Abstract
The oxidation of acetaminophen was studied at a glassy carbon electrode modified with multi-walled carbon nanotubes and a graphite paste. Cyclic voltammety, differential pulse voltammetry and square wave voltammetry at various pH values, scan rates, and the effect of the ratio of nanotubes to graphite were investigated in order to optimize the parameters for the determination of acetaminophen. Square wave voltammetry is the most appropriate technique in giving a characteristic peak at 0.52 V at pH 5. The porous nanostructure of the electrode improves the surface area which results in an increase in the peak current. The voltammetric response is linear in the range between 75 and 2000 ng.mL−1, with standard deviations between 0.25 and 7.8%, and a limit of detection of 25 ng.mL−1. The method has been successfully applied to the analysis of acetaminophen in tablets and biological fluids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multi-walled carbon nanotubes (MWCNTs) are one of the more novel forms of carbon materials and as such have attracted substantial research attention, in particular for the determination applications for carbon nanomaterials. Due to their unique properties, such as their large surface area, mass transfer capabilities, significant mechanical strength, high catalytic capability and excellent electrical conductivity, they have the ability to facilitate electron transfer that ensures a wide range of applications in electrochemical analysis [1–6].
Acetaminophen, N-acetyl-p-aminophenol, or paracetamol is an analgesic compound that is regularly used to relieve headache, backache, arthritis and post-operative pain. The compound is effective in reducing fever associated with bacterial and viral infections [7]. The ready availability of acetaminophen has resulted in increased use of the compound in suicide attempts [8]. Consequently it is vital to develop a reliable and sensitive technique for the determination of acetaminophen in a variety of matrices.
The quantitative analysis of acetaminophen has been achieved using spectrophotometry, liquid chromatography, near infrared transmittance spectroscopy, titrimetry and voltammetry. In addition several flow injection methods for the determination of acetaminophen have been reported using fluorometric [9], UV–Vis spectrophotometry [10], Fourier transform infrared spectrophotometry [11] or electrochemical detection techniques [12]. However some of these methods are time-consuming and since acetaminophen is electroactive and can be oxidized under suitable conditions the use of electrochemical detection can be considered appropriate due rapid response and high sensitivity capabilities. Furthermore the potential for miniaturization, ease of manufacture and low cost electrochemical techniques can be considered as attractive method for the determination of acetaminophen [6, 12].
Modified electrodes have been distinguished by the application of nanomaterials on the electrode surface and that are capable of mediating rapid electron transfer [13–15]. Various electrodes have been used for the determination of acetaminophen and include modified glassy carbon [16, 17], boron-doped diamond [18–20], carbon ionic liquid [21], modified electrode surface with zirconium alcoxide porous gels and carbon-coated nickel magnetic nanoparticles [22, 23], gold nanoparticle modified carbon paste [24], gold electrodes modified with self assembled monolayer [25] and MWCNTs and carbon nanoparticle modified electrodes [26–30].
In this study, we describe the use of MWCNTs and graphite to produce a thin layer on the surface of a glassy carbon (GC) electrode for the electrochemical determination of acetaminophen in tablets and biological fluids. The use of MWCNTs:graphite/GC electrode revealed an improved electrochemical response for acetaminophen. Therefore a simple, rapid and sensitive electrochemical method was developed for the quantitative determination of acetaminophen. Several experimental parameters that influenced the optimization of the analysis of acetaminophen determination including pH, scan rate, electrochemical technique and the ratio of MWCNTs to graphite were investigated.
Experimental
Apparatus
A μ-AUTOLAB TYPE III in combination with GPES software version of 4.9 (www.metrohm-autolab.com) was used for all electrochemical experiments. A three electrode cell was used for the analysis and incorporated a hand-made working MWCNTs:graphite/GC electrode, a saturated Ag/AgCl/KCl as a reference electrode and a platinum wire that was used as a counter electrode. Sonication was achieved using of ultrasonic bath system TECNO-GAZ, Tecna 6 (50–60 Hz, 230 ± 10% V, 0.138 KW). Furthermore a Philips model X-30 scanning electron microscope was used to capture images.
Chemicals and reagents
All chemicals and reagents used in these studies were at least of analytical grade from Merck Co. (www.merck.com). Britton-Robinson buffer containing 0.04 M of each of acetic, ortho phosphoric and boric acids was adjusted to the required pH with a 0.2 M NaOH solution and was used as a supporting electrolyte. A working standard of acetaminophen powder was obtained from the Iranian Quality Control Laboratory of the Ministry of Health and Medical Education Department in Iran (www.behdasht.gov.ir). Acetaminophen tablets, each of containing 500 mg of the active pharmaceutical ingredient was purchased from a local pharmacy. Fresh frozen plasma was purchased from the Iranian Blood Research and Fractionation Holding Company (http://ibrf.ir/EN/Concern.asp). Drug free human urine was collected from healthy volunteers (25–30 years). Phosphoric acid, acetic acid, boric acid, ethyl acetate, sodium hydroxide, paraffin oil (density 0.84 ~ 0.89) and graphite powder (< 50 μm) were all purchased from Merck. Multi-walled carbon nanotubes (MWCNTs) prepared by chemical vapor deposition were purchased from Neutrino Co. (www.neunano.com, Iran). The MWCNTs had an outer wall diameter distribution of <10 nm, a length of between 5 and 15 μm and a special surface area of 180–190 m2.g−1 and amorphous carbon < 3%. Doubly distilled water was generated by purification through a Millipore water system and used for all analyses. All experiments were carried out at an ambient temperature of 25 ± 2 °C.
Preparation of MWCNTs: graphite/GC electrode
Several MWCNTs:graphite/GC electrodes with different ratios of MWCNTs:graphite mixtures were prepared and summarized in Table 1. Prior to application of the MWCNTs:graphite mixture onto the glassy carbon electrode (disk, r = 1 mm), the electrode surface was polished manually using alumina powder on the polishing cloth. Then, the electrode was sonicated for 2 min. in ethanol and rinsed with doubly distilled water. In order to manufacture the MWCNTs:graphite mixture, the materials were weighed and mixed together with a small amount of paraffin oil. Following completion of the mixing step a portion of the composite mixture was packed into the end of a polytetrafluoroethylene tube. The electrical contact was made by forcing a glassy carbon rod (r = 1 mm) into the polytetrafluoroethylene tube and the composite to ensure that a thin layer of the composite was formed on the surface of glassy carbon electrode. The surface morphology of the MWCNTs and MWCNTs:graphite mixture was investigated using scanning electron microscope.
Preparation of stock and standard solutions
A stock solution of acetaminophen (0.2 mg.mL−1) was prepared in doubly distilled water. For preparation of standard solutions, an aliquot of the stock solution of acetaminophen was transferred into a volumetric flask and made up to volume with buffer solution (pH 5). In all cases, the prepared solutions were protected from light using aluminium foil and stored at 4 °C for 3 days. Standard solutions in plasma or urine of 200 ng.mL−1, 1000 ng.mL−1 and 2000 ng.mL−1 were prepared by spiking of the drug into acetaminophen free plasma/urine with suitable dilution of an aqueous stock solution of acetaminophen.
Extraction procedure
In order to analyze acetaminophen in biological samples a 1 mL aliquot of 0.2 M NaOH was added to 0.8 mL of the standard plasma or urine solution. The mixture was vortexed for 3 min after which 3 mL ethyl acetate was added and mixture was vortexed for an additional 3 min. The mixture was centrifuged at 4500 rpm for 5 min to separate the aqueous and organic layers. After removal of the organic phase the extraction procedure was repeated with the residual aqueous phase. The ethyl acetate phases were pooled and dried under a gentle stream of nitrogen at 60 °C. After drying the samples were reconstituted with 20 mL of buffer solution at pH 5 and transferred to the electrochemical cell for analysis.
Tablet assay procedure
For the analysis of acetaminophen in tablets, 20 tablets were accurately weighed and powdered well. An amount of powder equivalent to the weight of one tablet was dissolved in doubly distilled water and then diluted with a Britton-Robinson buffer (pH 5) to produce a solution of acetaminophen with a concentration of 2000 ng.mL−1.
Calibration and system validation
The method was validated with respect to parameters including linearity, limit of detection, limit of quantification, precision, accuracy and selectivity. Square wave voltammograms of acetaminophen solutions with concentrations range 75–2000 ng.mL−1 in pH 5 at MWCNTs:graphite/GC (1:1) were recorded.
Results and discussion
Scanning electron microscopic characterization of MWCNTs and MWCNTs: graphite mixture
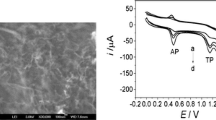
The properties and performance of a broad range of materials of different devices depends to large extent on their surface characteristics. The scanning electron microscopic images of MWCNTs and MWCNTs:graphite mixture were recorded and are shown in Fig. 1. The scanning electron microscopic image of MWCNTs is shown in part (a) of Fig. 1, whereas the related image of MWCNTs:graphite mixture is shown in part (b) of this figure. The presence of paraffin oil in the MWCNTs:graphite mixture is able to bridge the isolated carbon materials and MWCNTs are clearly visible in part (b) of Fig. 1.
Voltammetric study of the acetaminophen using different carbon electrodes
Voltammetric experiments including the generation of cyclic and differential pulse voltamograms were performed in solutions of pH ranging between 2 and 11 for 1000 ng.mL−1 samples of acetaminophen. Analyses were conducted for both glassy carbon and MWCNTs:graphite/GC electrodes with different ratios of MWCNTs:graphite mixture in Britton-Robinson buffer. By overlaying the cyclic voltammograms in different pH values at different electrodes (the results are not shown) it was possible to determine that the best pH for analysis was pH 5. The results showed sharper oxidation and reduction peaks in acidic pH at higher potentials. As the pH increases, the oxidation peak becomes wider and the signal weaker and the peak representing reduction disappeared. Furthermore the peaks shift to lower potentials. No peak for the reduction of acetaminophen was observed when solutions of pH higher than 6 were used. Therefore, the pH was varied between values of 3 and 7. These results are similar to those reported previously [8].
The resultant voltammogram for the cyclic voltammetric studies of a 1000 ng.mL−1 acetaminophen in buffer solution (pH 5) on different electrodes such as graphite, glassy carbon, MWCNTs:graphite (1:2, w/w), MWCNTs:graphite (2:1), MWCNTs:graphite (1:1) are indicated in Fig. 2. The results reveal that the best response was observed for analysis on MWCNTs:graphite/electrode with a ratio of (1:1). Therefore this electrode was chosen for the analysis of acetaminophen in this study.
Figure 3 depicts the cyclic voltammograms of acetaminophen in various pH values ranging from 2 to 6 using a MWCNTs:graphite/GC electrode. The obtained voltammograms demonstrate that the sharpest response was observed at pH 5 rather than other pH values. In accordance to Fig. 3, when the pH of buffer solutions is changed from 2 to 6, anodic peaks shift to more negative potential values. The inset of Fig. 3 shows a linear relationship between pH values and anodic peak potentials (Epa) and that the Epa decreased by about 55 mV per pH unit, with an equation of Epa = −0.055 pH + 0.863 (R2 = 0.999), indicating that the same numbers of electron and protons are involved in the oxidation of acetaminophen. The mechanism of acetaminophen oxidation involves the generation of N-acetyl-p-quinoneimine with two electrons and two protons and this phenomenon has been described in previous reports [8, 12, 31].
The effects of scan rate on the electrochemical response for acetaminophen were assessed by cyclic voltammetry and are shown in Fig. 4. The results reveal that a positive shift in the anodic peak potentials occurs which confirms the irreversibility of the process when the scan rate is increased. The linear relationship that exists between the oxidation peak current (Ipa) and the square root of the scan rate (v 1/2), is indicative of the fact that the oxidation process is predominantly diffusion-controlled in the whole range of scan rates studied [32]. The regression equation is Ipa = 0.1088v 1/2 + 0.3651 (R2 = 0.985).
The electrochemical responses of MWCNTs:graphite/GC electrode in various concentrations of acetaminophen were studied. The anodic current for acetaminophen was plotted versus concentration and linear regression analysis was performed on the resulting curves. A linear range of 75–2000 ng.mL−1 was obtained for acetaminophen with the slop of 0.1104 (10−8A/ng.mL−1). The R.S.D. values for three curves were ranging from 0.25 to 7.84%. A typical regression equation for the calibration curve was found to be Ipa = 0.1104C − 0.3178 (R2 = 0.999). The limit of quantitation and limit of detection of the electrode for acetaminophen were found to be 75 ng.mL−1 and 25 ng.mL−1, respectively. The precision of the method was investigated with respect to both repeatability (single electrode) and reproducibility (multiple electrodes). Repeatability was assessed by continuous electrodetermination of a standard solution of acetaminophen standard (2000 ng.mL−1) with the same MWCNTs:graphite/GC electrode for 12 analyses. The anodic current decreased by 1.65% after completing 12 scans with the RSD value of 2.05% which showed that the electrode responds with good repeatability. Reproducibility was investigated by electrodetermination of three replicate samples containing 75, 1000 and 2000 ng.mL−1 standards on 3 consecutive days using multiple electrodes where the mean concentrations were found to be 80.1, 1025 and 1994 ng.mL−1 with associated R.S.D. values of 7.17, 3.25, and 1.35%, respectively. The ruggedness of the method was assessed by comparison of the intra- and inter-day assay results for acetaminophen that had been performed by two analysts. The R.S.D. values for intra-and inter—day assays of acetaminophen in analyses performed in the same laboratory by two different analysts did not exceed 5%, thereby indicating the ruggedness of the method. The accuracy of the assay was determined by interpolation of replicate (n = 3) peak areas of three accuracy standards (75, 1000 and 2000 ng.mL−1) from a calibration curve prepared as previously described. In each case, the percent relevant error was calculated. The resultant concentrations were found to be 79.74 ± 5.737 ng.mL−1 (mean ± S.D.), 1034 ± 24.43 ng.mL−1 and 2016 ± 23.3 ng.mL−1 with percent relevant errors of 6.35, 3.4, and 0.8%, for the low, medium and high concentrations, respectively.
Analytical application
Some voltammetric procedures have been reported for the determination of acetaminophen in pharmaceutical tablets and biological fluids. However, to the best of our knowledge there has been no report in which the use of MWCNTs:graphite/GC electrode for the determination of acetaminophen has been documented. The most important reported electrochemical methods for the determination of acetaminophen are summarized in Table 2.
Assay of acetaminophen in tablets
The developed method was applied to the determination of acetaminophen in tablets. The result of the assay of acetaminophen tablets yielded a recovery of 100.02% (R.S.D. = 2.150%) of label claim for the tablets. The results of the assay indicate that the method is selective for the analysis of acetaminophen without interference from the excipients including cellulose, pregelatinized corn starch, sodium starch glycolate, magnesium stearate, polyethylene glycol, polysorbate 80, iron oxide, titanium dioxide and carnauba wax were used in manufacture of tablet formulations. In agreement with USP 29/NF, several combinations of acetaminophen and other drugs such as aspirin, caffeine, codeine phosphate, dextrometorphane HBr, diphenhydramine, phenylepherine and psudoephedrine are formulated in different dosage forms. Also, acetaminophen is widely used with ascorbic acid and cetirizine dihydrochloride [33]. So, the investigation of simple, rapid, sensitive and selective methods are important in the analysis of acetaminophen in the presence of other drugs. In this study, the applicability of the developed method in analyzing acetaminophen in the presence of the declared drugs assessed by cyclic voltammetric studies on the surface of MWCNTs:graphite/GC electrode in buffer solution (pH 5.0), which is not shown here, no interference peaks were recorded. The results therefore show that this method has a high degree of selectivity and sensitivity for acetaminophen in tablets.
Determination of acetaminophen in human plasma
The electrochemichal method was developed for the determination of acetaminophen and applied to the analysis of human plasma and urine samples. The recovery of acetaminophen from human plasma samples was measured by spiking drug-free samples with a known amount of acetaminophen. The resultant square wave voltammograms of extracted samples are shown in Fig. 5 (I) for buffer (a), blank plasma (b) and spiked sample (c, 2000 ng.mL−1). As can be seen there is no interference from the plasma sample. Three concentrations of acetaminophen spiked plasma were tested (200, 1000 and 2000 ng.mL−1). The percent recoveries for these studies are summarized in Table 3.
(I) The square wave voltammograms related to the blank of a buffer and b extracted plasma blank c extracted plasma spiked sample (2000 ng.mL−1). (II) Square wave voltammograms in blank of a buffer b urine blank previous extraction, c urine blank after extraction and d extracted urine spiked sample (2000 ng.mL−1)
Determination of acetaminophen in human urine
Figure 5 (II) depicts the square wave voltammograms of buffer and urine samples. The unextracted blank urine reveals a sharp peak in the range of potential for which acetaminophen is analyzed. This peak was present in three different sources of urine. Therefore the extraction of urine with ethyl acetate was necessary. Figure 5 (II) depicts the SWV of buffer (a) blank urine prior to extraction (b) blank urine after extraction (c) and spiked urine sample (d, 2000 ng.mL−1). Three concentrations of acetaminophen spiked urine were tested (200, 1000 and 2000 ng.mL−1). The percent recovery for these studies is shown in Table 3.
Conclusion
The high electroactive surface area and excellent electronic conductivity of multi-walled carbon nanotubes made their use in these studies, feasible. Several different ratios of MWNCTs:graphite/GC electrodes were made and voltammetric studies conducted using these electrodes were compared to those generated using glassy carbon electrode. The best ratio to use for the MWCNTs:graphite was 1:1 and all other studies were conducted using this electrode. The results were similar but more sensitive than those obtained for glassy carbon electrode using the square wave voltammetry in buffer solution pH 5. The limit of detection for the analysis of acetaminophen by this method was 25 ng.mL−1. The electrode was successfully used for the determination of acetaminophen in tablets directly without any separation steps and also in biological fluids with good percent recovery and RSD. The sample preparation is easy and the method is reproducible.
References
Liu Y, Wei W, Zhai X, Zeng J (2009) A novel carbon nanotube-modified biosensor containing a dsDNA-Ni(II) complex membrane, and its use for electro-catalytic oxidation of methanol in alkaline medium. Microchim Acta 164:167
Mohammadi A, Bayandori Moghaddam A, Dinarvand R, Rezaei-Zarchi S (2009) Direct electron transfer of polyphenol oxidase on carbon nanotube surfaces: application in biosensing. Int J Electrochem Sci 4:895
Sun D, Zhang H (2007) Electrochemical determination of acetaminophen using a glassy carbon electrode coated with a single-wall carbon nanotube-dicetyl phosphate film. Microchim Acta 158:131
Sun D, Sun Z (2008) Electrochemical determination of Pb2+ using a carbon nanotube/Nafion composite film-modified electrode. J Appl Electrochem 38:1223
Zhang WD, Chen J, Jiang LC, Yu YX, Zhang JQ (2010) A highly sensitive nonenzymatic glucose sensor based on NiO-modified multi-walled carbon nanotubes. Microchim Acta 168:259
Wan Q, Wang X, Yu F, Wang X, Yang N (2009) Effects of capacitance and resistance of MWNT-film coated electrodes on voltammetric detection of acetaminophen. J Appl Electrochem 39:1145
Fanjul-Bolado P, Lamas-Ardisana PJ, Hernandez-Santos D, Costa-Garcia A (2009) Electrochemical study and flow injection analysis of paracetamol in pharmaceutical formulations based on screen-printed electrodes and carbon nanotubes. Anal Chim Acta 638:133
Xiong H, Xu H, Wang L, Wang S (2009) Characterization and sensing properties of a carbon nanotube paste electrode for acetaminophen. Microchim Acta 167:129
Palgarin JAM, Bermejo LFG (1996) Flow-injection stopped-flow spectrofluorimetric kinetic determination of paracetamol based on its oxidation reaction by hexacyanoferrate (III). Anal Chim Acta 333:59
Canada MJA, Reguera PMI, Ruiz Medina A (2000) Fast determination of paracetamol by using a very simple photometric flow-through sensing device. J Pharm Biomed Anal 22:59
Ramos ML, Tyson JF, Curran DJ (1998) Determination of acetaminophen by flow injection with on-line chemical derivatization: Investigations using visible and FT IR spectrophotometry. Anal Chim Acta 364:107
Li M, Jing L (2007) Electrochemical behavior of acetaminophen and its detection on the PANI-MWCNTs composite modified electrode. Electrochim Acta 52:3250
Haran BM, Gopu G, Vedhi C (2009) Determination of analgesics in pharmaceutical formulations and urine samples using nano polypyrrole modified glassy carbon electrode. J Appl Electrochem 39:1177
Mohammadi A, Bayandori Moghaddam A, Kazemzad M, Dinarvand R, Badraghi J (2009) Synthesis of nickel oxides nanoparticles on glassy carbon as an electron transfer facilitator for horseradish peroxidase: direct electron transfer and H2O2 determination. Mat Sci Eng C 29:1752
Mohammadi A, Bayandori Moghaddam A, Dinarvand R, Badraghi J, Atyabi F, Saboury AA (2008) Bioelectrocatalysis of methyldopa by adsorbed tyrosinase on the surface of modified glassy carbon with carbon nanotubes. Int J Electrochem Sci 3:1248
Silva MLS, Carcia MBQ, Lima JLFC, Barrado E (2006) Modified tubular electrode in a multi-commutated flow system. Determination of acetaminophen in blood serum and pharmaceutical formulations. Anal Chim Acta 573–574:383
Cao BCL, Medeiros RA, Rocha-Filho RC, Mazo LH (2009) Simultaneous voltammetric determination of paracetamol and caffeine in pharmaceutical formulations using a boron-doped diamond electrode. Talanta 78:748
Radovan C, Cofan C, Cinghita D (2008) Simultaneous determination of acetaminophen and ascorbic acid at an unmodified boron-doped diamond electrode by differential pulse voltammetry in buffered media. Electroanalysis 20:1346
Cofan C, Radovan C (2008) Simultaneous chronoamperometric sensing of ascorbic acid and acetaminophen at a boron-doped diamond electrode. Sensors 8:3952
Guan XS, Zhang H, Zheng J (2008) Electrochemical behavior and differential pulse voltammetric determination of paracetamol at a carbon ionic liquid electrode. Anal Bioanal Chem 391:1049
Kumar SA, Tang CF, Chen SM (2008) Electroanalytical determination of acetaminophen using nano-TiO2/polymer coated electrode in the presence of dopamine. Talanta 76:997
Sima V, Cristea C, Lapadus F, Marian IO, Marian A, Sandulescu R (2008) Electroanalytical properties of a novel biosensor modified with zirconium alcoxide porous gels for the detection of acetaminophen. J Pharm Biomed Anal 48:1195
Wang SF, Xie F, Hu RF (2007) Carbon-coated nickel magnetic nanoparticles modified electrodes as a sensor for determination of acetaminophen. Sens Actuators B: Chem 123:495
Xu Z, Yue Q, Zhuang Z, Xiao D (2009) Flow injection amperometric determination of acetaminophen at a gold nanoparticle modified carbon paste electrode. Microchim Acta 164:387
Jia L, Zhang XH, Li Q, Wang SF (2007) Determination of acetaminophen by square wave voltammetry at a gold electrode modified by 4-amino-2-mercaptopyrimidine self-assembled monolayers. J Anal Chem 62:266
Li M, Jing L (2007) Electrochemical behavior of acetaminophen and its detection on the PANI-MWCNTs composite modified electrode. Electrochim Acta 52:3250
Duan LS, Xie F, Zhou F, Wang SF (2007) The electrochemical behavior of acetaminophen on multi-walled carbon nanotubes modified electrode and its analytical application. Anal Lett 40:2653
Kachoosangi RT, Wildgoose GG, Comptom RG (2008) Sensitive adsorptive stripping voltammetric determination of paracetamol at multiwalled carbon nanotube modified basal plane pyrolytic graphite electrode. Anal Chim Acta 618:54
Muralidharan B, Gopu G, Vedhi C, Manisankar P (2008) Determination of analgesics using a montmorillonite modified electrode. Appl Clay Sci 42:206
Ghorbani-Bidkorbeh F, Shahrokhian S, Mohammadi A, Dinarvand R (2010) Simultaneous voltammetric determination of tramadol and acetaminophen using carbon nanoparticles modified glassy carbon electrode. Electrochim Acta 55:2752
Miner D, Rice J, Riggin R, Kissinger P (1981) Voltammetry of acetaminophen and its metabolites. Anal Chem 53:2258
Radi A, El-Shahawi MS, Elmogy T (2005) Differential pulse voltammetric determination of the dopaminergic agonist bromocriptine at glassy carbon electrode. J Pharm Biomed Anal 37:195
The United States Pharmacopoeia Convention, United States Pharmacopoeia 30, Acetaminophen Tablets, The United States Pharmacopoeia Convention, Rockville, USA, 2006
Acknowledgements
The authors would like to acknowledge financial assistance from Tehran University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 53 kb)
Rights and permissions
About this article
Cite this article
Moghaddam, A.B., Mohammadi, A., Mohammadi, S. et al. The determination of acetaminophen using a carbon nanotube:graphite-based electrode. Microchim Acta 171, 377–384 (2010). https://doi.org/10.1007/s00604-010-0445-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0445-7