Abstract
Since the first report of the spontaneous appearance of venous thrombophlebitis as a sign of visceral cancer by Trousseau in 1865, many other studies have documented the existence of cancer-associated coagulation disorders. In this review, we describe the hypercoagulable state associated with colorectal cancer, from three perspectives: first, the incidence, risk factors and prevention of clinically symptomatic thromboembolic conditions associated with cancer, such as venous thromboembolism and arterial thrombosis; second, the association between hypercoagulable conditions, such as thrombocytosis, hyperfibrinogenemia, or d-dimer elevation, and the clinical progression and poor prognosis of cancer patients; third, the experimental approach to elucidate the role of various coagulation-related factors in the process of cancer progression, focusing specifically on the role of platelets and tissue factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that patients with solid tumors frequently present with an abnormal activation of the coagulation system, and consequently have a high incidence of thromboembolism. Since the first report on the spontaneous appearance of venous thrombophlebitis as a sign of visceral cancer by Trousseau in 1865 [1], many other studies have documented the existence of cancer-associated coagulation disorders. Reports related to the association between malignancy and coagulation abnormalities can be divided into the following three categories: first, the association between the incidence and risk factors of clinically symptomatic thromboembolic conditions and cancer. Patients without any evident cancer who develop symptomatic idiopathic thromboembolism have an approximately 10 % risk of subsequently being diagnosed with cancer [2]. The second category is the association between hypercoagulable conditions and the clinical progression and prognosis in cancer patients. A number of coagulation abnormalities, such as thrombocytosis, hyperfibrinogenemia, and an elevation of the d-dimer levels, fibrin degradation products (FDP) or von Willebrand factor (vWF) levels have been reported to correlate with the progression or a poor prognosis of different types of solid tumors, including esophageal, gastric, bladder, ovarian, lung and colorectal cancer [3–9]. The third category is papers describing the experimental approaches used to elucidate the role(s) of various coagulation-related factors in the process of cancer progression. Platelets and fibrinogen, by inhibiting the natural killer (NK) cell-mediated elimination of tumor cells, have been shown to increase the metastatic potential of cancer in a mouse experimental metastasis model [10]. In this review, we discuss the available literature concerning coagulation abnormalities and cancer progression, focusing specifically on colorectal cancer.
Colorectal cancer and thromboembolic conditions
Thromboembolism is a common condition observed in patients with malignancies. The incidence of venous thromboembolism (VTE) in patients with a cancer burden has been reported to be significantly higher than that in non-cancer patients [11], and a 2.2–12 % prevalence of occult cancer detection within 2 years after the venous thromboembolic event has been reported [12]. The thromboembolic event may be venous or arterial. VTEs include deep vein thrombosis (DVT) and pulmonary embolism (PE), while arterial events include stroke, myocardial infarction and arterial embolism. The actual incidence of VTE in the postoperative period in colorectal cancer patients varies among the reports, depending largely on the different follow-up periods or races of the patients. However, the surgical procedures used for colorectal cancer are abdominal, cancer-related and often involve the pelvis; therefore, the risk of thromboembolism is high. In cases without perioperative anticoagulation therapy, the incidence of symptomatic DVT occurring within 30 days after surgery was 1.4 % [13], and with a cumulative incidence of 3.1 % during a 2-year follow-up [14]. In a large population-based cohort study, including 68,142 colorectal cancer patients, the incidence of VTE was shown to be the highest during the first 6 months after the diagnosis [14]. Therefore, in the American College of Chest Physicians (ACCP) evidence-based clinical practice guidelines, colorectal cancer surgery patients are considered to be the highest-risk group for VTE, and even for those cases without a high risk of major bleeding complications, the introduction of pharmacologic prophylaxis with low-molecular-weight heparin (LMWH) for periods as long as 4 weeks is recommended [15]. There is another guideline for the prophylaxis of VTE in cancer patients [16], but neither of the guidelines take into account the levels of coagulation-related biomarkers, such as d-dimer elevation or thrombocytosis. Because a number studies have documented that the incidence of VTE in cancer patients correlates with the levels of hemostatic biomarkers, such as the d-dimer level, prothrombin fragment 1 + 2 level or platelet count [17], the establishment of a novel guideline for VTE prevention that includes recommendations for individualized treatment with pharmacological prophylaxis based on the individual coagulation abnormalities is desired.
Another coagulation-relevant complication is arterial thrombosis. Although most of the thromboembolic diseases associated with cancer are venous events, distinct from other cancers, colorectal cancer patients have also been shown to develop thrombosis associated with chemotherapy. One of the mainstream agents used for the treatment of advanced and recurrent colorectal cancer, bevacizumab, has been reported to increase the incidence of arterial thrombosis. The pooled analysis of randomized controlled trials performed in 2007, including a total of 1,745 patients, demonstrated an increased risk of arterial thrombosis by the use of bevacizumab [18]. In a recently published meta-analysis, which included 13,026 patients from 20 randomized trials, the overall relative risk for arterial thromboembolic events with bevacizumab-based therapy versus controls was 1.46, and bevacizumab treatment significantly increased the incidence of arterial thromboembolism [19].
Coagulation disorders and colorectal cancer progression
The reports on the association between coagulation abnormalities and the progression or a poor prognosis of colorectal cancer are summarized in Table 1. Several reports have shown that an elevation of the platelet count was associated with cancer progression [20, 21], but in most of these reports, the number of cases analyzed was limited. Recently, we analyzed the preoperative platelet counts in a relatively large cohort of colorectal cancer patients [22], and observed that the presence of preoperative thrombocytosis independently correlated with the tumor diameter and depth of invasion, but not with lymph node or distant metastasis, suggesting that thrombocytosis may reflect the local progression of colorectal cancer, but not its metastatic state. In our study, thrombocytosis was also found to be a predictive factor for a shorter disease-free survival and shorter cancer-specific survival, independent of the TNM classification. Similar results were reported in studies of the d-dimer and fibrinogen levels [23–26]. For example, Oya et al. [23] demonstrated that preoperative d-dimer elevation significantly correlated with the tumor size and pathological T factor, but not with lymph node metastasis, and it also independently correlated with a shorter overall survival. In our series, preoperative hyperfibrinogenemia significantly correlated with the depth of invasion and a shorter disease-free survival [25], and Tang et al. [26] reported that preoperative plasma fibrinogen elevation correlated with positive venous invasion and a worse 5-year survival. Taken together, these results suggest that thrombocytosis, hyperfibrinogenemia and d-dimer elevation are all events that simultaneously occur along with local cancer progression, but are not necessarily associated with the metastatic progression of cancer. These abnormalities of the coagulation system also correlate with a worse prognosis of colorectal cancer, independent of the TNM factors. These abnormalities are likely an integral part of the colorectal cancer-associated coagulation disorder, however, no report has concurrently investigated the correlation between the platelet counts, d-dimer level and fibrinogen level in colorectal cancer patients.
The association of other coagulation-related factors with colorectal cancer remains controversial. Wang et al. [27] reported that elevation of vWF in the peripheral blood correlated with a higher stage, the presence of multiple metastases and a shorter survival. However, Schellerer et al. [9] failed to demonstrate any correlation. Although prothrombin fragment F1 + 2 has been reported to be elevated in gynecological [28] and gastric cancer [29], its role in colorectal cancer has not yet been investigated.
There have been several reports concerning the efficacy of chemotherapy or radiotherapy and the presence of pretreatment coagulation abnormalities in other types of cancers. For example, a pretherapeutic high d-dimer level significantly correlated with a poor response to neoadjuvant chemotherapy in patients with advanced esophageal cancer [30], and pretreatment thrombocytosis correlated with a shorter survival in patients with non-small cell lung cancer who received radiotherapy with curative intention [31]. However, until a few years ago, there had been no such study involving patients with colorectal cancer, partly because adjuvant therapy was not routinely indicated in colon cancer patients. Since preoperative chemoradiotherapy (CRT) has recently become a standard therapy for rectal cancer, we investigated the correlation between coagulation abnormalities and the response to neoadjuvant CRT, and found that pre-CRT thrombocytosis and post-CRT fibrinogen elevation strongly correlated with a low regression rate, poor pathological response and higher rate of local recurrence [32, 33]. Further investigations to elucidate the role of the hypercoagulable state in the response to chemo- and/or radiotherapy in colorectal cancer will be necessary.
Experimental models of coagulation abnormalities in colorectal cancer
Presently, many abnormalities of the coagulation system have been reported to be associated with cancer progression and prognosis, and recently, several clotting factor gene polymorphisms were reported to significantly increase the risk of colorectal cancer, supporting the importance of coagulation abnormalities in cancer development [34]. Although the involvement of various procoagulant factors in the process of cancer development has been speculated (Fig. 1), the detailed mechanism(s) remain unclear, but increasing evidence indicates that two key coagulation factors, namely, platelets and tissue factor (TF), play a central role in the malignancy-associated hypercoagulable state. A metastasis-promoting ability of platelets has been suggested. The in vivo depletion of platelets markedly reduced experimental metastasis, and the formation of platelet aggregates around tumor cells has been shown to inhibit the in vitro tumor lysing activity of natural killer cells [35]. Several experimental models have also demonstrated that vWF, by connecting tumor cells and platelets, can promote the aggregation of platelets around tumor cells, protecting cancer cells from the innate immune system [36, 37]. However, the clinical reports indicate that abnormal thrombocytosis strongly correlates with local cancer progression of the primary tumor, but not with distant metastasis [20, 22]; thus, the protective effect of platelets does not seem to be relevant in the biology of colorectal cancer metastasis.
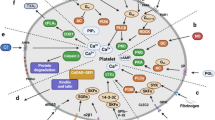
A schematic diagram of the coagulation cascade activated locally in cancer tissue. Tissue factors expressed in cancer tissue initiate a coagulation cascade, resulting in the generation of thrombin and formation of a fibrin polymer matrix. The thrombin and exposed subendothelial matrix activate platelets, which secrete various biologically active substances, such as PDGF, EGF, TGF-β, S1P and microparticles
Upon activation, platelets release various biologically active metabolites, which can activate cancer cells. The α-granules released by activated platelets contain storage pools of active substances, including angiogenic factors, such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF), which contribute to the formation of new vessels by cancer tissue. α-granules also contain a variety of cytokines, such as TGF-β, IL-1, RANTES (CCL5), IL-8 and MIP-1α, which promote the proliferation and migration of cancer cells, or proteases such as matrix metalloproteinase, which are involved in the invasion of cancer cells [38]. Upon stimulation, platelets also secrete sphingosine-1-phosphate (S1P) and lysophosphatidic acid (LPA), active lipids which promote strong migratory and proliferative activities, as well as the transactivation of the EGF receptor [39–42]. Microparticles (MPs) are circulating cellular membrane fragments (0.1–1 mm) which convey the plasma membrane and cytoplasmic components of apoptotic cells. The majority of MPs in the peripheral blood are platelet-derived MPs (PMPs), known as procoagulant metabolites, which are strongly correlated with the tumor aggressiveness and a poor clinical outcome [43]. PMPs can adhere to cancer cells, increasing their adhesiveness to endothelial cells and the extracellular matrix. PMPs also facilitate tumor cell invasiveness by increasing the metalloproteinase synthesis and secretion [44].
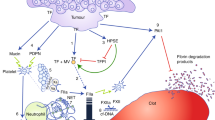
Another important procoagulant factor is TF, also called clotting factor III, which is a membrane-bound glycoprotein that is the primary initiator of the extrinsic coagulation cascade. TFs are expressed in 70 % of tumor cells and 53 % of tumor vascular endothelial cells in colorectal cancer. The overexpression of TFs in colorectal cancer is speculated to be dependent on the mutations of oncogenes, such as K-ras or p53 [45–47]. TFs expressed in cancer tissue react with clotting factor VIIa and activate a sequential coagulation cascade, resulting in thrombin generation and fibrin polymer formation. Thrombin activates platelets in cancer tissues and promotes the release of the above-mentioned chemical mediators. It was also reported that the expression of TFs is closely associated with the vascularization of cancer tissue. Increased levels of TFs resulted in the promotion of angiogenesis and a larger tumor size in mice injected with colorectal cancer cells, whereas TF knockdown largely abolished tumor angiogenesis [47]. In patients with high TF-expressing pancreatic cancer, the incidence of symptomatic VTE was reported to be markedly higher than that in patients with low TF expression [48]. Therefore, the local activation of the coagulation cascade induced by TFs appears to result in a systemic hypercoagulable state.
Another important coagulation trigger is collagen, which is abundantly present in the tumor stroma. Tumor vessels frequently lack endothelial cell integrity, and collagen can infiltrate into the blood stream through holes in the endothelium, activating platelets, together with vWF (Fig. 1) [49]. Inversely, the increased vascular permeability results in the leakage of plasma proteins, including prothrombin and fibrinogen, into the subendothelial extracellular matrix. Prothrombin is converted to thrombin by the activated coagulation pathway, and may result in platelet activation and the production of fibrin from fibrinogen [50]. Thrombin can also bind directly to cancer cells, thereby increasing their adhesion to the microvascular endothelium and expression of hypoxia-inducible factor-1α [51, 52]. Furthermore, factor VII, a counterpart of TFs, is reported to be synthesized by colorectal cancer cells, and promotes tumor invasion and metastasis [53]. Thus, the interactions of these many and complex factors may result in the promotion of the local progression and metastasis of tumors.
Because these findings suggest that a hypercoagulable state promotes cancer development and progression, there is a possibility that the suppression of abnormal hypercoagulation by anticoagulant or antiplatelet drugs can result in cancer prevention or improvement of the prognosis of cancer. The use of anticoagulants such as warfarin or clopidogrel has been shown to improve the biochemical control of prostate cancer treated with radiotherapy [54], and LMWH or warfarin has been reported to improve the survival in lung cancer patients both by preventing VTE-related mortality and by inducing anti-metastatic effects [55, 56]. Recently, it was reported that aspirin use was significantly associated with a longer survival in colorectal cancer patients with a phosphatidylinositol 3-kinase mutation [57]. Further investigations concerning the clinical application of anticoagulant drugs as anti-cancer remedies are awaited.
Conclusion
Although the first report of the association of a hypercoagulable state and malignancy was in 1865 [1], it still remains a hot topic in cancer research. Activated coagulation and cancer tissue form a mutual positive-feedback loop, and strategies that interfere with this loop may be an interesting new strategy for cancer prevention or treatment. The potential efficacy of anticoagulant or antiplatelet drugs for the treatment of cancer has not been clearly determined, and further investigations are warranted.
References
Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723–9.
Piccioli A, Lensing AW, Prins MH, et al. Extensive screening for occult malignant disease in idiopathic venous thromboembolism: a prospective randomized clinical trial. J Thromb Haemost. 2004;2:884–9.
Aminian A, Karimian F, Mirsharifi R, et al. Significance of platelet count in esophageal carcinomas. Saudi J Gastroenterol. 2011;17:134–7.
Wang L, Huang X, Chen Y, Jin X, Li Q, Yi TN. Prognostic value of TP/PD-ECGF and thrombocytosis in gastric carcinoma. Eur J Surg Oncol. 2012;38:568–73.
Komurcuoglu B, Ulusoy S, Gayaf M, Guler A, Ozden E. Prognostic value of plasma d-dimer levels in lung carcinoma. Tumori. 2011;97:743–8.
Yamashita H, Kitayama J, Kanno N, Yatomi Y, Nagawa H. Hyperfibrinogenemia is associated with lymphatic as well as hematogenous metastasis and worse clinical outcome in T2 gastric cancer. BMC Cancer. 2006;6:147.
Tsihlias J, Grossman HB. The utility of fibrin/fibrinogen degradation products in superficial bladder cancer. Urol Clin North Am. 2000;27:39–46.
Tas F, Kilic L, Bilgin E, et al. Clinical and prognostic significance of coagulation assays in advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2013;23:276–81.
Schellerer VS, Mueller-Bergh L, Merkel S, et al. The clinical value of von Willebrand factor in colorectal carcinomas. Am J Transl Res. 2011;3:445–53.
Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–85.
Alikhan R, Cohen AT, Combe S, et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study. Arch Intern Med. 2004;164:963–8.
Monreal M, Lensing AW, Prins MH, et al. Screening for occult cancer in patients with acute deep vein thrombosis or pulmonary embolism. J Thromb Haemost. 2004;2:876–81.
Davenport DL, Vargas HD, Kasten MW, Xenos ES. Timing and perioperative risk factors for in-hospital and post-discharge venous thromboembolism after colorectal cancer resection. Clin Appl Thromb Hemost. 2012;18:569–75.
Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24:1112–8.
Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e227S–77S.
Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11:56–70.
Ay C, Pabinger I. Predictive potential of haemostatic biomarkers for venous thromboembolism in cancer patients. Thromb Res. 2012;129(Suppl 1):S6–9.
Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–9.
Schutz FA, Je Y, Azzi GR, Nguyen PL, Choueiri TK. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22:1404–12.
Kandemir EG, Mayadagli A, Karagoz B, Bilgi O, Turken O, Yaylaci M. Prognostic significance of thrombocytosis in node-negative colon cancer. J Int Med Res. 2005;33:228–35.
Qiu MZ, Yuan ZY, Luo HY, et al. Impact of pretreatment hematologic profile on survival of colorectal cancer patients. Tumour Biol. 2010;31:255–60.
Sasaki K, Kawai K, Tsuno NH, Sunami E, Kitayama J. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J Surg. 2012;36:192–200.
Oya M, Akiyama Y, Okuyama T, Ishikawa H. High preoperative plasma d-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol. 2001;31:388–94.
Stender MT, Larsen TB, Sorensen HT, Thorlacius-Ussing O. Preoperative plasma d-dimer predicts 1-year survival in colorectal cancer patients with absence of venous thromboembolism (VTE): a prospective clinical cohort study. J Thromb Haemost. 2012;10:2027–31.
Yamashita H, Kitayama J, Taguri M, Nagawa H. Effect of preoperative hyperfibrinogenemia on recurrence of colorectal cancer without a systemic inflammatory response. World J Surg. 2009;33:1298–305.
Tang L, Liu K, Wang J, Wang C, Zhao P, Liu J. High preoperative plasma fibrinogen levels are associated with distant metastases and impaired prognosis after curative resection in patients with colorectal cancer. J Surg Oncol. 2010;102:428–32.
Wang WS, Lin JK, Lin TC, et al. Plasma von Willebrand factor level as a prognostic indicator of patients with metastatic colorectal carcinoma. World J Gastroenterol. 2005;11:2166–70.
Gadducci A, Marrai R, Baicchi U, Gagetti O, Facchini V, Genazzani AR. Prothrombin fragment F1 + 2 and thrombin-antithrombin III complex (TAT) plasma levels in patients with gynecological cancer. Gynecol Oncol. 1996;61:215–7.
Kwon HC, Oh SY, Lee S, et al. Plasma levels of prothrombin fragment F1 + 2, d-dimer and prothrombin time correlate with clinical stage and lymph node metastasis in operable gastric cancer patients. Jpn J Clin Oncol. 2008;38:2–7.
Tomimaru Y, Yano M, Takachi K, et al. Correlation between pretherapeutic d-dimer levels and response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. Dis Esophagus. 2008;21:281–7.
Holgersson G, Sandelin M, Hoye E, et al. Swedish lung cancer radiation study group: the prognostic value of anaemia, thrombocytosis and leukocytosis at time of diagnosis in patients with non-small cell lung cancer. Med Oncol. 2012;29:3176–82.
Kawai K, Kitayama J, Tsuno NH, Sunami E, Nagawa H. Hyperfibrinogenemia after preoperative chemoradiotherapy predicts poor response and poor prognosis in rectal cancer. Int J Colorectal Dis. 2011;26:45–51.
Kawai K, Kitayama J, Tsuno NH, Sunami E, Watanabe T. Thrombocytosis before pre-operative chemoradiotherapy predicts poor response and shorter local recurrence-free survival in rectal cancer. Int J Colorectal Dis. 2012
Vossen CY, Hoffmeister M, Chang-Claude JC, Rosendaal FR, Brenner H. Clotting factor gene polymorphisms and colorectal cancer risk. J Clin Oncol. 2011;29:1722–7.
Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–300.
Gasic GJ. Role of plasma, platelets, and endothelial cells in tumor metastasis. Cancer Metastasis Rev. 1984;3:99–114.
Gasic GJ, Gasic TB, Galanti N, Johnson T, Murphy S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int J Cancer. 1973;11:704–18.
Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl 1):S13–33.
Shida D, Kitayama J, Yamaguchi H, et al. Sphingosine 1-phosphate transactivates c-Met as well as epidermal growth factor receptor (EGFR) in human gastric cancer cells. FEBS Lett. 2004;577:333–8.
Yamashita H, Kitayama J, Shida D, et al. Sphingosine 1-phosphate receptor expression profile in human gastric cancer cells: differential regulation on the migration and proliferation. J Surg Res. 2006;130:80–7.
Shida D, Kitayama J, Yamaguchi H, et al. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003;63:1706–11.
Mori K, Kitayama J, Shida D, Yamashita H, Watanabe T, Nagawa H. Lysophosphatidic acid-induced effects in human colon carcinoma DLD1 cells are partially dependent on transactivation of epidermal growth factor receptor. J Surg Res. 2006;132:56–61.
Helley D, Banu E, Bouziane A, et al. Platelet microparticles: a potential predictive factor of survival in hormone-refractory prostate cancer patients treated with docetaxel-based chemotherapy. Eur Urol. 2009;56:479–84.
Dashevsky O, Varon D, Brill A. Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. Int J Cancer. 2009;124:1773–7.
Seto S, Onodera H, Kaido T, et al. Tissue factor expression in human colorectal carcinoma: correlation with hepatic metastasis and impact on prognosis. Cancer. 2000;88:295–301.
Rao B, Gao Y, Huang J, et al. Mutations of p53 and K-ras correlate TF expression in human colorectal carcinomas: TF downregulation as a marker of poor prognosis. Int J Colorectal Dis. 2011;26:593–601.
Yu JL, May L, Lhotak V, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–41.
Khorana AA, Ahrendt SA, Ryan CK, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13:2870–5.
Verheul HM, Hoekman K, Lupu F, et al. Platelet and coagulation activation with vascular endothelial growth factor generation in soft tissue sarcomas. Clin Cancer Res. 2000;6:166–71.
Wojtukiewicz MZ, Sierko E, Klement P, Rak J. The hemostatic system and angiogenesis in malignancy. Neoplasia. 2001;3:371–84.
Klepfish A, Greco MA, Karpatkin S. Thrombin stimulates melanoma tumor-cell binding to endothelial cells and subendothelial matrix. Int J Cancer. 1993;53:978–82.
Chang LH, Chen CH, Huang DY, Pai HC, Pan SL, Teng CM. Thrombin induces expression of twist and cell motility via the hypoxia-inducible factor-1alpha translational pathway in colorectal cancer cells. J Cell Physiol. 2011;226:1060–8.
Tang JQ, Fan Q, Wu WH, et al. Extrahepatic synthesis of coagulation factor VII by colorectal cancer cells promotes tumor invasion and metastasis. Chin Med J. 2010;123:3559–65.
Choe KS, Correa D, Jani AB, Liauw SL. The use of anticoagulants improves biochemical control of localized prostate cancer treated with radiotherapy. Cancer. 2010;116:1820–6.
Noble S. Low-molecular-weight heparin and survival in lung cancer. Thromb Res. 2012;129(Suppl 1):S114–8.
Maurer LH, Herndon JE 2nd, Hollis DR, et al. Randomized trial of chemotherapy and radiation therapy with or without warfarin for limited-stage small-cell lung cancer: a Cancer and Leukemia Group B study. J Clin Oncol. 1997;15:3378–87.
Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–606.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawai, K., Watanabe, T. Colorectal cancer and hypercoagulability. Surg Today 44, 797–803 (2014). https://doi.org/10.1007/s00595-013-0606-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-013-0606-5