Abstract
We report on a biosensor for organophosphate pesticides (OPs) by exploiting their inhibitory effect on the activity of acetylcholinesterase (AChE). A boron-doped diamond (BDD) electrode was modified with a nanocomposite prepared from carbon spheres (CSs; with an average diameter of 500 nm) that were synthesized from resorcinol and formaldehyde, and then were coated with gold nanoparticles (AuNPs) by chemically growing them of the CSs. Compared to a bare BDD electrode, the electron transfer resistance is lower on this new electrode. Compared to an electrode without Au-NPs, the peak potential is negatively shifted by 42 mV, and the peak current is increased by 55 %. This is ascribed to the larger surface in the AuNP-CS nanocomposite which improves the adsorption of AChE, enhances its activity, and facilitates electrocatalysis. Under optimum conditions, the inhibitory effect of chlorpyrifos is linearly related to the negative log of its concentration in the 10−11 to 10−7 M range, with a detection limit of 1.3 × 10−13 M. For methyl parathion, the inhibition effect is linear in the 10−12 to 10−6 M range, and the detection limit is 4.9 × 10−13 M. The biosensor exhibits good precision and acceptable operational and temporal stability.

A novel acetylcholinesterase-based biosensor based on a boron-doped diamond electrode modified with gold nanoparticles and carbon spheres was firstly prepared to detect organophosphate pesticides. This biosensor exhibited higher sensitivity, lower detection limit, good reproducibility and acceptable stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphate pesticides (OPs) are widely used to ensure high agricultural productivity because of their insecticidal activity [1]. However, irrational use of OPs may cause the problem of pesticide residue, which threatens environment and human health. In view of the major concerns regarding their toxicity, it is necessary to develop fast, sensitive, and economical methods for OPs monitoring and analysis. Although numerous methods including chromatography and mass spectroscopy are generally performed for OPs detection with good sensitivity and accuracy, they are very expensive, time-consuming and laboratory-oriented methods [2–4]. Acetylcholinesterase (AChE) based electrochemical biosensors are excellent candidates in low-cost and on-site detection due to their rapid response, ease of operation and small size [5, 6].
For fabrication of stable and sensitive biosensors, the strategy for immobilization of the enzyme onto the electrode surface is one of the most important factors. Because gold nanoparticles (AuNPs) [7, 8] can provide large surface area, high loading efficiency and fast electron transfer, a lot of AuNPs-based nanomaterials have been successfully used to immobilize AChE [9–11]. On the other hand, numerous functionalized carbon nanotubes-based nanomaterials have also been widely used to immobilize AChE and other molecules due to their advantages such as unique structural and electromechanical properties, and good biocompatibility [12–18]. Recently, some studies show that carbon spheres (CSs) possess better tunability of particle size and shape, homogeneity of particle size, and porous nanostructure for large loading of guest molecules, as compared with carbon nanotubes [19–22]. These advantages make CSs potentially beneficial for immobilizing enzymes.
On the other hand, as a novel electrode material, the boron-doped diamond (BDD) electrode has been demonstrated to be superior to the glassy carbon electrode and other electrodes in terms of high signal-to-noise ratio, long-term stability, high sensitivity, and good reproducibility [23–25].
We describe here a novel biosensor for OPs based on a BDD electrode modified with AuNPs-CSs nanocomposite. CSs with desired diameter and narrow particle size distribution are synthesized by the extension of the Stöber method of the polymerization of resorcinol and formaldehyde, which is a facile and versatile procedure for making polymer beads and is considered to be low cost and suitable supports for loading biological molecules [26]. Then, AuNPs are supported on CSs surface by chemically growing method to obtain AuNPs-CSs nanocomposite. Using BDD as working electrode, AChE biosensor is firstly constructed based on AuNPs-CSs for OPs detection.
Experimental
Materials and reagents
Acetylcholinesterase (Type C3389, 500 U·mg−1 from electric eel), acetylthiocholine chloride and bovine serum albumin were purchased from Sigma–Aldrich (http://www.sigma-aldrich.com/) and used as received. Chlorpyrifos and methyl parathion (≥99 %) were obtained from Dr. Ehrenstorfer (Augsburg, Germany, http://www.ehrenstorfer.com/). 0.1 M phosphate buffer solution was prepared by mixing stock solutions of NaH2PO4 and Na2HPO4 and adjusting the pH with 0.1 M HCl or 0.1 M NaOH. All other chemicals were of analytical-reagent grade and used without further purification. Double distilled water was used throughout the experiments.
Apparatus
All the electrochemical experiments were performed on a CHI 660D Electrochemical Workstation (Shanghai Chenhua Instrument Corporation, China). A three-electrode system comprised platinum wire as auxiliary electrode, Ag/AgCl as reference electrode and the modified or unmodified BDD as working electrode.
The prepared materials were characterized by transmission electron microscopy (TEM, JEM-2100UHR, Japan) operating at 200 kV. The structures of AuNPs were determined on an X-ray diffraction (XRD) using Cu Kα radiation source on Rigaku D/max-2500.
Preparation of the gold nanoparticles-carbon spheres nanocomposite
Carbon spheres (CSs) were synthesized by using resorcinol/formaldehyde as precursors according to the extension of the Stöber method reported recently by Liu et al. [26]. The prepared CSs (0.023 g) were added into 40 mL double distilled water solution under stirring vigorously for several hours to allow complete dispersion. Then, 0.6 mL of 1 % HAuCl4 was added into 40 mL of CSs suspension with vigorous stirring, followed by the addition of 0.2 mL of an aqueous solution of K2CO3 (0.2 M). Under vigorous stirring, 0.4 mL of a freshly prepared aqueous solution of NaBH4 (0.5 mg·mL−1) was then quickly added to the mixture 3–5 times until the solution turned wine-red in color. The AuNPs-coated CSs were obtained by the above-mentioned approaches. After being rinsed repeatedly with deionized water, the AuNPs-coated CSs dispersion were diluted to 20 mL with water, then HAuCl4 (1 wt.%, 0.3 mL) and NH2OH·HCl (0.04 M, 1.2 mL) were added under stirring for 15 min to increase and stabilize the amount of AuNPs grown on the CSs surface. The AuNPs-CSs nanocomposite was obtained by the above-mentioned approaches.
Fabrication of acetylcholinesterase biosensor
BDD was sequentially ultrasonicated in acetone, double distilled water, and dried at room temperature. The AChE/AuNPs-CSs/BDD biosensor was prepared as follows: Firstly, 5 mg AuNPs-CSs nanocomposite was ultrasonically dispersed in 10 mL double distilled water for 30 min to form a homogeneous ink. The BDD electrode was spread with 8 μL AuNPs-CSs nanocomposite solution, and dried at room temperature. Then 10.0 μL AChE solution (containing 1 % bovine serum albumin to maintain the stability of AChE) was dropped on AuNPs-CSs/BDD electrode and incubated at 25 °C for 30 min. After evaporation of water, the modified electrode was washed with phosphate buffer solution to remove the unbound AChE, and the obtained AChE/AuNPs-CSs/BDD biosensor was stored at 4 °C when not in use.
Measurement procedure
Using chlorpyrifos and methyl parathion as model compound, the performance of the prepared AChE/AuNPs-CSs/BDD biosensor for OPs detection were studied. Firstly, the prepared AChE/AuNPs-CSs/BDD was immersed in phosphate buffer solution (pH 7.3) containing different concentrations of standard chlorpyrifos and methyl parathion solution for 10 min and then transferred to an electrochemical cell of phosphate buffer solution (pH 7.3) containing 1.0 mM acetylthiocholine chloride as substrate to study the electrochemical response by differential pulse voltammetry. The peak current produced by thiocholine oxidation, hydrolysis product of acetylthiocholine chloride, is related with the activity of immobilized AChE, which can be used as an indicator for quantitative measurement of the inhibition action of OPs on the activity of immobilized AChE. The inhibition of chlorpyrifos and methyl parathion is calculated as follows: inhibition (%) = [(I0−I)/I0] × 100 %. where I0 is the peak current of thiocholine oxidation and I is that with chlorpyrifos and methyl parathion inhibition.
Results and discussion
Characterization of gold nanoparticles-carbon spheres nanocomposite
Figure 1a presented the TEM image of the CSs. It can be seen that the obtained CSs had smooth surfaces with an average diameter of 500 nm. Figure 1b showed the TEM image of AuNPs-CSs. It can be seen that little agglomeration of particles was observed on the CSs supports, indicating that the AuNPs were adhered to the CSs surface. The TEM image indicated that the spherical morphology was basically retained after the incorporation of AuNPs. Figure 1c showed the XRD patterns of AuNPs. The crystal structure of AuNPs was confirmed by the diffraction peaks at 38.4°, 44.4°, 64.8°, 77.6° and 81.8°. These peaks correspond to (111), (200), (220), (311) and (222) facets of the face-centered cubic structure of AuNPs crystal, respectively.
Characterization of the AChE/AuNPs-CSs/BDD biosensor
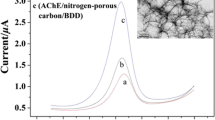
Figure 2 showed the Nyquist plots of different electrodes in the frequency range of 0.05 Hz to 10 KHz using 1 × 10−2 M [Fe(CN)6]3−/4− redox couple as the electrochemical probe. The electron transfer resistance of the AuNPs-CSs/BDD (Fig. 2c) was about 410 Ω, which was smaller than that of the bare BDD (about 591 Ω, Fig. 2a) and that of the CSs/BDD (about 485 Ω, Fig. 2b), suggesting that the presence of AuNPs-CSs nanocomposite on the electrode surface could improve the reactive site, reduce the interfacial resistance, and make the electron transfer easier. The remarkable increase of semicircle diameter on the AChE/AuNPs-CSs/BDD electrode (about 1,020 Ω, Fig. 2d) confirmed the successful immobilization of AChE.
Electrochemical behaviors on different electrodes
Differential pulse voltammetry response of 1.0 mM acetylthiocholine chloride in pH 7.3 phosphate buffer solution on different electrodes was studied, and the results are shown in Fig. 3. Obvious oxidation peaks were showed on different electrodes, which came from the oxidation of thiocholine, hydrolysis product of acetylthiocholine chloride, catalyzed by the immobilized AChE. The oxidation peak current of thiocholine was 1.542 μA at ~0.224 V on the AChE/BDD electrode (Fig. 3a), and 2.419 μA at ~0.182 V on the AChE/AuNPs-CSs/BDD electrode (Fig. 3b). In comparison with AChE/BDD electrode, on AChE/AuNPs-CSs/BDD electrode, the peak potential negatively shifted by 42 mV, and peak current increased by 55 %, which were ascribed to that the AuNPs-CSs nanocomposite could increase the surface area, promote AChE adsorption, enhance the activity of AChE, promote the electrocatalytic ability, and accelerate the electron transfer. When AChE/AuNPs-CSs/BDD was immersed in 1 nM chlorpyrifos solution for 10 min, the oxidation peak current of thiocholine decreased from 2.419 μA to 1.079 μA, and the inhibition was 55.39 % (Fig. 3c). This was ascribed that chlorpyrifos could irreversibly inhibit AChE activity, which reduced the yield of thiocholine.
The effect of inhibition time on AChE/AuNPs-CSs/BDD biosensor response
The effect of inhibition time on AChE/AuNPs-CSs/BDD biosensor response was investigated. The AChE/AuNPs-CSs/BDD biosensor was incubated in a constant concentration of 1 nM chlorpyrifos (Fig. 4a) and 1 nM methyl parathion (Fig. 4b) standard solution for different time. As shown in Fig. 4a and b, the inhibition showed obvious increase with the increase of inhibition time within 10 min. However, there was no obvious inhibition improvement when the inhibition time is longer than 10 min, indicating an equilibration state. So, the optimum inhibition time was chosen as 10 min.
Calibration curve for chlorpyrifos and methyl parathion detection
Based on the optimum conditions, the inhibition effect of OPs on the response of the biosensor was investigated. Figure 5 showed the relationship between inhibition of the AChE/AuNPs-CSs/BDD biosensor and different concentrations of chlorpyrifos (Fig. 5a) and methyl parathion (Fig. 5b). Obviously, the inhibition increased sharply at low pesticide concentration then changed slowly at high pesticide concentration with increasing chlorpyrifos and methyl parathion concentrations ranging from 10−13 to 10−5 M, which indicated that the bonding interaction between chlorpyrifos or methyl parathion and AChE tended to saturation. For chlorpyrifos, good linear relationship between inhibition% and –Log [chlorpyrifos] was obtained in the range of 10−11−10−7 M with the regression equation of Inhibition (%) = −9.522x +137.736 (%) (R2 = 0.993), and the detection limit was 1.29 × 10−13 M (calculated for 15 % inhibition rate). For methyl parathion, good linear relationship between inhibition % and –Log [methyl parathion] was obtained in the range of 10−12−10−6 M with the regression equation of Inhibition (%) = −8.6x +120.874 (%) (R2 = 0.994), and the detection limit was 4.9 × 10−13 M (calculated for 15 % inhibition rate). The performance of the fabricated biosensor was compared with those of other reported AChE biosensors. As shown in Table 1, the performance of AChE/AuNPs-CSs/BDD was superior to other reports [11, 13, 15, 27–32]. This result may be attributed to the advantages of AuNPs-CSs including high accessible surface area, the effective immobilization of AChE, good biocompatibility to retain the AChE activity, and satisfying conductivity.
Precision and stability of the AChE/AuNPs-CSs/BDD biosensor
The repeatability of the AChE/AuNPs-CSs/BDD biosensor was assessed through repetitively measuring chlorpyrifos levels for eight times in 1.0 mM acetylthiocholine chloride after being treated with 1 nM chlorpyrifos for 10 min. The results indicated that the method possessed good repeatability with relative standard deviation of 4.93 %. The reproducibility was estimated by fabricating six AChE/AuNPs-CSs/BDD biosensors, and the relative standard deviation was 7.27 %. The prepared AChE/AuNPs-CSs/BDD biosensor was stored at 4 °C when not in use. After a 30-day storage period, the biosensor retained 84.72 % of its initial current response, indicating the acceptable stability.
Analysis of real samples
The contents of chlorpyrifos and methyl parathion in cucumber juice samples were detected by AChE/AuNPs-CSs/BDD biosensor. A standard addition method was adopted to assess the reliability of the prepared biosensor. As shown in Table 2, for chlorpyrifos detection, the recoveries were found to be between 82.4 % and 91.2 %. For methyl parathion detection, the recoveries were found to be between 85.6 % and 93.4 %. These results indicated that this method could be used for assay of real samples.
Conclusion
A simple and novel AChE biosensor based on AuNPs-CSs nanocomposite was developed for OPs detection. The results showed that AuNPs-CSs not only increased satisfying conductivity and the accessible surface area of the electrodes, but effectively promoted immobilization of AChE and enhanced the enzymatic activity. The prepared AChE/AuNPs-CSs/BDD biosensor exhibited higher sensitivity, lower detection limit, good reproducibility and acceptable stability toward OPs detection, which may meet the requirements of rapid OPs detection for food safety and environment protection. The present results suggest that the design and preparation of nanocomposite is helpful for the fabrication of sensitive and stable biosensors. Ongoing and further studies will develop a variety of useful nanomaterials for the sensing platform.
References
Pan D, Ma SM, Bo XJ, Guo LP (2011) Electrochemical behavior of methyl parathion and its sensitive determination at a glassy carbon electrode modified with ordered mesoporous carbon. Microchim Acta 173:215
Arduini F, Amine A, Moscone D, Palleschi G (2010) Biosensors based on cholinesterase inhibition for insecticides, nerve agents and aflatoxin B1 detection (review). Microchim Acta 170:193
Huang B, Zhang W-D, Chen C-H, Yu Y-X (2010) Electrochemical determination of methyl parathion at a Pd/MWCNTs-modified electrode. Microchim Acta 171:57
Wei W, Zong XM, Wang X, Yin LH, Pu YP, Liu SQ (2012) A disposable amperometric immunosensor for chlorpyrifos-methyl based on immunogen/platinum doped silica sol–gel film modified screen-printed carbon electrode. Food Chem 135:888
Du D, Wang M, Cai J, Qin Y, Zhang A (2010) One-step synthesis of multiwalled carbon nanotubes–gold nanocomposites for fabricating amperometric acetylcholinesterase biosensor. Sensors Actuators B 143:524
Sun X, Du S, Wang X (2012) Amperometric immunosensor for carbofuran detection based on gold nanoparticles and PB-MWCNTs-CTS composite film. Eur Food Res Technol 235:469
Li J, Yang Z-J, Zhang Y-C, Yu S-H, Xu Q, Qu Q-S, Hu X-Y (2012) Tin disulfide nanoflakes decorated with gold nanoparticles for direct electrochemistry of glucose oxidase and glucose biosensing. Microchim Acta 179:265
Cao XD, Ye YK, Liu SQ (2011) Gold nanoparticle-based signal amplification for biosensing. Anal Biochem 417:1
Buiculescu R, Chaniotakis NA (2012) The stabilization of Au NP–AChE nanocomposites by biosilica encapsulation for the development of a thiocholine biosensor. Bioelectrochemistry 86:72
Norouzi P, Pirali-Hamedani M, Ganjali MR, Faridbod FA (2010) A novel acetyl cholinesterase biosensor based on chitosan–gold nanoparticles film for determination of monocrotophos using FFT continuous cyclic voltammetry. Int J Electrochem Sci 5:1434
Gong J, Wang L, Zhang L (2009) Electrochemical biosensing of methyl parathion pesticide based on acetylcholinesterase immobilized onto Au–polypyrrole interlaced network-like nanocomposite. Biosens Bioelectron 24:2285
Du D, Ye X, Cai J, Liu J, Zhang A (2010) Acetylcholinesterase biosensor design based on carbon nanotube-encapsulated polypyrrole and polyaniline copolymer for amperometric detection of organophosphates. Biosens Bioelectron 25:2503
Viswanathan S, Radecka H, Radecki J (2009) Electrochemical biosensor for pesticides based on acetylcholinesterase immobilized on polyaniline deposited on vertically assembled carbon nanotubes wrapped with ssDNA. Biosens Bioelectron 24:2772
Zamfir L-G, Rotariu L, Bala C (2011) A novel, sensitive, reusable and low potential acetylcholinesterase biosensor for chlorpyrifos based on 1-butyl-3-methylimidazolium tetrafluoroborate/multiwalled carbon nanotubes gel. Biosens Bioelectron 26:3692
Chauhan N, Pundir CS (2011) An amperometric biosensor based on acetylcholinesterase immobilized onto iron oxide nanoparticles/multiwalled carbon nanotubes modified gold electrode for measurement of organophosphorus insecticides. Anal Chim Acta 701:66
Ivanov AN, Younusov RR, Evtugyn GA, Arduini F, Moscone D, Palleschi G (2011) Acetylcholinesterase biosensor based on single-walled carbon nanotubes–cophthalocyanine for organophosphorus pesticide detection. Talanta 85:216
Yang Z-J, Huang X-C, Li J, Zhang Y-C, Yu S-H, Xu Q, Hu X-Y (2012) Carbon nanotubes-functionalized urchin-like In2S3 nanostructure for sensitive and selective electrochemical sensing of dopamine. Microchim Acta 177:381
Li J, Yang Z-J, Tang Y, Zhang Y-C, Hu X-Y (2013) Carbon nanotubes-nanoflake-like SnS2 nanocomposite for direct electrochemistry of glucose oxidase and glucose sensing. Biosens Bioelectron 41:698
Xu QN, Yan F, Lei JP, Leng C, Ju HX (2012) Disposable electrochemical immunosensor by using carbon sphere/gold nanoparticle composites as labels for signal amplification. Chem Eur J 18:4994
Ho JA, Lin YC, Wang LS, Hwang KC, Chou PT (2009) Carbon nanoparticle-enhanced immunoelectrochemical detection for protein tumor marker with CdS biotracers. Anal Chem 81:1340
Tang SC, Vongehr S, Meng XK (2010) Controllable incorporation of Ag and Ag-Au nanoparticles in carbon spheres for tunable optical and catalytic properties. J Mater Chem 20:5436
Du D, Zou ZX, Shin YS, Wang J, Wu H, Engelhard MH, Liu J, Aksay IA, Lin YH (2010) Sensitive immunosensor for cancer biomarker based on dual signal amplification strategy of graphene sheets and multienzyme functionalized carbon nanospheres. Anal Chem 82:2989
Wei M, Sun LG, Xie ZY, Zhi JF, Fujishima A, Einaga Y, Fu DG, Wang XM, Gu ZZ (2008) Selective determination of dopamine on boron-doped diamond electrode modified with gold nanoparticle/polyelectrolyte coated polystyrene colloid. Adv Funct Mater 18:1414
Ivandini TA, Rao TN, Fujishima A, Einaga Y (2006) Electrochemical oxidation of oxalic acid at highly boron-doped diamond electrodes. Anal Chem 78:3467
Spataru N, Sarada BV, Popa E, Tryk DA, Fujishima A (2001) Voltammetric determination of L-cysteine at conductive diamond electrodes. Anal Chem 73:514
Liu J, Qiao SZ, Liu H, Chen J, Orpe A, Zhao D, Liu QG (2011) Extension of the stöber method to the preparation of monodisperse resorcinol–formaldehyde resin polymer and carbon spheres. Angew Chem Int Ed 50:5947
Lin Y, Lu F, Wang J (2004) Disposable carbon nanotube modified screen-printed biosensor for amperometric detection of organophosphorus pesticides and nerve agents. Electroanalysis 16:145
Gong J, Liu T, Song D, Zhang X, Zhang L (2009) One-step fabrication of three dimensional porous calcium carbonate–chitosan composite film as the immobilization matrix of acetylcholinesterase and its biosensing on pesticide. Electrochem Commun 11:1873
Raghu P, Swamy BEK, Reddy TM, Chandrashekar BN, Reddaiah K (2011) Sol–gel immobilized biosensor for the detection of organophosphorous pesticides: a voltammetric method. Bioelectrochemistry 83:19
Kuswandi B, Fikriyah CI, Gani AA (2008) An optical fiber biosensor for chlorpyrifos using a single sol–gel film containing acetylcholinesterase and bromothymol blue. Talanta 74:613
Chauhan N, Narang J, Pundir CS (2011) Immobilization of rat brain acetylcholinesterase on ZnS and poly(indole-5-carboxylic acid) modified Au electrode for detection of organophosphorus insecticides. Biosens Bioelectron 29:82
Liu T, Xu MR, Yin HS, Ai SY, Qu XJ, Zong SS (2011) A glassy carbon electrode modified with graphene and tyrosinase immobilized on platinum nanoparticles for sensing organophosphorus pesticides. Microchim Acta 175:129
Acknowledgments
This research was supported by National Natural Science Foundation of China (Grant No. 21105022), Doctor Foundation of Henan University of Technology (2010BS019), and Plan for Scientific Innovation Talent of Henan University of Technology (2012CXRC01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, M., Zeng, G. & Lu, Q. Determination of organophosphate pesticides using an acetylcholinesterase-based biosensor based on a boron-doped diamond electrode modified with gold nanoparticles and carbon spheres. Microchim Acta 181, 121–127 (2014). https://doi.org/10.1007/s00604-013-1078-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1078-4