nontoxicity, biocompatibility, mechanical strength, and good water permeability. Hu et al. described the electrochemical characteristics of graphene-chitosan [29]. Xu et al. found that graphene-chitosan composite film would be a promising platform for protein immobilization and biosensor preparation [30]. Chitosan owns many primary amino groups, and has a pKa value of about 6.3. At pH below the pKa, free amino groups of chitosan are protonated, and chitosan is soluble. When the solution pH is raised near or above the pKa, many of the amino groups are deprotonated, and chitosan becomes insoluble. Usually, electrochemical deposition of chitosan was performed via water reduction [31–34]. At a reducing potential, H+ in the solution was consumed at the cathode. Using the locally generated H+ gradient, acidic side chains of chitosan were titrated. So the solubility of chitosan changed, eventually led to the controlled deposition of chitosan film, other substances such as graphene and even enzymes could be effectively entrapped into chitosan film during the electrodeposition [35]. Due to its desirable properties, chitosan can be an excellent polymer for the obtention of graphene-polymer with electrochemical reduction of GO-polymer, and can provide a good biocompatible microenvironment for enzyme to construct biosensors [4, 29, 30]. However, to our best knowledge, there are no works reported on the preparation of graphene-chitosan nanocomposite from GO-chitosan by one-step electrodeposition, and no reports on the construction of a graphene-chitosan-GOx biosensor by one-step electrodeposition, which can also realize direct electrochemistry of GOx and maintain the bioactivity of GOx well.
Here, we first obtained graphene-chitosan nanocomposite from GO-chitosan dispersion solution by direct electrodeposition and first described a fast and easy way for the fabrication of graphene-chitosan-GOx film by one-step electrodeposition under controlled potential. The direct electron transfer of GOx immobilized on graphene-chitosan hybrids was achieved, GOx maintained its bioactivity well and exhibited reversible two-proton and two-electron transfer reaction. The electrochemical property of the electrodeposited graphene-chitosan-GOx film was studied in detail. By combining the benefits of graphene and chitosan, the method presented a number of attractive features for glucose determination, such as simplicity, low detection limit, high sensitivity, fast response time and wide linear range.
Experimental
Reagents
Graphene oxide was synthesized from natural fl ake graphite by the Hummers method [36]. Chitosan from crab shells (85 % deacetylated) was purchased from Sigma (China-mainland, www.sigmaaldrich.com). Glucose oxidase (EC 1.1.3.4; type II from Aspergillusniger, activity ≈ 100 U mg−1) and D-glucose were purchased from Amresco (USA, www.amresco-inc.com). All other chemicals were of analytic grade and were used without further purification. Double − distilled water was used throughout the experiments. 0.05 M, pH 7.0 phosphate buffer solution was used as the electrolyte. Chitosan solution was prepared by dissolving chitosan solid in 0.10 M acetic acid (HAc). The stock GOx solution was prepared in the phosphate buffer solution and stored at 4 °C.
Apparatus and instrumentations
All the electrochemical measurements were performed on a CHI 660A electrochemical workstation (Shanghai Chenhua Instrument Company, China). A conventional three electrode system was used, which consisted of a modified or bare glassy carbon electrode (3 mm in diameter) as working electrode, a platinum foil as counter electrode, and an Ag/AgCl (3 M KCl) electrode as reference electrode. All potentials were measured against Ag/AgCl. Amperometric studies were carried out under stirred conditions. All experiments were done at room temperature (∼25 °C).
The micrograph of graphene-chitosan film was investigated by transmission electron microscopy (TEM, Philips EM400ST microscopy).
One-step electrodeposition of graphene-chitosan-glucose oxidase film onto glassy carbon electrode
Prior to electrodeposition, the GCE was polished with 0.3 μm and 0.05 μm α-Al2O3 powder until a mirror-shiny surface was obtained, then ultrasonicated in ethanol and twice-distilled water for 10 min, respectively. The polished GCE was then cleaned by cyclic voltammetry between −0.6 V and +0.6 V at 50 mV s−1 in 0.05 M phosphate buffer solution (pH 7.0) until stable cyclic voltammograms were obtained. The prepared GCE was dried with nitrogen gas and used for modification immediately.
5 mg of GO was dispersed in 5 mL of 0.2 % (w/v) chitosan solution with ultrasonication to form 1 mg mL−1 GO-chitosan solution. Then the GO-chitosan solution prepared above and 5 mg mL−1 GOx were mixed together to form a GO-chitosan-GOx solution. The prepared GCE was immersed into the GO-chitosan-GOx solution while stirring and a fixed potential of −1.0 V was applied for 400 s. After the electrodeposition, the graphene-chitosan-GOx/GCE was obtained. The graphene-chitosan-GOx/GCE was stored at 4 °C in a refrigerator under dry conditions when not in use.
For comparison, chitosan/GCE and graphene-chitosan/GCE were obtained by electrodepositing chitosan or GO-chitosan onto the GCE under −1.0 V for 400 s.
Results and discussion
Characterization of graphene-chitosan/GCE
It is well known that chemical reduction of GO sheets in aqueous solutions results in their irreversible agglomerate [21]. Therefore, it is reasonable to suppose that when the GO sheets in direct contact with an electrode accept electrons to suffer from electrochemical reduction, the resulted graphene sheets will also be insoluble, and thus directly attach to the electrode surface [22]. Figure 1(a) and (b) are the SEM and TEM images of the graphene-chitosan composite electrodeposited on the GCE surface. The SEM image of the graphene-chitosan film reveals a wrinkled texture that was associated with the presence of flexible and ultrathin graphene sheets. The TEM image confirms the typical crumpled and wrinkled graphene sheet structure on the rough surface of the chitosan film. The Raman spectra of graphene-chitosan composite (Fig. 1(c)) shows a strong diamondoid (D) band at 1,290 cm−1 and a weak graphitic (G) band at 1,600 cm−1, which are attributed to two vibration modes of graphene. The D band associates with vibrations of carbon atoms with dangling bonds in plane terminations of disordered graphite and the G band corresponds to an E2g mode of graphite and is related to the vibration of sp2-bonded carbon atoms in a 2D hexagonal lattice, such as in a graphite layer [37]. The Raman result is also similar to the previous reports [28]. All these results indicate that graphene-chitosan nanocomposite has been prepared on electrode from GO-chitosan dispersion by direct one-step electrodeposition.
Figure 2 shows the cyclic voltammograms (CVs) obtained by four different modified GCEs in 5 mM [Fe(CN)6]3−/4− containing 0.1 M KCl at 50 mV s−1. As shown, a pair of well-defined redox peaks is observed for the bare GCE (Fig. 2, line a), which is due to the reversible one-electron redox behavior of ferricyanide ion. After electrodepositing chitosan onto the bare GCE, a largely blocked interfacial charge transfer between the bare GCE and ferricyanide ion is observed (Fig. 2, line b), due to chitosan film can act as a barrier. Compared with chitosan or even the bare GCE surface, increased redox peak currents are observed on graphene-chitosan/GCE (Fig. 2, line c), indicating that graphene facilitates the conductivity and the electron transfer process. When electrodepositing graphene-chitosan-GOx onto GCE (Fig. 2, line d), redox peak currents decrease and redox peak potentials separation increase compared with those of the graphene-chitosan/GCE. This result indicates that the GOx is steadily adsorbed into the graphene-chitosan film, causing some of inhibition of the electron transfer of the redox couple.
Direct electrochemistry of glucose oxidase immobilized on graphene-chitosan film
The flavin group in the enzyme GOx (FAD) undergoes a redox reaction where two protons and two electrons are exchanged [4, 38]. The electrochemistry response of GOx immobilized on the solid surface is due to the redox reaction of FAD [4, 39]. Direct electron transfer between GOx and the substrate can be achieved under appropriate conditions and be used to prepare biosensing devices [4, 6]. Figure 3 presents the CVs of four different GCEs in N2-saturated phosphate buffer solution (pH 7.0). No peaks are observed for bare GCE, chitosan/GCE and graphene-chitosan/GCE (Fig. 3, line a–c). The background current of graphene-chitosan/GCE is much higher than that of the chitosan/GCE, which is attributed to the large surface area of the graphene-chitosan film. Well-defined and quasi-reversible redox peaks are observed at graphene-chitosan-GOx/GCE (Fig. 3, line d), which indicate the favorable direct electron transfer between the electrode and the redox centers of GOx. The redox peaks are attributed to the electrochemical redox reaction of FAD, the cathodic peak (Epc) at −0.517 V is attributed to the conversion of FAD to FADH2 and the anodic peak (Epa) at −0.465 V is ascribed to the conversion of FADH2 to FAD. Furthermore, the formal potential (E0′) obtained by averaging potential values of the Epc and Epa is −0.491 V. This E0′ value is close to the standard electrode potential of FAD/FADH2 at pH 7.0 (25.8 °C) [7] and similar to the previously reported results [4, 6–9]. All these results suggest that GOx molecules retain their bioactivity on the graphene-chitosan film.
As is well known, the direct electron transfer of GOx is a two electron along with two-proton reaction that undergoes a redox reaction as follows [4]:
Therefore, The influence of buffers pH on the electrochemical characteristic of graphene-chitosan-GOx/GCE is also studied. Both reduction and oxidation peaks shift negatively as pH increases. The formal potential (E0′) has a linear relationship with pH from 5.0 to 10.0 (see supplemental Figure S1). The slope of E0′ versus pH is −61.9 mV pH−1, which is close to the theoretical value of −58.6 mV pH−1 according to the reaction Eq. (1) [39] for a reversible, indicating two protons and two electrons attending in the electron transfer process.
The mechanism of glucose determination
We describe here, amperometry technique was used to measure glucose. The mechanism is shown in Scheme 1.
The biocatalytical process for the oxidation of glucose in the presence of GOx can be summarized as following two processes:
First, the flavin group (FAD) in the enzyme is reduced of by reaction with glucose to the reduced form of the enzyme GOx(FADH2)
Then, the flavin is reoxidated by molecular oxygen to regenerate the oxidized form of the enzyme GOx(FAD)
The quantification of glucose can be achieved via electrochemical detection of the enzymatically liberated H2O2:
Optimization of the graphene-chitosan-glucose oxidase/GCE electrodeposition conditions
All the GO-chitosan, chitosan and GOx contents in the deposition bath have significant effect on the performance of the biosensor. Supplemental Figure S2 (A-C) depicts the effects of GO-chitosan, chitosan and GOx concentrations on the current response of graphene-chitosan-GOx/GCE to 0.2 mM glucose, respectively. As can be seen, the peak currents rise sharply with the increased concentrations of GO-chitosan, chitosan and GOx, and the maximum responses are approached at the concentration of 1 mg mL−1, 0.2 % (w/v) and 5 mg mL−1, respectively. Thus, 1 mg mL−1 GO-chitosan, 0.2 % (w/v) chitosan and 5 mg mL−1 GOx were chosen as the optimal concentrations for the biosensor.
The effect of the electrodeposition time is also illustrated in supplemental figure S2 (D). The current response increases evidently as the electrodeposition time increases from 100 to 400 s, and no significant improvement of the current response is observed from 400 to 900 s. These would be caused by two reasons: first, the amount of GOx entrapped in the film increases as the film thickness increased at longer deposition time, and second, too thick film would affect the activity of GOx; On the other hand,, longer deposition time resulted in longer response time. Therefore, the optimum electrodeposition time was chosen as 400 s in our experiments.
Optimization of glucose determination conditions
To improve the performance of the biosensor, the effect of the determination conditions such as the applied potential and the pH value on the current response of the graphene-chitosan-GOx/GCE to 0.2 mM glucose has been investigated in detail.
The effect of the applied potential is shown in supplemental Figure S3 (A). With the increase of the applied potential from 0.3 V to 0.5 V, the response current increases accordingly. This means that the response of the enzyme electrode is resulted from the electrochemical oxidation of hydrogen peroxide. When the applied potential is higher than 0.5 V, the response current begins to level off and the maximum response current is obtained at 0.5 V. So 0.5 V was selected as the optimum applied potential.
The effect of pH value is studied in supplemental Figure S3 (B). The response current increases with pH from 5.0 to 7.0 and decreases from 7.0 to 9.0 in 0.05 M phosphate buffer solution, the maximum current response is obtained at pH 7.0. Thus, pH 7.0 was selected in the subsequent experiments.
Amperometric response of the glucose biosensor
The experiment was performed under the optimized conditions in a stirred system. Figure 4(a) shows the current–time plots for the graphene-chitosan-GOx/GCE with successive injection of 0.2 mM glucose into a stirring phosphate buffer solution (pH 7.0). As can be seen, The response time is very fast and the steady-state current reaches another steady-state value less than 3 s. The detection limit is 4.0 × 10−7 M (S/N = 3), which is 50-fold lower compared to the biosensor prepared by drop − casting method [4]. The calibration curve of the graphene-chitosan-GOx/GCE has been shown in Fig. 4(b). The graphene-chitosan-GOx/GCE displays an expanded linear response range of 4.0 × 10−7 M to 2 × 10−3 M with a correlation coefficient of 0.998, which is also much wider than the biosensor prepared by drop − casting method [4].
a The steady-state current–time response of graphene-chitosan-GOx/GCE with successive injection of 0.2 mM glucose into stirring 0.05 M phosphate buffer solution (pH 7.0). The applied potential was 0.5 V. b The calibration curve of graphene-chitosan-GOx/GCE to different concentration of glucose in 0.05 M phosphate buffer solution (pH 7.0)
The performances of the electrode and other electrodes for the detection of glucose are compared and listed in Table 1. Compared with the other glucose biosensors based on GOx shown in Table 1, the graphene-chitosan-GOx/GCE has a much lower determination limit and a wider linear range, which may attribute to the exciting electronic properties of graphene-chitosan hybrids and the excellent bioactivity of GOx immobilized on graphene-chitosan hybrids.
Reproducibility and stability of the biosensor
The reproducibility and storage stability of the biosensor have also been studied. The relative standard deviation (RSD) of the current response of graphene-chitosan-GOx/GCE to 0.2 mM glucose at 0.5 V was 3.8 % for 10 successive measurements. For six electrodes modified identically, the R.S.D. was 4.4 %. The stability of the graphene-chitosan-GOx/GCE under storage conditions (0.05 M phosphate buffer solution, pH 7.0, 4 °C) was investigated. The current response for 0.2 mM glucose was measured everyday up to 30 days. Only 5 % of the current response was lost after 1 week. After 30 days, the current response still remained above 80 % of its initial response. The relatively good storage stability implies that the graphene-chitosan-GOx film is very stable and it is strongly bound to the GCE surface. But, when the pH value of solution was lower than 6, the stability of the biosensor became very poor. This disadvantage was induced by the detachment of chitosan film under acid condition.
Effect of electroactive interferents and real sample study
Selectivity is important in practical use of the biosensors. Oxidizable compounds, such as ascorbic acid, uric acid, citric acid and acetaminophen, are usually co-existed with glucose in real samples. The current response obtained in the mixture of glucose and the interfering species was compared with the results obtained in the pure glucose solution. As shown in Table 2, all these interferences have little influence on the glucose determination. This modified electrode shows satisfactory anti-interference ability and can realize the specific detection of glucose.
Human serum samples were assayed to demonstrate the practical use of the proposed biosensor. The results of the glucose determination and the recovery of the serum samples are summarized in Table 3. As shown, the results obtained by the biosensor are in good agreement with those measured by the biochemical analyzer in the hospital and the recovery is satisfied.
Conclusion
Here, we obtain graphene-chitosan nanocomposite from GO-chitosan dispersion solution by direct electrodeposition and describe a fast and easy way for the fabrication of graphene-chitosan-GOx film by one-step electrodeposition under controlled potential. The direct electron transfer reaction of GOx immobilized on graphene-chitosan hybrids is observed, and GOx maintained its bioactivity well. The results indicate that the graphene-chitosan film provides a favorable microenvironment for the enzyme absorption and promotes the direct electron transfer between redox enzyme and the surface of electrode. The electrochemical property of the electrodeposited graphene-chitosan-GOx film is studied in detail. By combining the benefits of graphene and chitosan, the prepared electrode presented a number of attractive features for glucose determination, such as simplicity, low detection limit, wide linear range, long-time stability, high reproducibility and satisfactory anti-interference ability. The direct one-step electrodeposition graphene-polymer nanocomposite from GO-polymer nanocomposite method developed here may offer a new approach for developing novel types of highly sensitive and stable electrochemical biosensors.
References
Pei J, Tian F, Thundat T (2004) Glucose biosensor based on the microcantilever. Anal Chem 76:292
Center for Disease Control (Ministry of Health, China http://www.eds.org.cn/), 2005. Guideline for Management of Chinese Diabetes
Ahmad M, Pan CF, Luo ZX, Zhu J (2010) A single ZnO nanofiber-based highly sensitive amperometric glucose biosensor. J Phys Chem C 114:9308
Kang XH, Wang J, Wu H, Aksay IA, Liu J, Lin YH (2009) Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens Bioelectron 25:901
Liu XY, Zeng XD, Mai NN, Liu Y, Kong B, Li YH, Wei WZ, Luo SL (2010) Amperometric glucose biosensor with remarkable acid stability based on glucose oxidase entrapped in colloidal gold-modified carbon ionic liquid electrode. Biosens Bioelectron 25:2675
Shan CS, Yang HF, Song JF, Han DX, Ivaska A, Niu L (2009) Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal Chem 81:2378
Dai ZH, Ni J, Huang XH, Lu GF, Bao JC (2007) Direct electrochemistry of glucose oxidase immobilized on a hexagonal mesoporous silica-MCM-41 matrix. Bioelectrochemistry 70:250
Huang YX, Zhang WJ, Xiao H, Li GX (2005) An electrochemical investigation of glucose oxidase at a CdS nanoparticles modified electrode. Biosens Bioelectron 21:817
Liu Y, Wang MK, Zhao F, Xu ZA, Dong SJ (2005) The direct electron transfer of glucose oxidase and glucose biosensor based on carbon nanotubes/chitosan matrix. Biosens Bioelectron 21:984
Pulcu GS, Elmore BL, Arciero DM, Hooper AB, Elliott SJ (2007) Direct electrochemistry of tetraheme cytochrome c554 from nitrosomonas europaea: redox cooperativity and gating. J Am Chem Soc 129:1838
Zeng XD, Li XF, Liu XY, Liu Y, Luo SL, Kong B, Yang SL, Wei WZ (2009) A third-generation hydrogen peroxide biosensor based on horseradish peroxidase immobilized on DNA functionalized carbon nanotubes. Biosens Bioelectron 25:896
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 442:282
Ramanathan T, Abdala AA, Stankovich S, Dikin DA, Herrera-Alonso M, Piner RD (2008) Functionalized graphene sheets for polymer nanocomposites. Nature Nanotech 3:327
Pérez-López B, Merkoçi A (2012) Carbon nanotubes and graphene in analytical sciences. Microchim Acta 179:1
Gan T, Hu SS (2011) Electrochemical sensors based on graphene materials. Microchim Acta 175:1
Du Y, Luo XL, Xu JJ, Chen HY (2007) A simple method to fabricate a chitosan-gold nanoparticles film and its application in glucose biosensor. Bioelectrochemistry 70:342
Chen XH, Matsumoto N, Hu YB, George SW (2002) Electrochemically mediated electrodeposition/electropolymerization to yield a glucose microbiosensor with improved characteristics. Anal Chem 74:368
Salimi A, Hallaj R, Soltanian S, Mamkhezri H (2007) Nanomolar detection of hydrogen peroxide on glassy carbon electrode modified with electrodeposited cobalt oxide nanoparticles. Anal Chim Acta 594:24
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Chen LY, Tang YH, Wang K, Liu CB, Luo SL (2011) Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem Communica 13:133
Li D, Kaner RB (2008) Graphene-based materials. Science 320:1170
Niyogi S, Bekyarova E, Itkis ME, Mcwilliams JL, Hamon MA, Haddon RC (2006) Solution properties of graphite and graphene. J Am Chem Soc 128:7720
Schniepp HC, Li JL, Mcallister MJ, Sai H, Herrera-Alonso M, Adamson DH, Prud’homme RK, Car R, Saville DA, Aksay IA (2006) Functionalized single graphene sheets derived from splitting graphite oxide. J Phys Chem B 110:8535
Xu Y, Bai H, Lu GW, Li C, Shi GQ (2008) Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J Am Chem Soc 130:5856
Kang XH, Mai ZB, Zou XY, Cai PX, Mo JY (2007) Electrochemical biosensor based on multi-walled carbon nanotubes and Au nanoparticles synthesized in chitosan. J Nanosci Nanotechnol 7:1618
Liu CB, Wang K, Luo SL, Tang YH, Chen LY (2011) Direct electrodeposition of graphene enabling the one − step synthesis of graphene − metal nanocomposite films. Small 7:1203
Hu HT, Wang XB, Wang JC, Liu FM, Zhang M, Xu CH (2011) Microwave-assisted covalent modification of graphene nanosheets with chitosan and its electrorheological characteristics. Appl Surf Sci 257:2637
Xu HF, Dai H, Chen GN (2010) Direct electrochemistry and electrocatalysis of hemoglobin protein entrapped in graphene and chitosan composite film. Talanta 81:334
Wu LQ, Gadre AP, Yi H, Kastantin MJ, Rubloff GW, Bentley WE, Payne GF, Ghodssi R (2002) Voltage-dependent assembly of the polysaccharide chitosan onto an electrode surface. Langmuir 18:8620
Wu LQ, Yi H, Li S, Rubloff GW, Bentley WE, Ghodssi R, Payne GF (2003) Spatially selective deposition of a reactive polysaccharide layer onto a patterned template. Langmuir 19:519
Wu LQ, Lee K, Wang X, English DS, Losert W, Payne GF (2005) Chitosan-mediated and spatially selective electrodeposition of nanoscale particles. Langmuir 21:3641
Yi H, Wu LQ, Ghodssi R, Rubloff GW, Payne GF, Bentley WE (2004) A robust technique for assembly of nucleic acid hybridization chips based on electrochemically templated chitosan. Anal Chem 76:365
Lu XB, Zhang Q, Zhang L, Li JH (2006) Direct electron transfer of horseradish peroxidase and its biosensor based on chitosan and room temperature ionic liquid. Electrochem Communica 8:874
William S, Hummers JR, Richard EO (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Ferrari AC (2007) Raman spectroscopy of graphene and graphite: disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun 143:47
Ianniello RM, Lindsay TJ, Yacynych AM (1982) Differential pulse voltammetric study of direct electron transfer in glucose oxidase chemically modified graphite electrodes. Anal Chem 54:1098
Liu Q, Lu X, Li J, Yao X, Li JH (2007) Direct electrochemistry of glucose oxidase and electrochemical biosensing of glucose on quantum dots/carbon nanotubes electrodes. Biosens Bioelectron 22:3203
Wu J, Zou YH, Gao N, Jiang JH, Shen GL, Yu RQ (2005) Electrochemical performances of C/Fe nanocomposite and its use for mediator-free glucose biosensor preparation. Talanta 68:12
Wu BY, Hou SH, Yin F, Li J, Zhao ZX, Huang JD, Chen Q (2007) Amperometric glucose biosensor based on layer-by-layer assembly of multilayer films composed of chitosan, gold nanoparticles and glucose oxidase modified Pt electrode. Biosens Bioelectron 22:838
Acknowledgments
This work was supported by the National Natural Science Foundation of China (50878079, 51078129, 21047004) and the National Basic Research Program of China (2009CB421601).
Author information
Authors and Affiliations
Corresponding author
Additional information
Shanli Yang and Zhenzhen Lu contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 87 kb)
Rights and permissions
About this article
Cite this article
Yang, S., Lu, Z., Luo, S. et al. Direct electrodeposition of a biocomposite consisting of reduced graphene oxide, chitosan and glucose oxidase on a glassy carbon electrode for direct sensing of glucose. Microchim Acta 180, 127–135 (2013). https://doi.org/10.1007/s00604-012-0911-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0911-5