Abstract
Background
Disorders in the blood coagulation system are often associated with malignancy. Patients with colorectal cancer (CRC) have been shown to have abnormal data for various coagulation tests.
Methods
We retrospectively analyzed the relation between the preoperative plasma fibrinogen level and tumor recurrence in 569 patients with CRC who underwent curative surgical resection and were followed up without adjuvant chemotherapy.
Results
The plasma fibrinogen level showed a positive association with tumor recurrence, age, sex, T stage, and TNM classification. When divided with the median value, hyperfibrinogenemia is positively correlated with tumor recurrence, although it lost independence in the multivariate analysis. In the C-reactive protein (CRP)-negative population, hyperfibrinogenemia is independently correlated with tumor recurrence and recurrence-free survival. In contrast, hyperfibrinogenemia has no effect on recurrence in CRP-positive patients.
Conclusions
Hyperfibrinogenemia is clinically relevant in tumor recurrence before a systemic inflammatory response and thus can be a useful predictor of recurrence in the preinflammatory stage of CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the most common forms of cancer in the world. Surgical removal is the main treatment modality when overt dissemination of cancer beyond the surgically resected area is not evident. However, even after macroscopically curative resection of the tumor, a certain proportion of the patients with CRC develop recurrent disease, with reduced survival. Adjuvant chemotherapy has been shown to be effective to reduce the recurrence rate in those patients. Because the toxicity of anticancer drugs is not negligible, however, selecting patients with high recurrence possibility who may obtain real benefit from adjuvant chemotherapy is clinically important.
Pathologic staging is the most powerful predictor of clinical outcome in CRC [1], and many randomized trials have shown that adjuvant chemotherapy has successfully reduced the rate of recurrent disease with stage III CRC [2–4], although the effectiveness for stage II disease remains debatable. To identify the patients with high risk of recurrence without a pathologically determined stage, some serologic markers have been used to predict clinical outcome in CRC. Carcinoembryonic antigen (CEA) is one of the widespread markers in CRC. Although the prognostic significance of preoperative CEA is still controversial, the preoperative serum CEA level has been reported to be an independent prognostic marker in much of the current literature [5–10]. However, preoperative serum CEA is negative in more than half of patients with CRC [7, 11], and patients with CEA-negative CRC do develop recurrent disease at a constant rate. Therefore, preoperative CEA is not a powerful marker to select candidates for adjuvant chemotherapy or other additional treatments.
Previously, we demonstrated that elevated plasma fibrinogen level was associated with lymph node metastasis and liver metastasis, but not with peritoneal metastasis, in gastric cancer [12, 13]. Furthermore, hyperfibrinogenemia was also associated with worse clinical outcome in serosal invasion-negative advanced gastric cancer [13], suggesting that hyperfibrinogenemia had an impact on hematogenous and lymphatic metastasis and/or relapse in this population. Fibrinogen is known to be elevated during the systemic inflammatory response, and thus hyperfibrinogenemia is supposed to result in increased tumor volume [14]. However, recent studies support the hypothesis that fibrinogen can assist hematogeneous metastasis [15, 16]. For CRC, the major determinant of clinical outcome is mainly hematogenous and/or lymph node recurrence. Thus, we hypothesized that hyperfibrinogenemia is associated with the outcome of CRC as well. Hence, we retrospectively examined the preoperative plasma fibrinogen level of patients with CRC who underwent curative resection. Furthermore, because the plasma fibrinogen level is critically affected by inflammation, a high plasma fibrinogen level might be only a secondary event in tumor progression due to inflammatory cytokines. To clarify this point, we simultaneously evaluated the serum C-reactive protein (CRP) level, a major inflammatory marker, and assessed whether the inflammation is clinical or subclinical in patients with CRC.
Patients and methods
A total of 569 patients, from February 1988 to January 2002, with colorectal adenocarcinoma were enrolled in this study. None of the patients had distant metastasis. They underwent curative resection of the tumor at the First Department of Surgery, University of Tokyo Hospital, Tokyo. At our institution, most patients with T3 or T4 rectal cancer undergo preoperative irradiation therapy, and these patients were excluded from this study. Patients with neoadjuvant chemotherapy or another synchronous cancer were also excluded from the study.
The patients were followed up for 0.1–18.1 years (median 5.3 years). The follow-up consisted of a complete history, physical examination, complete blood count, chemistry tests, and tumor markers—CEA and cancer antigen (CA) 19-9—every 3 months for 1 year and then biannually thereafter. Computed tomography (CT) was done biannually for 3 years and then annually thereafter. If clinically indicated, additional CT was performed for surveillance. Endoscopic surveillance was done annually. Patients with acute inflammatory disease and liver cirrhosis were also excluded to minimize confounding factors because the plasma fibrinogen level was critically affected by the presence of inflammation and liver cirrhosis. In addition, the impact of the preoperative plasma fibrinogen level on the outcome were evaluated in all patients as well as in a population with negative CRP values to evaluate the difference between having or not having clinical inflammation. Patients in this study were all followed up without adjuvant chemotherapy.
For this study we referred to the classifications established by the Japanese Research Society for Colorectal Carcinoma, which define T2 as a tumor invading the muscularis propria, T3 as a tumor invading through the muscularis propria into the subserosa or into nonperitonealized pericolic or perirectal tissues, and T4 as a tumor directly invading other organs or structures and/or perforating visceral peritoneum.
The preoperative plasma fibrinogen level, serum CEA, and CRP were measured from early morning samples taken before breakfast 5–10 days before surgery. A serum CEA level <5 ng/ml and CRP level <0.3 mg/dl were taken as normal.
The association of the fibrinogen level with clinicopathologic factors was assessed with Fisher’s exact test or Pearson’s chi-squared test. Plasma fibrinogen levels were compared with one-way analysis of variance (ANOVA) followed by Tukey’s HSD test. Age was compared by unpaired Student’s t-test or Wilcoxon’s rank sum test. A multivariate stepwise logistic regression analysis was performed to identify independently associated variables that were correlated with tumor recurrence. Recurrence-free survival rates were calculated from the date of the operation. Data on patients who were alive without recurrence at the end of our study or at the last follow-up visit were censored for recurrence-free survival analysis. The Cox regression model was used to investigate the relation between fibrinogen and recurrence-free survival. We include the interaction term between fibrinogen (continuous) and CRP category (<0.3/≥0.3) to estimate the hazard ratio (HR) of fibrinogen within each CRP level. Values of p < 0.05 were considered significant. Statistical analysis was carried out using JMP 6.0.0 and SAS (version 9.1.3).
Results
Association of preoperative plasma fibrinogen level with clinical parameters in patients with colorectal cancer
The preoperative plasma fibrinogen level in patients with CRC showed a positive association with many factors, including age, sex, T stage, and TNM classification. For example, the fibrinogen level was increasingly elevated as the T stage of the tumor increased (Fig. 1). The median (interquartile range) plasma fibrinogen level in the 569 patients studied was 336 mg/dl (283–397 mg/dl). In the following analysis, the patients were divided into two groups with this median value (>336 mg/dl and ≤336 mg/dl) to clarify the associated variables with hyperfibrinogenemia. As shown in Table 1, the hyperfibrinogenemia was increased in older and male patients with advanced tumor stage. Hyperfibrinogenemia is also strongly associated with higher serum CRP and CEA levels.
Impact of hyperfibrinogenemia on tumor recurrence in colorectal cancer
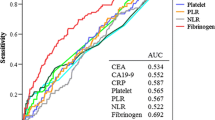
Among the 569 patients with CRC, 112 patients developed recurrent tumors during their follow-up. The site of the recurrence was the liver in 56, lung in 30, lymph node in 15, peritoneum in 10, local in 16, bone in 2, and brain in 2, respectively. Eleven patients had recurrences at two sites, and four patients had recurrences at three sites. We next evaluated the associations of clinicopathologic variables with the tumor recurrence. In agreement with previous reports, the depth of invasion, lymphatic invasion, venous invasion, and lymph node metastasis were associated with tumor recurrence by univariate analysis (Table 2). In addition to these pathologic parameters, hyperfibrinogenemia and an elevated CEA level were positively associated with tumor recurrence, although they did not show independence by multivariate analysis. An elevated CRP level did not show a significant association with recurrence.
Impact of hyperfibrinogenemia on tumor recurrence in CRP-negative colorectal cancer
Hyperfibrinogenemia was significantly associated with a high CRP level in our series, as shown in Table 1 (p < 0.0001). However, 150 (37.6%) of 399 patients with normal CRP values also exhibited hyperfibrinogenemia. We next evaluated the association of clinicopathological variables with tumor recurrence in a CRP-negative population. Hyperfibrinogenemia was associated with tumor recurrence in this population by univariate analysis (Table 3). Multivariate analysis indicated that hyperfibrinogenemia had an independent association with tumor recurrence with an odds ratio (OR) of 1.98 [95% confidence interval (CI) 1.15–3.43, p < 0.05], whereas all of the other variables lost their independence. This relation was more marked when the site of recurrence was confined to a hematogenously metastasized organ (liver, lung, bone, brain) (OR 2.23, 95% CI 1.22–4.11, p < 0.01). Cox regression analysis showed that the plasma fibrinogen level was associated with recurrence-free survival among those with CRP-negative status—hazard ratio (HR) 1.38 (/100 mg/dl), 95% CI 1.07–1.78, p < 0.05—whereas no association was found in those with CRP-positive status (Table 4).
Discussion
Previous studies have indicated that patients with CRC often show abnormal coagulation parameters, including platelet counts [17] and D-dimer [18], fibrinopeptide A [19], fragment 1 + 2, thrombin–antithrombin complex, and soluble fibrin assays [20]. Those reports have also shown that the abnormalities of these coagulation factors are associated with worse clinical outcome [17, 18, 21]. In particular, preoperative plasma fibrin and fibrinogen degradation products (FDP) levels were increased in patients with metastatic diseases [21]. Because elevated plasma fibrin and FDP levels indicate enhanced fibrinogen conversion by activated coagulation cascade and fibrinolytic activity, these facts suggest that CRC cells have a potential to activate and interact with the host hemostatic system for progression.
Fibrinogen, an essential hemostatic factor, is converted to fibrin (a final product of the hemostatic pathway) by activated thrombin. Interestingly, however, a large-scale study to determine the association of plasma fibrinogen and CRC has not been documented, although a Chinese study described an increased fibrinogen level in advanced-stage CRC [22]. In this study, we found that the plasma fibrinogen level is positively associated with tumor recurrence and the T stage in a CRP-negative population. The plasma fibrinogen level was critically affected by the presence of inflammation, suggesting that the systemic inflammatory response of the host to CRC might elevate the plasma fibrinogen level. It has been shown that the serum level of CRP, another systemic imflammatory marker, is associated with distant metastasis as well as lymph node metastasis and consequently is correlated with a worse clinical outcome of the CRC [23]. An elevated serum CRP level has also been reported to be a biomarker for recurrence development with some types of tumor [24, 25]. However, it is still controversial whether a high CRP level is an independent predictor for poor prognosis [26–28]. In our study, a positive CRP value did not have any relation with the event of tumor recurrence or recurrence-free survival after curative surgical resection. However, among 115 patients with Dukes D CRC during the same period, 64 patients (55.7%) had a positive CRP value, and their clinical outcome was much worse than that of the patients in this study (data not shown). In contrast, the CRP-positive rate was much smaller in Dukes A (15.6%), Dukes B (34.3%), and Dukes C (29.3%) CRCs, which is consistent with other studies. This suggests that a high serum CRP level might indicate a systemic inflammatory response to the tumor burden according to the disease stage and may simply be a by-product of cancer spread, making it functionally irrelevant to the metastatic process.
Our data demonstrate that the fibrinogen levels are considerably different from CRP levels in CRC patients. In fact, 134 of 170 patients (78.8%) with positive CRP values showed hyperfibrinogenemia. However, 399 of 569 patients (70.1%) in this series were CRP-negative preoperatively even with CRC, and 150 of the 399 CRP-negative patients (37.6%) showed a high plasma fibrinogen level in our series. Moreover, a preoperative high plasma fibrinogen level was associated with tumor recurrence of CRP-negative CRC whereas it had no impact in the CRP-positive population. Both plasma fibrinogen and CRP are proteins synthesized predominantly in the liver. Both are up-regulated by proinflammatory cytokines, such as interleukin-6 (IL-6) and IL-1β [29]. However, our results raise the possibility that the plasma fibrinogen level is regulated differently from CRP to respond to the cancer as a systemic event in CRC patients. In fact, Kleemann et al. has shown that ciprofibrate, a statin drug that has been shown to reduce the risk of CRC development [30], decreases IL-1-induced CRP synthesis but does not affect IL-1-induced fibrinogen synthesis in human hepatocytes [31].
Hyperfibrinogenemia may enhance the metastatic process of CRC through several possible mechanisms. Recent studies in fibrinogen-deficient mice provided clear evidence that fibrinogen plays a crucial role in hematogenous and lymphatic metastasis of cancer cells [15, 16]. The role of fibrinogen in the development of hematogenous metastasis is mainly protection against natural killer (NK) cells [32]. Fibrinogen is a dimeric molecule with multiple integrin- or non-integrin-binding motifs, and malignant cells often express high levels of fibrinogen receptors, such as α5β1, αvβ3 integrins or intercellular adhesion molecule-1 (ICAM-1). If fibrinogen were to bind to ICAM-1 on endothelial cells, it might promote stable adhesion of tumor cells to the endothelium of target organs. In addition, tumor cells and platelets can form large aggregates through binding fibrinogen because platelet αIIbβ3 integrin receptors have a high affinity for fibrinogen. These aggregates effectively form microemboli in target organs, which can protect tumor cells from the innate immune system [33, 34]. Moreover, tumor cells have been shown to produce tumor procoagulant, causing fibrin formation around the tumor cell surface and thus make them adhere to the surrounding tissue [35]. In this situation also, hyperfibrinogenemia may be advantageous for tumor metastasis. In fact, hyperfibrinogenemia showed an independent association with lymph node and liver metastasis in human gastric cancer [12, 13]. Together with the data on CRC in this study, one can supposed that fibrin(ogen) may function to protect circulating cancer cells (from various malignancies) in the blood as well as those at other sites before the increasing tumor mass induces a systemic inflammatory response.
Conclusion
Hyperfibrinogenemia is clinically relevant to recurrence of CRC. Our results show that the plasma fibrinogen level may be considered a possible biomarker to predict disseminating tumor cells before distant metastasis manifests in patients without a systemic inflammatory response. CRC patients with hyperfibrinogenemia might be potential candidates for neoadjuvant/adjuvant chemotherapy.
References
Sakamoto J, Ohashi Y, Hamada C et al (2004) Efficacy of oral adjuvant therapy after resection of colorectal cancer: 5-year results from three randomized trials. J Clin Oncol 22:484–492
Laurie JA, Moertel CG, Fleming TR et al (1989) Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil: the North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol 7:1447–1456
Akasu T, Moriya Y, Ohashi Y et al (2006) Adjuvant chemotherapy with uracil-tegafur for pathological stage III rectal cancer after mesorectal excision with selective lateral pelvic lymphadenectomy: a multicenter randomized controlled trial. Jpn J Clin Oncol 36:237–244
Moertel CG, Fleming TR, Macdonald JS et al (1995) Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med 122:321–326
Chu DZ, Erickson CA, Russell MP et al (1991) Prognostic significance of carcinoembryonic antigen in colorectal carcinoma: serum levels before and after resection and before recurrence. Arch Surg 126:314–316
Wolmark N, Fisher B, Wieand HS et al (1984) The prognostic significance of preoperative carcinoembryonic antigen levels in colorectal cancer: results from NSABP (National Surgical Adjuvant Breast and Bowel Project) clinical trials. Ann Surg 199:375–382
Wang WS, Lin JK, Chiou TJ et al (2000) Preoperative carcinoembryonic antigen level as an independent prognostic factor in colorectal cancer: Taiwan experience. Jpn J Clin Oncol 30:12–16
Louhimo J, Carpelan-Holmstrom M, Alfthan H et al (2002) Serum HCG beta, CA 72-4 and CEA are independent prognostic factors in colorectal cancer. Int J Cancer 101:545–548
Harrison LE, Guillem JG, Paty P et al (1997) Preoperative carcinoembryonic antigen predicts outcomes in node-negative colon cancer patients: a multivariate analysis of 572 patients. J Am Coll Surg 185:55–59
Wanebo HJ, Rao B, Pinsky CM et al (1978) Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med 299:448–451
Takahashi T, Kato T, Kodaira S et al (1996) Prognostic factors of colorectal cancer: results of multivariate analysis of curative resection cases with or without adjuvant chemotherapy. Am J Clin Oncol 19:408–415
Yamashita H, Kitayama J, Nagawa H (2005) Hyperfibrinogenemia is a useful predictor for lymphatic metastasis in human gastric cancer. Jpn J Clin Oncol 35:595–600
Yamashita H, Kitayama J, Kanno N et al (2006) Hyperfibrinogenemia is associated with lymphatic as well as hematogenous metastasis and worse clinical outcome in T2 gastric cancer. BMC Cancer 6:147
Chen Z, Malhotra PS, Thomas GR et al (1999) Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res 5:1369–1379
Palumbo JS, Kombrinck KW, Drew AF et al (2000) Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 96:3302–3309
Palumbo JS, Potter JM, Kaplan LS et al (2002) Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res 62:6966–6972
Costantini V, Zacharski LR, Moritz TE et al (1990) The platelet count in carcinoma of the lung and colon. Thromb Haemost 64:501–505
Oya M, Akiyama Y, Okuyama T et al (2001) High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol 31:388–394
Abbasciano V, Bianchi MP, Trevisani L et al (1995) Platelet activation and fibrinolysis in large bowel cancer. Oncology 52:381–384
Iversen LH, Thorlacius-Ussing O (2002) Relationship of coagulation test abnormalities to tumour burden and postoperative DVT in resected colorectal cancer. Thromb Haemost 87:402–408
Blackwell K, Hurwitz H, Lieberman G et al (2004) Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer 101:77–82
Wang Q, Xie R, Zhang QY (2005) Clinical significance of plasma fibrinogen level in patients with colorectal cancer. Zhonghua Zhong Liu Za Zhi 27:544–546
Nozoe T, Matsumata T, Kitamura M et al (1998) Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg 176:335–338
Shimada H, Kitabayashi H, Nabeya Y et al (2003) Treatment response and prognosis of patients after recurrence of esophageal cancer. Surgery 133:24–31
Nakanishi H, Araki N, Kudawara I et al (2002) Clinical implications of serum C-reactive protein levels in malignant fibrous histiocytoma. Int J Cancer 99:167–170
Stamatiadis AP, St. Toumanidou M, Vyssoulis GP et al (1990) Value of serum acute-phase reactant proteins and carcinoembryonic antigen in the preoperative staging of colorectal cancer: a multivariate analysis. Cancer 65:2055–2057
Chung YC, Chang YF (2003) Serum C-reactive protein correlates with survival in colorectal cancer patients but is not an independent prognostic indicator. Eur J Gastroenterol Hepatol 15:369–373
McMillan DC, Canna K, McArdle CS (2003) Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg 90:215–219
Black S, Kushner I, Samols D (2004) C-reactive protein. J Biol Chem 279:48487–48490
Poynter JN, Gruber SB, Higgins PD et al (2005) Statins and the risk of colorectal cancer. N Engl J Med 352:2184–2192
Kleemann R, Gervois PP, Verschuren L et al (2003) Fibrates down-regulate IL-1-stimulated C-reactive protein gene expression in hepatocytes by reducing nuclear p50-NFkappa B-C/EBP-beta complex formation. Blood 101:545–551
Palumbo JS, Talmage KE, Massari JV et al (2005) Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105:178–185
Yano H, Kitayama J, Hatano K et al (2001) Clustered cancer cells show a distinct adhesion behavior from single cell form under physiological shear conditions. J Exp Clin Cancer Res 20:407–412
Nieswandt B, Hafner M, Echtenacher B et al (1999) Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59:1295–1300
Gale AJ, Gordon SG (2001) Update on tumor cell procoagulant factors. Acta Haematol 106:25–32
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan and by a grant from the Ministry of Health and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamashita, H., Kitayama, J., Taguri, M. et al. Effect of Preoperative Hyperfibrinogenemia on Recurrence of Colorectal Cancer Without a Systemic Inflammatory Response. World J Surg 33, 1298–1305 (2009). https://doi.org/10.1007/s00268-009-9992-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-009-9992-7