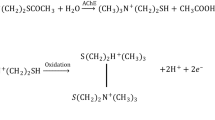
Abstract
The authors describe an amperometric biosensor for the determination of organophosphate pesticides (OPs) via inhibition of the enzyme acetylcholinesterase (AChE). The enzyme was immobilized on nitrogen-doped porous carbon and then placed on boron-doped diamond (BDD). The Michaelis-Menten constant for immobilized AChE is 0.177 mmol L−1, indicating that AChE has a stronger enzymatic activity and affinity due to the introduction of nitrogen-doped porous carbon. The biosensor was applied to the determination of the OPs dichlorvos and fenitrothion. The efficiency of the inhibition by dichlorvos increases linearly in the 100 pg·L-1 to 10.0 μg·L-1 concentration range. The detection limit is as low as 1.50 pg·L-1. The inhibition by fenitrothion can be detected in the same concentration range, and the detection limit is 4.40 pg·L-1 (calculated for 10% inhibition). The unique sensitivity of this assay make it a most attractive tool for the detection of OPs.

Nitrogen-doped porous carbon is used to develop an AChE/biosensor based on a boron-doped diamond electrode. The biosensor shows higher sensitivity, lower detection limit, good reproducibility and acceptable stability towards organophosphate pesticides detection. (AChE: acetylcholinesterase; BDD: boron-doped diamond)
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphate pesticides (OPs) have been widely used in agricultural production to improve its harvest. However, OPs may pollute soils and various water resources and get into the food industry via environmental pollution, resulting in health issues to both humans and animals [1,2,3]. Many common techniques such as gas chromatography, high-performance liquid chromatography, liquid chromatography coupled with mass spectroscopy are used to detect OPs due to their superiority with high sensitivity, good stability and favorable precision [4, 5]. However, the requirement of expensive and bulky instrumentation, complex pretreatment processes, highly trained personnel, and high detection costs make them unsuitable for rapid analysis of field samples. Electroanalytical techniques have been of great interest due to their advantages of comparative simplicity, rapid response and low cost [6,7,8,9,10,11]. Based on the inhibition effect of OPs on acetylcholinesterase (AChE) activity, the AChE-based electrochemical biosensors have attracted considerable attention in the past few years as the promising alternative to detect OPs [12,13,14,15].
Effective immobilization of enzyme to solid electrode surface is crucial for the fabrication of AChE-based biosensor. With their excellent properties of large surface area and fast electron transfer, many carbon-based nanomaterials such as carbon nanotubes [16, 17], graphene [18], carbon spheres [19] and other nanomaterials [20] have been successfully used to immobilize AChE. In our previous study, we developed a novel biosensor by using porous carbon to immobilize AChE [21]. Unfortunately, it was found that the poor dispersion of porous carbon affected its effective immobilization of the enzyme. Some studies reported that nitrogen element doping not only maintained the specific pore structure, but also increased the charge density of the porous carbon. This improves the conductivity, enhances the surface hydrophilicity, and improves the biocompatibility of the porous carbon [22, 23].
Herein, the nitrogen-doped porous carbon was prepared by using the silica spheres as hard template and the ionic liquid 1-butyl-3-methylimidazolium dicyanamide (BMIMdca) as precursors. The AChE was immobilized on the surface of BDD electrode by using the nitrogen-doped porous carbon to construct AChE/nitrogen-doped porous carbon/BDD sensor for the detection of dichlorvos and fenitrothion. The electrochemical behavior of acetylthiocholine chloride (ATCl) was studied by using the chronoamperometry method to obtain the Michaelis-Menten value of AChE/nitrogen-doped porous carbon/BDD.
Experimental materials and methods
Reagents
Dichlorvos and fenitrothion ( ≥ 99 %) were obtained from Dr. Ehrenstorfer (Augsburg, Germany, http://ehrenstorfergmbh. lookchem.com/). ATCl and AChE (Type C3389, 236 U·mg−1 from electric eel) were obtained from Sigma-Aldrich (St. Louis, USA, http://www.sigmaaldrich.com/). BMIMdca was obtained from Shanghai Cheng Jie Chemical Co. LTD. Phosphate buffer solution (pH 7.5) was prepared with double distilled water, containing 0.1 mol·L−1 Na2HPO4 and 0.1 mol·L−1 KH2PO4. All other reagents of analytical grade were purchased from Sinopharm Chemical Reagent Inc. (Beijing, China).
Apparatus
All the electrochemical experiments were performed on a CHI 660E Electrochemical Workstation (Shanghai Chenhua Instrument Corporation, China). A three-electrode system was comprised of boron-doped diamond (BDD) as working electrode, platinum wire as auxiliary electrode, and Ag/AgCl as reference electrode. Surface morphology and microstructure of samples were characterized by field-emission scanning electron microscopy (FESEM, JSM-7001F, JEOL Ltd., Japan) and Transmission electron microscopy (TEM, JSM-2100UHR, JEOL Ltd., Japan).
Preparation of the AChE/nitrogen-doped porous carbon/BDD electrode
The nitrogen-doped porous carbon was prepared by using the SiO2 spheres as hard template and the ionic liquid BMIMdca as precursors [23]. Firstly, SiO2 spheres with a diameter of about 300 nm were synthesized by Stöber method. SiO2 spheres (1.00 g) and BMIMdca (1.00 g) were mixed in 50.0 mL water, and then stirred at room temperature for 4 h. The mixtures were heated to 100 °C to remove the water. The resultant products were heat treated at 800 °C for 3 h in a quartz tube furnace under nitrogen atmosphere. The samples were treated with 10 wt% HF solution to remove the silica hard template. Finally, nitrogen-doped porous carbons were obtained by filtration, washing, and drying. 1.00 mg of the prepared material was added to 1.00 mL of double distilled water with magnetic stirring to prepare the 1 mg·mL-1 nitrogen-doped porous carbon solution.
The BDD electrode was prepared on silicon (100) wafers by a microwave-assisted plasma chemical vapor deposition technique using a commercial microwave plasma reactor (ASTeX Corp., Woburn, MA) at 5 kW with high purity hydrogen as the carrier gas. The prepared BDD was sequentially ultrasonicated in acetone and double distilled water before use.
The mixture of 5.00 μL of 1 mg·mL-1 nitrogen-doped porous carbon solution and 6.00 μL of 40.0 U·mL-1 AChE enzyme solution was dropped on the surface of the cleaned BDD electrode and dried at room temperature, and the AChE/nitrogen-doped porous carbon/BDD sensor was obtained.
Chronoamperometry response of ATCl on the AChE/nitrogen-doped porous carbon/BDD electrode
The typical amperometric current–time curve for the AChE/nitrogen-doped porous carbon/BDD electrode was obtained at a certain working voltage after the successive addition of ATCl to 5.00 mL phosphate buffered solution (pH 7.50) with stirring. The Michaelis-Menten constant (Km) was calculated according to the experimental results.
Results and discussion
Characterization of the nitrogen-doped porous carbon
Figure 1 are the SEM image and TEM image of the nitrogen-doped porous carbon. It can be seen from Fig. 1A that the nitrogen-doped porous carbon had abundant and well-distributed pores with a pore diameter of about 150 nm. The TEM image of Fig. 1B shows the nitrogen-doped porous carbon maintained the porous structure and the interconnected framework.
Electrochemical impedance spectroscopy on the different electrodes
Electrochemical impedance spectroscopy (EIS) of the different electrodes in 1.00×10−2 mol·L−1 [Fe(CN)6]3-/4- solution containing 0.100 mol·L−1 KCl are shown in Fig. 2. The electron transfer resistance of the porous carbon/BDD was about 199 Ω (b), which was smaller than that of the bare BDD (about 365 Ω, a). This suggests that the presence of porous carbon on the electrode improves the number of reactive sites, reduces the interfacial resistance, and facilitates the electron transfer. The minimum resistance of 79.0 Ω (c) was obtained on the nitrogen-doped porous carbon/BDD electrode. This was mainly ascribed that the introduction of nitrogen can increase the charge density of the porous carbon, thereby improving the conductivity and accelerating the electron transfer rate of the electrode surface. However, when AChE was immobilized on the surface of the nitrogen-doped porous carbon/BDD electrode, the resistance increased to 591 Ω (d). This indicates that the non-conducting, negatively charged AChE prevented [Fe(CN)6]3-/4- from reaching the electrode surface and hindered the electron transfer.
The differential pulse curve (DPV) responses of ATCl on the different electrodes
Figure 3 shows the DPV responses of ATCl on the different electrodes. On the AChE/BDD electrode (a), the DPV showed an oxidation peak at 0.240 V and the peak current was 0.750 μA, which comes from the oxidation of thiocholine, hydrolysis product of ATCl, catalyzed by the immobilized AChE. An obvious oxidation peak also appeared at 0.230V on the AChE/porous carbon/BDD electrode (b) and the peak current was 1.10 μA. The peak current increased by 46.7% on the AChE/porous carbon/BDD electrode, as compared with that on the AChE/BDD electrode. This was because that the well-ordered pore structure of porous carbon can provide more reactive site for the immobilization of AChE. In comparison with the AChE/porous carbon/BDD electrode, the oxidation peak current of the AChE/nitrogen-doped porous carbon/BDD electrode (c) reached 2.34 μA at 0.220V, increased by 113%. This indicates that nitrogen-doped porous carbon can enhance the conductivity and improve the detection sensitivity of enzyme sensor because of its large specific surface area and high charge density due to the introduction of nitrogen.
The chronoamperometry response of ATCl on the AChE/nitrogen-doped porous carbon/BDD electrode
The chronoamperometric response on the AChE/nitrogen-doped porous carbon/BDD electrode were performed at the different potential. After the baseline was stabilized, 50.0 μL 0.100 mol·L−1 ATCl was added dropwise to the electrolytic cell every 30 s for three times. As showen in Fig. 4, the signal current increased with increasing of the potential and reached the maximum at 0.220 V, then decreased when the potential was increased further. The background current increased with the increase of the potential. At 0.220 V, the AChE/nitrogen-doped porous carbon/BDD electrode had a best signal-to-noise ratio (Inset B). Therefore, 0.220 V was chosen as the optimum working potential in this experiment.
Figure 5A shows the chronoamperometry curve of AChE/nitrogen-doped porous carbon/BDD electrode at 0.220 V with increasing the ATCl concentration. As can be seen from Fig. 5B, the linear relationship between the concentration of ATCl and peak current was obtained in the range of 0.00200~2.20 mmol·L−1 and 2.20~5.40 mmol·L−1 with the linear curve I=1.59C+0.174 (r=0.999) and I=0.465C+2.70 (r=0.999). When the ATCl concentration was saturated, the amperometric response gradually tended to a plateau. For the higher ATCl concentrations the shape of the amperometric response was indicative of the Michaelis-Menten kinetic characteristics. The Km value was calculated according to the equation as follows:
Here, Iss represents the steady-state current after the addition of the analyte; Imax represents the maximum current after the addition of the analyte; C represents the total concentration of the analyte added. The Km value of 0.177 mmol·L−1 was obtained and lower than that of AChE-Ionic liquid-Graphene-CHIT/GCE [24] (0.770 mmol·L−1), AChE-MWCNTs-Au-CHIT/GCE [25] (0.270 mmol·L−1) and AChE-MSF-CHIT/GCE [26] (0.380 mmol·L−1), which indicated that AChE had stronger enzymatic activity and affinity due to the introduction of nitrogen-doped porous carbon.
Detection of dichlorvos and fenitrothion
Figure 6 shows the DPV responses of the AChE/nitrogen-doped porous carbon/BDD electrode before and after inhibition by dichlorvos and fenitrothion. As shown in Fig. 6A, the oxidation peak current of thiocholine was 2.490 μA without adding dichlorvos standard solution. When the AChE/nitrogen-doped porous carbon/BDD was immersed in 1.00 × 10-6 g·L−1 dichlorvos solution for 8 min, the peak current decreased to 0.700 μA, and the inhibition was 71.9%. Fig. 6B shows the DPV responses of the electrode before and after inhibition by adding 1.00 × 10-6 g·L−1 fenitrothion. It can be seen that the peak current decreased from 2.37 μA to 0.770 μA, and the inhibition was 67.5%. In the absence of OPs, AChE.can catalyze the hydrolysis of ATCl and produce the electro-active thiocholine, which occurs electrochemical oxidation and produce a strong signal. In the presence of OPs, OPs can inhibit the activity of AChE, and then decrease the oxidation of thiocholine. The oxidation peak current of thiocholine is inversely proportional to the concentration of OPs. By monitoring the oxidation peak current of thiocholine before and after inhibition, the OPs concentration can be determined.
Calibration plots for dichlorvos and fenitrothion
Under the optimal experimental conditions, the calibration curve of dichlorvos and fenitrothion were established on the AChE/nitrogen-doped porous carbon/BDD electrode, and the results are shown in Fig. 7. It was obvious that the inhibition increased with the increase of the concentration of pesticides. As shown in Fig. 7A, good linear relationship between the inhibition and –Lg [CDichlorvos] exists in the range of 1.00 × 10-10 ~ 1.00 × 10-5 g·L−1 with the regression equation of Inhibition (%) = 10.9 Lg [CDichlorvos] + 139 (%) (R2=0.999), and the detection limit was 1.50 × 10-12 g·L−1. Figure 7B shows the good linear relationship between the inhibition and –Lg [CFenitrothion] in the range of 1.00 × 10-10 ~ 1.00 × 10-5 g·L−1 with the regression equation of Inhibition (%) = 10.9 Lg [CFenitrothion] +134 (%) (R2=0.999), and the detection limit is 4.42×10-12 g·L−1.
Compared to previous reports [21, 27,28,29,30,31], the AChE/nitrogen-doped porous carbon/BDD biosensor was superior to other sensors, and the result was shown in Table 1. The lower detection limit of the developed biosensor may be attributed to the synergistic action of the advantages of the nitrogen-doped porous carbon and the superiority of the BDD electrode. The pore structure and good biocompatibility of the nitrogen-doped porous carbon can provide more reactive site for AChE immobilization, provide a compatible microenvironment and maintain the activity of AChE. The introduction of nitrogen can increase the charge density of the porous carbon, thereby improving the conductivity and accelerating the electron transfer rate of the electrode surface. On the other hand, the BDD electrode has been demonstrated to be superior to the glassy carbon electrode and other electrodes in terms of high signal-to-noise ratio, long-term stability, high sensitivity, and good reproducibility [32], which contributes to the lower detection limit of the AChE/nitrogen-doped porous carbon/BDD biosensor. Especially, compared with our previous work [20], except the lower detection limit, the preparation of nitrogen-doped porous carbon is more convenient and stable than the preparation of composite [BSmim]HSO4-AuNPs-porous carbon.
Repeatability, reproducibility, stability and regeneration of the electrode
Under the same conditions, the reproducibility was estimated by fabricating six different AChE/nitrogen-doped porous carbon/BDD electrodes, and the R.S.D. was 5.38%. The repeatability of AChE/nitrogen-doped porous carbon/BDD electrode was assessed through repetitively measuring dichlorvos levels for six times in 1.00 mmol·L−1 ATCl after being treated with 1.00 × 10-6 g·L−1 dichlorvos for 8 min. The result indicated that the method possessed good repeatability with relative standard deviation (R.S.D.) of 1.07%. The biosensor was stored at 4 °C when not in use. After a 30-day storage period, the biosensor retained 92.3% of its initial current response, indicating the acceptable stability. Three electrodes are used for determination of repeatibility and storage stability. According to the literatures [33,34,35], the inhibited AChE was reactivated by pralidoxime iodide. In this work, the AChE/nitrogen-doped porous carbon/BDD biosensor inhibited by 1.00 × 10-6 g·L−1 dichlorvos was immersed in 5.00 mmol·L−1 pralidoxime iodide solution for 8 min to completely dissociate the enzyme-inhibitor complex for the regeneration of AChE activity. The results showed that the inhibited AChE can be regenerated 93.0% of its original activity. The result indicated that the biosensor had acceptable regeneration.
Interference study
The interference effects of common ions such as CO3 2- (0.500 mmol·L−1), PO4 3- (0.500 mmol·L−1), NO3 - (0.500 mmol·L−1), K+ (0.500 mmol·L−1), Mg2+ (0.500 mmol·L−1), Ca2+ (0.500 mmol·L−1), Cl- (0.500 mmol·L−1) were discussed. For the detection of dichlorvos and fenitrothion, after adding the interfering ions, the peak current values were respectively maintained between 96.7% ~ 104% and 96.0% ~ 103% of the original current value (Fig. S4). This indicates that the AChE/Nitrogen-doped porous carbon/BDD sensor has an acceptable anti-interference ability for the detection of OPs.
Analysis of real samples
The sensor was employed to detect the content of dichlorvos in lettuce leaves and the content of fenitrothion in shanghaiqing leaves. As shown in the table S1 and the table S2, the recoveries of dichlorvos and fenitrothion were found to be between 96.1%~100% and 90.8%~107%, respectively. These results indicated that the biosensor can be used for the analysis of pesticide residues in real samples.
Conclusion
In summary, nitrogen-doped porous carbon was prepared and firstly used to develop an AChE-based/ biosensor for organophosphates (OPs) using a boron doped diamond electrode (BDD). The Michaelis-Menten constant for the enzyme on the biosensor indicated that AChE had stronger enzymatic activity and affinity due to the introduction of nitrogen-doped porous carbon. Using dichlorvos and fenitrothion as model compounds, the biosensor shows higher sensitivity, lower detection limit, good reproducibility and acceptable stability towards OPs detection. Because the detection mechanism depends on the inhibition of phosphate groups of OPs on AChE, this biosensor is broad-spectrum and selective for all OPs. The specificity for different OPs detection may be improved by using other biomolecules like aptamers as biosensors, which is the focus of the future research.
References
Barros LAD, Martins I, Rath S (2010) A selective molecularly imprinted polymer-solid phase extraction for the determination of fenitrothion in tomatoes. Analytical and Bioanalytical Chemistry 397:1355
Eskandari H, Naderi-Darehshori A (2012) Preparation of magnetite/poly(styrene-divinylbenzene) nanoparticles for selective enrichment-determination of fenitrothion in environmental and biological samples. Analytica Chimica Acta 743:137
Kesik M, Kanik FE, Turan J, Kolb M, Timur S, Bahadir M (2014) An acetylcholinesterase biosensor based on a conducting polymer using multiwalled carbon nanotubes for amperometric detection of organophosphorous pesticides. Sensors & Actuators B Chemical 205:39
Dutta RR, Puzari P (2013) Amperometric biosensing of organophosphate and organocarbamate pesticides utilizing polypyrrole entrapped acetylcholinesterase electrode. Biosensors & Bioelectronics 52:166
Abolghasemi MM, Hassani S, Bamorowat M (2016) Efficient solid-phase microextraction of triazole pesticides from natural water samples using a Nafion-loaded trimethylsilane-modified mesoporous silica coating of type SBA-15. Microchimica Acta 183:889
Geremedhin W, Amare M, Admassie S (2013) Electrochemically pretreated glassy carbon electrode for electrochemical detection of fenitrothion in tap water and human urine. Electrochimica Acta 87:749
Krejcova Z, Barek J, Vyskocil V (2016) Voltammetric determination of fenitrothion and study of its interaction with dna at a mercury meniscus modified silver solid amalgam electrode. Monatshefte für Chemie - Chemical Monthly 147:1
Salehzadeh H, Ebrahimi M, Nematollahi D, Salarian AA (2016) Electrochemical study of fenitrothion and bifenox and their simultaneous determination using multiwalled carbon nanotube modified glassy carbon electrode. Journal of Electroanalytical Chemistry 767:188
Khan I, Pandit UJ, Wankar S, Das R, Limaye SN (2017) Fabrication of electrochemical nanosensor based on polyaniline film-coated AgNP-MWCNT-modified GCE and its application for trace analysis of fenitrothion. Ionics 23:1293
Govindasamy M, Chen SM, Mani V, Akilarasan M, Kogularasu S, Subramani B (2016) Nanocomposites composed of layered molybdenum disulfide and graphene for highly sensitive amperometric determination of methyl parathion. Microchimica Acta 184:725
Pelle FD, Carlo MD, Sergi M, Compagnone D, Escarpa A (2016) Press-transferred carbon black nanoparticles on board of microfluidic chips for rapid and sensitive amperometric determination of phenyl carbamate pesticides in environmental samples. Microchimica Acta 183:3143
Carter JP, Noronha-Blob L, Audia VH, Dupont AC, McPherson DW, Natalie KJ Jr, Rzeszotarski WJ, Spagnuolo CJ, Waid PP, Kaiser C (2007) Immobilization of acetylcholinesterase on gold nanoparticles embedded in sol-gel film for amperometric detection of organophosphorous insecticide. Biosensors & Bioelectronics 23:130
Shamagsumova RV, Shurpik DN, Padnya PL, Stoikov II, Evtugyn GA (2015) Acetylcholinesterase biosensor for inhibitor measurements based on glassy carbon electrode modified with carbon black and pillar[5]arene. Talanta 144:559
Huo D, Li Q, Zhang Y, Hou C, Lei Y (2014) A highly efficient organophosphorus pesticides sensor based on CuO nanowires-SWCNTs hybrid nanocomposite. Sensors & Actuators B Chemical 199:410
Srivastava R, Kaur B (2015) Polyaniline-zeolite nanocomposite material based acetylcholinestrase biosensor for the sensitive detection of acetylcholine and organophosphates. New Journal of Chemistry 39:6899
Chauhan N, Pundir CS (2011) An amperometric biosensor based on acetylcholinesterase immobilized onto iron oxide nanoparticles/multi-walled carbon nanotubes modified gold electrode for measurement of organophosphorus insecticide. Analytica Chimica Acta 701:66
Rui X, Kang TF, Lu LP, Cheng SY (2012) Immobilization of acetylcholinesterase via biocompatible interface of silk fibroin for detection of organophosphate and carbamate pesticides. Applied Surface Science 258:6040
Yang L, Wang G, Liu Y (2013) An acetylcholinesterase biosensor based on platinum nanoparticles-carboxylic graphene-nafion-modified electrode for detection of pesticides. Biosensors & Bioelectronics 437:144
Wei M, Zeng G, Lu Q (2014) Determination of organophosphate pesticides using an acetylcholinesterase-based biosensor based on a boron-doped diamond electrode modified with gold nanoparticles and carbon spheres. Microchimica Acta 181:121
Arduini F, Cinti S, Scognamiglio V, Moscone D (2016) Nanomaterials in electrochemical biosensors for pesticide detection: advances and challenges in food analysis. Microchimica Acta 183:2063
Wei M, Wang J (2015) A novel acetylcholinesterase biosensor based on ionic liquids-AuNPs-porous carbon composite matrix for detection of organophosphate pesticides. Sensors & Actuators B Chemical 211:290
Tang J, Liu J, Li C, Li Y, Tade MO, Dai S (2015) Synthesis of nitrogen-doped mesoporous carbon spheres with extra-large pores through assembly of diblock copolymer micelles. Angewandte Chemie 54:588
Liu Y, Zhang Y, Zhai C, Li X, Mao L (2016) Nitrogen-doped porous carbons supported pt nanoparticles for methanol oxidation in alkaline medium. Materials Letters 166:16
Li Y, Han G (2012) Ionic liquid-functionalized graphene for fabricating an amperometric acetylcholinesterase biosensor. Analyst 137:3160
Du D, Wang M, Cai J, Qin Y, Zhang A (2010) One-step synthesis of multiwalled carbon nanotubes-gold nanocomposites for fabricating amperometric acetylcholinesterase biosensor. Sensors & Actuators B Chemical 143:524
Wu S, Zhang L, Qi L, Tao S, Lan X, Liu Z (2011) Ultra-sensitive biosensor based on mesocellular silica foam for organophosphorous pesticide detection. Biosensors & Bioelectronics 26:2864
Guan H, Zhang F, Yu J, Chi D (2012) The novel acetylcholinesterase biosensors based on liposome bioreactors-chitosan nanocomposite film for detection of organophosphates pesticides. Food Research International 49:15–21
Wu S, Huang F, Lan X, Wang X, Wang J, Meng C (2013) Electrochemically reduced graphene oxide and nafion nanocomposite for ultralow potential detection of organophosphate pesticide. Sensors & Actuators B Chemical 177:724
Kumaravel A, Chandrasekaran M (2011) A biocompatible nano TiO2/nafion composite modified glassy carbon electrode for the detection of fenitrothion. Journal of Electroanalytical Chemistry 650:163
Zhao L, Zhao F, Zeng B (2014) Synthesis of water-compatible surface-imprinted polymer via click chemistry and raft precipitation polymerization for highly selective and sensitive electrochemical assay of fenitrothion. Biosensors & Bioelectronics 62:19
Kaur N, Thakur H, Kumar R, Prabhakar N (2016) An electrochemical sensor modified with poly(3,4-ethylenedioxythiophene)-wrapped multi-walled carbon nanotubes for enzyme inhibition-based determination of organophosphates. Microchim Acta 183:2307
Wei M, Sun L-G, Xie Z-Y, Zhi J-F, Fujishima A, Einaga Y, Fu D-G, Wang X-M, Gu Z-Z (2008) Selective determination of dopamine on a boron-doped diamond electrode modified with gold nanoparticle/polyelectrolyte-coated polystyrene colloids. Advanced Functional Materials 18:141
Yang Y, Asiri AM, Du D, Lin Y (2014) Acetylcholinesterase biosensor based on a gold nanoparticle-polypyrrole-reduced graphene oxide nanocomposite modified electrode for the amperometric detection of organophosphorus pesticides. Analyst 139:3055
Gong J, Wang X, Li X, Wang K (2012) Highly sensitive visible light activated photoelectrochemical biosensing of organophosphate pesticide using biofunctional crossed bismuth oxyiodide flake arrays. Biosensors and Bioelectronics 38:43
Dong J, Liu T, Meng XM, Zhu JY, Shang K, Ai SY, Cui SL (2012) Amperometric biosensor based on immobilization of acetylcholinesterase via specific binding on biocompatible boronic acid-functionalized Fe@Au magnetic nanoparticles. Journal of Solid State Electrochemistry 16:3783
Acknowledgements
This research was supported by the Fundamental Research Funds for the Henan Provincial Colleges and Universities in Henan University of Technology (2016RCJH04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 625 kb)
Rights and permissions
About this article
Cite this article
Wei, M., Feng, S. Amperometric determination of organophosphate pesticides using a acetylcholinesterase based biosensor made from nitrogen-doped porous carbon deposited on a boron-doped diamond electrode. Microchim Acta 184, 3461–3468 (2017). https://doi.org/10.1007/s00604-017-2380-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2380-3