Abstract
An amperometric detector with a gold nanoparticle modified carbon paste electrode (GNMCPE) was applied applied to flow injection analysis for the determination of acetaminophen. An obvious shift of the peak potential and increase of the current peak were observed for the GNMCPE in comparison to that of the bare carbon paste electrode. The experimental conditions, such as species of buffer, pH, flow rate, detection volume, injection volume, and injection time were investigated. Under the optimized conditions, the calibration curve was obtained over the concentration range of 0.1–80 mg L−1 of acetaminophen with a linear correlation coefficient of 0.9994. The detection limit (3σ) was estimated to be 0.05 mg L−1 (n = 7). The recoveries of acetaminophen were between 98.40% and 104.1%, and the relative standard deviation varied between 1.66% and 2.74% for the different samples. This method was applied to analyze six types of tablets obtained from a local drugstore. The contents of acetaminophen were found to be 0.498, 0.323, 0.249, 0.324, 0.319 and 0.323 g of each tablet, respectively. These results are consistent with the values obtained by high performance liquid chromatography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In 1958, Adams [1] first introduced the carbon paste electrode (CPE) which usually comprises a Teflon bore, into which is inserted a copper, steel or carbon contact. The bore is filled with a paste made by mixing powdered carbon with an adequate mineral oil. In 1964, Kuwana and French [2] added modifier materials to the carbon paste in order to improve the selectivity and sensitivity of CPE. Since then, modifier materials have been widely researched in electroanalysis and in the context of chemical sensors [3–5]. In recent years, nanomaterials with special physical and chemical properties have been an attractive field of research [6–9]. Gold nanoparticles, one of the new promising materials, were used to modify electrodes due to their stable physical and chemical properties, small dimensional size and useful catalytic activities [10–12].
Acetaminophen (N-acetyl-p-aminophenol, APAP) is an extensively used antipyretic analgesic used in the symptomatic management of pain and fever [13]. Several methods have been proposed for the determination of acetaminophen, including titrimetry [14, 15], fluorimetry [16–18], spectrophotometry [19–22], chromatography [23–26], Fourier transform infrared spectrometry [27, 28], voltammetry [29, 30], capillary electrophoresis [31, 32], colorimetry [33] and flow-injection systems with colorimetric detection, and spectrophotometric detection [34]. However, the above mentioned methods have some disadvantages, for example, relatively expensive instruments, time-consuming procedures, and inconvenient operation. Flow injection analysis (FIA) with amperometric detection is an alternative method for the acetaminophen determination primarily because of the generally simple, inexpensive instrumentation and time-saving operation. In this paper, a gold nanoparticles modified carbon paste electrode (GNMCPE) was applied to FIA with an in-house amperometric detector for the determination of acetaminophen. The GNMCPE has some advantages, such as easy preparation, relatively fast response and low cost. The method of FIA-GNMCPE was applied to the determination of acetaminophen in six commercial pharmaceuticals. A satisfactory result was obtained which is consistent with that of high performance liquid chromatography (HPLC).
Experimental
Chemicals and reagents
All chemicals were of analytical grade and used without further purification. Sodium dihydrogen phosphate, sodium hydroxide, hydrochloric acid, anhydrous ethanol, acetone, ascorbic, urea and glucose were purchased from Chengdu Kelong Chemical Reagent Company (Chengdu, China). Acetaminophen was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Carbon powder (particle size of 60 μm) and heavy mineral oil were purchased from Shanghai Chemical Reagent Company (Shanghai, China). Hydrogen tetrachloroaurate (HAuCl4) was purchased from Sinopharm Chemical Reagent. Co., Ltd. (Shanghai, China).
All solutions were prepared using deionized water. Phosphate buffer solutions were prepared from sodium dihydrogen phosphate and adjusted to pH with 30% sodium hydroxide solution and 3 mol L−1 hydrochloric acid. The solution of standard acetaminophen was freshly prepared by dissolving 50 mg acetaminophen in 50 mL of anhydrous ethanol, and diluting to 100 mL with deionized water.
Apparatus
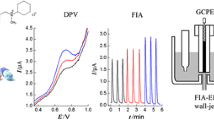
Electrochemical measurements were carried out with a microcomputer-based electrochemical analyzer (LK98BII, Tianjin Lanlike Chemical and Electron High Technology Co., Ltd, Tianjin, China, http://lanlike.instrument.com.cn). The amperometric measurements were performed in a flow-through cell with three electrodes (Fig. 1): a GNMCPE or a bare CPE as the working electrode, a saturated calomel electrode (SCE) as the reference electrode, and a platinum electrode as the auxiliary electrode. The amperometric detection flow-through cell was fabricated with a polyethylene tube, silicon rubber tube and polytetrafluoroethylene (PTFE) pipe with an inner diameter of 0.8 mm and an outer diameter of 1 mm. X-ray diffraction (XRD) analysis was performed on a Dandong Fangyuan 800 X-ray diffractometer (Dandong, China) with Cu Kα radiation. The transmission electron microscopy (TEM) images were obtained from a Hitachi H-800 transmission electron microscope (Tokyo, Japan) operating at 200 kV. The HPLC system was an Agilent Technologies 1100 series purchased from Agilent Technologies Co. Ltd. (Shanghai, China). The flow injection analysis system was supplied by Xintong Science Instrument Company (Beijing, China). The sonicator was purchased from Kunshan Ultrasonic Instrument Co. Ltd. (KQ218, 100W, 40 kHz, Jiangsu, China, http://www.ks-csyq.com). The microwave oven was purchased from Panasonic Electric Industrial Co., Ltd (Panasonic National, 950 W, Osaka, Japan).
Schematic diagram of the amperometric detection flow-through cell used in the flow injection system. 1 Platinum auxiliary electrode; 2 modified carbon paste electrode; 3 saturated calomel reference electrode (SCE); 4 polyethylene tube; 5 flow inlet; 6 PTFE pipe; 7 copper rod; 8 polyethylene tube; 9 flow outlet; 10 silicon rubber tube; 11 carbon paste
Procedures
Fabrication of gold nanoparticles modified carbon powder
A given weight of pure carbon powder was first carefully washed with acetone, ethanol and acetone in sequence, then three times with deionized water, transferred into an oven and dried at 80 °C. The dried powder was immediately separated into four parts and each part placed into a small beaker. The HAuCl4 solution of equal volume with different concentrations (1.3 × 10−3, 2.6 × 10−3, 5.2 × 10−3 and 9.6 × 10−3 mol L−1) was added into each beaker, respectively, and mixed simultaneously. Then the four beakers were kept in a microwave oven and heated for 5 min. The modified carbon powders were washed with deionized water until the yellow-green colors of the decanted solution had disappeared completely. Finally the products were kept in an oven and dried at 80 °C to obtain the gold nanoparticles modified carbon powder.
Preparation of the GNMCPE
GNMCPE was prepared as follows: firstly, 0.5 g of gold nanoparticles modified carbon powder and 0.18 mL of mineral oil were carefully mixed. Then, the mixture was homogenized by carefully mixing in an agate mortar and pressing with a pestle. Finally, the well-prepared paste was packed into a polyethylene cannula (inner diameter 2.5 mm, outer diameter 4.0 mm, length 70 mm) equipped with a copper rod (diameter 2.5 mm) as an external electric contact. This rod can move up and down to renew the surface of the electrode. The bare CPE was prepared in a similar way.
A shiny surface was obtained by smoothing the electrode onto a clean weighing paper, and then washing with deionized water several times. After several times of use, a new electrode surface can be obtained by piling out a small amount of the paste, rubbing away the excess against a clean weighing paper and polishing the electrode on the paper to obtain a shiny surface again.
FIA with amperometric detection for acetaminophen
As shown in Fig. 2, the flow injection analysis system consisted of a flow-through cell for amperometric detection, a main peristaltic pump, an auxiliary peristaltic pump, a sampling valve and an injection port with a 150 μL injection loop.
Deionized water was used as the carrier solution with a flow rate of 4.0 mL min−1, phosphate buffer solution (0.1 mol L−1, pH 4.7) was regulated by a reagent delivery module at a flow rate of 4.0 mL min−1.
Preparation of samples
All commercial samples were purchased from a local drugstore. These tablets were Riyebaifunin (Shanghai Ltd., Batch No. 0510565, Shanghai, China), Baijiahei (Jiangsu Ltd., Batch No. H10940251, Jiangsu, China), Gankang (Jilin Ltd., Batch No. H22026193, Jilin, China), Tainuo (Shanghai Ltd., Batch No. H20010115, Shanghai, China), Gandinuo (Xinjiang Ltd., Batch No. H19991427, Xinjiang, China) and Haiwangyindeifei (Shenzheng Ltd., Batch No. H44023557, Shenzheng, China). The tablet sample was crushed and powdered in a mortar, respectively. An 0.1 g amount of finely powdered sample was accurately weighed into a 100 mL flask, 50 mL of anhydrous ethanol was added before sonicating for 20 min, then the solution was diluted to volume with deionized water and filtered through an 0.45 μm membrane filter. Finally the filtrate was provided for assaying according to the method proposed in this paper and HPLC, respectively.
HPLC for acetaminophen
To evaluate the accuracy of the discussed method, the contents of acetaminophen in six commercial pharmaceuticals were determined by HPLC. The chromatographic parameters of the HPLC system are given in the electronic supported material S1.
Results and discussion
The properties of gold nanoparticles modified carbon paste and bare carbon paste
The XRD pattern of bare carbon paste (a) and gold nanoparticles modified carbon paste (b) is shown in Fig. 3. The characteristic peaks at 38.10°, 44.20° and 64.20° indicate that the modification of gold nanoparticles on carbon paste was successful. The morphologies of the bare carbon paste and gold nanoparticles modified carbon paste are also shown in Fig. 3, respectively. The average crystallite size of the gold nanoparticles was about 20 nm (Fig. 3B). When comparing Fig. 3A with Fig. 3B, the gold nanoparticles (two are marked with white arrows in Fig. 3B) throughout the carbon paste can be observed. Thus, we can safely draw the conclusion that gold nanoparticles had attached to the carbon paste electrode.
Electrocatalysis of acetaminophen at GNMCPE
The reaction of acetaminophen (APAP) is an electrochemical oxidation by a two-electron, two-proton process to generate N-acetyl-p-quinoneimine (NAPQI) [35, 36]. The oxidation mechanism is shown in Scheme 1.
First the electrocatalytic activities of CPE and GNMCPE towards the oxidation of acetaminophen in 0.1 mol L−1 phosphate buffer solution of pH 4.7 were demonstrated. Figure 4 displays the cyclic voltammograms (CVs) of 50 mg L−1 acetaminophen in 0.1 mol L−1 phosphate buffer solution of pH 4.7 at CPE (dotted line) and GNMCPE (solid line) respectively. It was found that both exhibited well-defined peak currents. For the CPE, an anodic peak occurred at approximately +0.8 V (peak 1) versus Ag/AgCl. However, in the case of the GNMCPE, a very well-defined oxidation peak at approximately +0.75 V (peak 2) versus Ag/AgCl was observed. The anodic peak (peak 1 and peak 2) represented the oxidation of APAP to NAPQI, while the cathodic peak (peak 3 and peak 5) represented the reverse reaction (NAPQI to APAP). At lower pHs, NAPQI would easily be protonated as the electroactive intermediate (c), which is then hydrolyzed to form hydrated NAPQI (d) which is finally transformed into benzoquinone (e). The last reaction (step E) was an electrochemical reduction by a two-electron, two-proton process to produce hydroquinone (f). The peak appeared at nearly +0.05 V (peak 4) maybe because of the oxidation of hydroquinone to benzoquinone. In addition, the voltammograms obtained with the GNMCPE provided higher signal-to-background (S/B) ratios in the same electrode area. Furthermore, the cyclic voltammograms of acetaminophen solution at different scan rates are shown in Fig. S1. The peak current for anodic oxidation of acetaminophen was proportional to the square root of the scan rate. This illustrated that the electrochemical oxidation reaction was diffusion-controlled.
We also studied the electrochemical properties of acetaminophen at GNMCPE with various concentrations of HAuCl4 (1.3 × 10−3, 2.6 × 10−3, 5.2 × 10−3 and 9.6 × 10−3 mol L−1) by cyclic voltammetry. The results showed that the oxidation potential of acetaminophen first increased and then decreased with the increase of HAuCl4 concentrations. In addition, the oxidation potential gradually shifted to a negative potential. Therefore, we chose the GNMCPE-modified concentration of HAuCl4 solutions to be 2.6 × 10−3 mol L−1 for the next experiments.
In order to improve its performance, various factors affecting the electrocatalytic activities of GNMCPE were investigated, including pH and sodium dihydrogen phosphate concentration. The effect of pH on the oxidation of acetaminophen was investigated over a pH range of 3.5 to 5.5. The results are presented in Fig. S2. The peak currents of acetaminophen first increased and then decreased with the increase in pH, and the maximum peak current was obtained when the pH was 4.7. The effect of phosphate buffer solution concentration was also investigated from 0.05 to 0.5 mol L−1 with a pH of 4.7. The results are presented in Fig. S3. It is clear that the maximum peak current was obtained when the concentration was 0.1 mol L−1. So 0.1 mol L−1 pH 4.7 phosphate buffer solution was selected and used for the following experiments.
Flow injection analysis with amperometric detection
In order to get the best response of the electrode in FIA, the following factors were optimized: flow rate, injection volume, injection time, and detection volume.
The effect of the flow rate on the response was verified using different flow rates (from 1.2 to 4.0 mL min−1) for the same sample volume. As can be seen from Fig. S4, as the flow rate increased, the peak current became higher and the shape of the peak became narrower. The maximum peak height was obtained with the flow rate of 4.0 mL min−1. So this flow rate (the maximum flow rate for the instrument) was selected. The sample injection volume varied from 150 to 400 μL (shown in Fig. S5). A volume of 150 μL was employed for further experiments. The effect of injection time on the magnitude of the amperometric response was investigated. The result shows that the peak current increased rapidly when increasing the injection time from 1 to 3 s (see Fig. S6). A further increase of the injection time has no effect on peak current. Thus, a 3 s injection time was chosen for further experiments. The amperometric response decreased with the increase of the volume of the flow-through cell from 30 to 80 μL (see Fig. S7). A cell volume of 30 μL was selected.
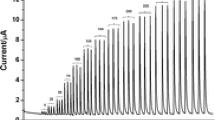
Once the conditions of the FIA procedure were optimized, amperometric detection of solutions containing different acetaminophen concentrations was carried out in phosphate buffer solution (pH 4.7) at the detection potential of +0.75 V versus Ag/AgCl. Figure 5 shows that the current values increased linearly with increasing acetaminophen concentrations. A linear response of the GNMCPE (Fig. 5a) to acetaminophen from 0.1 to 80 mg L−1 (ipGNMCPE (μA) = 0.1134 + 0.1123 C; r = 0.9994; n = 7) was obtained with a detection limit (3σ) of 0.05 mg L−1, where the unit of C was milligrams per liter. The relative standard deviation was less than 3%. Yet the response for CPE (Fig. 5b) to acetaminophen was only linear up to 40 mg L−1 (ipCPE (μA) = 0.1452 + 0.0754 C; r = 0.9928; n = 7) with a detection limit of 0.08 mg L−1. For higher concentrations (40 to 80 mg L−1), a non-linear relationship with decreasing slope was obtained. In conclusion, the GNMCPE compared with the CPE showed a relatively wide linear range for acetaminophen determination. Moreover, the GNMCPE improved the sensitivity and detection limit of acetaminophen determination.
Effect of concomitant substances, recovery and analysis of acetaminophen in pharmaceutical samples
In order to investigate the analytical application of this method, the specificity of GNMCPE to acetaminophen in the presence of some interfering substances such as ascorbic acid, urea and glucose was examined by carrying out the determination of 1 mg L−1 acetaminophen. Ascorbic acid and glucose represent electroactive materials, urea stands for non-electroactive matters. The results are compiled in Table 1. The interferences of ascorbic acid and glucose observed in the flow injection procedure for the determination of acetaminophen are obvious. However, the contents of these interferences in some relevant pharmaceuticals are commonly lower than the proportions of Table 1. This method can also be used for determination of acetaminophen.
The recovery tests of acetaminophen in the range of 5 to 25 mg L−1 were performed using the FIA-GNMCPE method. The results are listed in Table S1. The recoveries lay in the range of 98.40–104.1%, and the relative standard deviation was lower than 3%. This fact showed that the precision of this method was good, which testified to the applicability of FIA-GNMCPE to real samples.
FIA-GNMCPE was applied to the amperometric determination of acetaminophen in six commercial tablets with different compositions. The results are presented in Table 2. The analysis of acetaminophen for each sample was performed in triplicate. As can be observed, the relative errors were <1%, and the relative standard deviations were <2% in the results provided by FIA-GNMCPE. The results showed good accuracy and precision obtained with FIA-GNMCPE.
Conclusion
GNMCPE with an in-house amperometric detector was applied in flow injection analysis for the determination of acetaminophen. The GNMCPE shows a good electrochemical response to acetaminophen, and it exhibits a more sensitive and relatively fast response compared to CPE. The amperometric detection flow-through cell was made with inexpensive and easily obtained silicon rubber tubes of different diameters, its volume could be changed easily by adjusting the relative position of the silicon rubber tubes. The FIA-GNMCPE method developed represents excellent performance for the determination of acetaminophen in commercial tablets. The results are in agreement with HPLC. This method exhibited remarkable advantages including low cost, rapid response, simple detector configuration and high sensitivity. Apart from this, this method can be applied to the rapid determination of the filtration of analytes after ultrasonically assisted dissolution of the samples in water and anhydrous ethanol (without any previous reaction or derivatization process being necessary).
References
Adams RN (1958) Carbon paste electrodes. Anal Chem 30:1576
Kuwana T, French WH (1964) Electrooxidation or reduction of organic compounds into aqueous solutions using carbon paste electrode. Anal Chem 36:241
Wring SA, Hart JP, Birch BP (1990) Voltammetric behaviour of ascorbic acid at a graphite-epoxy composite electrode chemically modified with cobalt phthalocyanine and its amperometric determination in multivitamin preparations. Anal Chim Acta 229:63
Ulakhovich NA, Medyantseva EP, Budnikov GK (1993) Carbon paste electrode as a sensor in voltammetric analysis. J Anal Chem 48:682
Kalcher K, Kauffmann JM, Wang J, Svancara I, Vgtrass K, Neuhold C, Yang Z (1995) Sensors based on carbon-paste in electrochemical analysis—a review with particular emphasis on the period 1990–1993. Electroanal 7:5
Langer K, Barczyński P, Baksalary K, Filipiak M, Golczak S, Langer JJ (2007) A fast and sensitive continuous flow nanobiodetector based on polyaniline nanofibrils. Microchimica Acta 159:201
Wildgoose GG, Banks CE, Leventis HC, Compton RG (2006) Chemically modified carbon nanotubes for use in electroanalysis. Microchimica Acta 152:187
Zhao J, Yu JJ, Wang F, Hu SS (2007) Fabrication of gold nanoparticle-dihexadecyl hydrogen phosphate film on a glassy carbon electrode, and its application to glucose sensing. Microchimica Acta 156:277
Zhuang ZJ, Su XD, Yuan HY, Sun Q, Xiao D, Choi MMF (2008) An improved sensitivity non-enzymatic glucose sensor based on a CuO nanowire modified Cu electrode. The Analyst 133:126
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293
Liu Y, Geng T, Gao J (2007) Layer-by-layer immobilization of horseradish peroxidase on a gold electrode modified with colloidal gold nanoparticles. Microchimica Acta 161:241–248
Ding XQ, Yang M, Hu JB, Li QL, Mcdougall A (2007) Study of the adsorption of cytochrome c on a gold nanoparticle-modified gold electrode by using cyclic voltammetry, electrochemical impedance spectroscopy and chronopotentiometry. Microchimica Acta 158:65
Quintino Maria SM, Araki K, Toma HE, Angnes L (2002) Batch injection analysis utilizing modified electrodes with tetraruthenated porphyrin films for acetaminophen quantification. Electroanalysis 14:1629
Srivastava MK, Ahmed S, Singh D, Shukla IC (1985) Titrimetric determination of dipyrone and paracetamol with potassium hexacyanoferrate (III) in an acidic medium. Analyst 110:735
Bargot G, Auffret F, Burgot JL (1997) Determination of acetaminophen by thermometric titrimetry. Anal Chim Acta 343:125
Calatayud JM, Benito CG (1990) Flow-injection spectrofluorimetric determination of paracetamol. Anal Chim Acta 231:259
Vilchez JL, Blanc R, Avidad R, Navalón A (1995) Spectrofluorimetric determination of paracetamol in pharmaceuticals and biological fluids. J Pharm Biomed Anal 13:1119
Milch G, Szabó E (1991) Derivative spectrophotometric assay of acetaminophen and spectrofluorimetric determination of its main impurity. J Pharm Biomed Anal 9(10–12):1107
Ayaora Cañada MJ, Pascual Reguera MI, Ruiz Medina A, Fernández-de Córdova ML, Molina Díaz A (2000) Fast determination of paracetamol by using a very simple photometric flow-through sensing device. J Pharm Biomed Anal 22:59
Ni Y, Liu C, Kokot S (2000) Simultaneous kinetic spectrophotometric determination of acetaminophen and phenobarbital by artificial neural networks and partial least squares. Anal Chim Acta 419:185
Nagaraja P, Murthy KCS, Rangappa KS (1998) Spectrophotometric method for the determination of paracetamol and phenacetin. J Pharm Biomed Anal 17:501
Afshari JT, Liu TZ (2001) Rapid spectrophotometric method for the quantitation of acetaminophen in serum. Anal Chim Acta 443:165
Hewala II (1994) High-performance liquid chromatographic and derivative difference spectrophotometric methods for the determination of acetaminophen and its degradation product in aged pharmaceutical formulations. Anal Lett 27:561
Pérez JL, Bello MA (1999) Determination of paracetamol in dosage forms by non-suppressed ion chromatography. Talanta 48:1199
Dimitrova B, Doytchinova I, Zlatkova M (2000) Reversed-phase high-performance liquid chromatography for evaluating the distribution of pharmaceutical substances in suppository base-phosphate buffer system. J Pharm Biomed Anal 23:955
Ahrer W, Scherwenk E, Buchberger W (2001) Determination of drug residues in water by the combination of liquid chromatography or capillary electrophoresis with electrophoresis with electrospray mass spectrometry. J Chromatogr A 910:69
Ramos ML, Tyson JF, Curran DJ (1998) Determination of acetaminophen by flow injection with online chemical derivatization: investigations using visible and FTIR spectrophotometry. Anal Chim Acta 364:107
Zouhair B, Salvador G, Miguel de la G (1996) Flow injection-fourier transform infrared spectrometric determination of paracetamol in pharmaceuticals. Analyst 121:635
Ivaska A, Ryan T (1981) Application of a voltammetric flow-through cell to flow-injection-analysis (FIA). Czech Chem Commun 46:2865
Lau OW, Luk SF, Cheung YM (1989) Simultaneous determination of ascorbic acid, caffeine and paracetamol in drug formulations by differential-pulse voltammetry using a glassy carbon electrode. Analyst 114:1047
Kunkel A, Günter S, Wätzig H (1997) Quantitation of acetaminophen and salicylic acid in plasma using capillary electrophoresis without sample pretreatment improvement of precision. J Chromatogr A 768:125
Zhao SL, Bai WL, Yuan HY, Xiao D (2006) Detection of paracetamol by capillary electrophoresis with chemiluminescence detection. Anal Chim Acta 559:195
Knochen M, Giglio J, Reis BF (2003) Flow-injection spectrophotometric determination of paracetamol in tablets and oral solutions. J Pharm Biomed Anal 33:191
Bloonfield MS (2002) A sensitive and rapid assay for 4-aminophenol in paracetamol drug and tablet formulation, by flow injection analysis with spectrophotometric detection. Talanta 580:1301
Masawat P, Liawruangrath S, Vaneesorn Y, Liawruangrath B (2002) Design and fabrication of a low-cost flow-through cell for the determination of acetaminophen in pharmaceutical formulations by flow injection cyclic voltammetry. Talanta 58:1221
Li QL (1995) Electroanalytical chemistry. Beijing Normal University Press, China, p 227
Acknowledgments
This study was supported by the National Natural Science Foundation of China (20575042, 20775050) and the Science Foundation of the Chinese Education Commission (105141)
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material
Table S1
Recovery test of acetaminophen sample with amperometric detection on FIA-GNMCPE (n = 5) (DOC 27.5 KB)
Fig. S1
Cyclic voltammograms of the GNMCPE in 50 mg L−1 acetaminophen in potential range between −0.50 and +1.0 V at different scan rate: (a) 0.05, (b) 0.1, (c) 0.2, (d) 0.3, (e) 0.4 V s−1, respectively. Inset: plot of peak current on the square root of the scan rates (DOC 406 KB)
Fig. S2
Observed dependence of current on pH for 0.1 mol L−1 acetaminophen (DOC 39.0 KB)
Fig. S3
Observed dependence of current on phosphate buffer solution concentration (mol L−1) (DOC 42.0 KB)
Fig. S4
Flow injection analysis with amperometric detection results for a GNMCPE in 2.5 mg L−1 acetaminophen, 0.1 mol L−1 phosphate buffer solution (DOC 221 KB)
Fig. S5
Effect of injection volume of sample on a GNMCPE in 2.5 mg L−1 acetaminophen, 0.1 mol L−1 phosphate buffer solution (DOC 42.0 KB)
Fig. S6
Effect of injection time on the magnitude of amperometric response at a GNMCPE in 2.5 mg L−1 acetaminophen, 0.1 mol L−1 phosphate buffer solution (DOC 138 KB)
Fig. S7
Effect of detection volume of flow-through cell for flow injection analysis at a GNMCPE in 2.5 mg L−1 acetaminophen, 0.1 mol L−1 phosphate buffer solution (DOC 74.5 KB)
Rights and permissions
About this article
Cite this article
Xu, Z., Yue, Q., Zhuang, Z. et al. Flow injection amperometric determination of acetaminophen at a gold nanoparticle modified carbon paste electrode. Microchim Acta 164, 387–393 (2009). https://doi.org/10.1007/s00604-008-0072-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-008-0072-8