Abstract
Aims
We aimed at evaluating residual β-cell function in insulin-treated patients with type 2 diabetes (T2D) while determining for the first time the difference in C-peptide level between patients on basal–bolus compared to those on the basal insulin scheme, considered as an early stage of insulin treatment, together with assessing its correlation with the presence of complications.
Methods
A total of 93 candidates with T2D were enrolled in this cross-sectional study and were categorized into two groups based on the insulin regimen: Basal–Bolus (BB) if on both basal and rapid acting insulin, and Basal (B) if on basal insulin only, without rapid acting injections. HbA1c, fasting C-peptide concentration and other metabolic parameters were recorded, as well as the patient medical history.
Results
The average fasting C-peptide was 1.81 ± 0.15 ng/mL, and its levels showed a significant inverse correlation with the duration of diabetes (r = -0.24, p = 0.03). Despite similar disease duration and metabolic control, BB participants displayed lower fasting C-peptide (p < 0.005) and higher fasting glucose (P = 0.01) compared with B patients. Concentrations below 1.09 ng/mL could predict the adoption of a basal–bolus treatment (Area 0.64, 95%CI:0.521–0.759, p = 0.038, sensitivity 45% and specificity 81%).
Conclusions
Insulin-treated patients with long-standing T2D showed detectable level of fasting C-peptide. Measuring the β-cell function may therefore guide toward effective therapeutic options when oral hypoglycemic agents prove unsuccessful.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of type 2 diabetes (T2D) is increasing worldwide, mainly driven by that of obesity [1, 2], with significant repercussions on health and economy [3]. Many interventions are put into action to prevent or delay the onset of diabetes [4,5,6,7,8,9,10,11,12,13]; oral glucose lowering medications or non-insulin injectables are then used first to obtain glucose control upon diagnosis [14], whereas at later stages of progression insulin therapy is usually introduced [14]. The natural history of T2D is characterized by a progressive onset of hyperglycemia, resulting from a decline in β-cell function. It has been established that approximately 40–50% β-cell function is lost at the time of T2D diagnosis, with an additional estimated 4%–5% reduction each year afterwards [15]. Typically, the higher the magnitude of hyperglycemia, the worse the pancreatic impairment relative to insulin secretion. The Center for Disease Control (CDC) defines HbA1c ≥ 9% (75 mmol/mol) as poor control, and, according to the 2017 Healthcare Effectiveness and Information Set (HEDIS) data, a significant proportion of individuals fall into this category, making effective treatments urgently needed [16].
Insulin treatment is indicated in those who are no longer able to reach an appropriate HbA1c target through the use of oral antidiabetic drugs (OADs) and/or non-insulin injectables [14], and the introduction of basal insulin as add-on to OADs is usually suggested. This, in theory, is due to secondary β-cell failure, notwithstanding residual function not routinely assessed through direct measurement. Therefore, some patients run the risk of being prematurely or inappropriately treated with insulin, with the possible consequence of incurring into unwanted side effects such as weight gain and hypoglycemia [17]. Evaluating the residual β-cell function of insulin treated patients with T2M could aid in determining whether insulin therapy may be switched to other non-insulin therapeutic schemes, such as GLP-1 analogs or SGLT-2 inhibitors, possibly in association with the same insulin, a decision which is currently solely dependent on HbA1c levels.
Fasting plasma C-peptide is a well-established marker of residual β-cell function. Its values correlate with endogenous insulin production, type of diabetes, disease duration, and the age of the patient at the time of diagnosis [18].
This study aimed to evaluate the residual β-cell function in long standing insulin-treated T2D patients and to explore the utility of C-peptide measurement as a marker of diabetes related complications. Basal insulin usually is the first step in the initiation of insulin-based therapies, and this study for the first time identifies to what extent the C-peptide level differs between patients on basal–bolus compared to those on basal insulin scheme.
Materials and methods
Patients were consecutively enrolled at the outpatient service Unit of Endocrinology and Diabetes of Campus Bio-Medico University of Rome (UCBM). Inclusion criteria are as follows: a diagnosis of T2D according to ADA criteria [19]; age above 18 years; and current treatment with insulin. Those with a diagnosis of T2D following a pancreatectomy or whose T2D treatment had been modified within the preceding 3 months were excluded. Written consent from all participants was obtained before the commencement of the study. The study protocol was conducted in accordance with the principles of the Declaration of Helsinki and was approved by University Campus Bio-Medico’s ethical committee.
In this cross-sectional study, physicians examined the study subjects, and medical history was recorded; then, a registered dietitian measured anthropometric parameters. Body weight and height were obtained in the morning upon an overnight fast (last insulin injection at 8 pm the preceding day), and patients were allowed to wear light clothing but no shoes. The same stadiometer and calibrated scale were used for all patients. Fasting blood samples were collected by venipuncture, and were then transferred to the local laboratory, where glucose, HbA1c, C-peptide, total cholesterol, High-Density Lipoprotein (HDL), Low-Density Lipoprotein (LDL), triglycerides, and creatinine were measured according to the local standards of practice. The estimated glomerular filtration rate was calculated using the CKD-EPI formula.
The duration of T2D and a complete list of current pharmacological treatment were determined according to records retained by the physician and cross-checked with those provided by the patient. Presence of T2D related complications, namely cardiovascular disease, retinopathy, nephropathy, neuropathy, and also dyslipidemia, were assessed through in-person interview and physical examination, together with the consultation of their medical records, in accordance with current guidelines.
Patients were then categorized according to the treatment scheme: Basal–Bolus (BB group) if they were injecting with both basal insulin every 24 h and rapid acting insulin boluses at meal-time; and Basal (B group) if they only used basal insulin, with no rapid acting injections at meals.
The Statistical Package for Social Sciences (SPSS), v.20 was used for the statistical analysis. Results are presented as means ± SE. Variables were assessed for normality with the Kolmogorov–Smirnov test. Not normally distributed variables were log-transformed. A Student's t-test was used to compare subjects in the BB group with those in the B group. Chi-square or Fisher exact test were used for dichotomous variables analysis. A Pearson correlation method was used to analyze the correlation between continuous variables. A ROC area under curve (AUC) was performed to investigate which threshold of C-peptide level could predict the possibility of requiring a BB instead of a B only insulin treatment. Graphpad Prism was used to create graphs. P values < 0.05 were considered statistically significant.
Results
Ninety-three participants were enrolled, and patients’ features are summarized in Table 1. Briefly, mean age was 71.6 ± 0.9 years, BMI 30.84 ± 0.63 kg/m2, 55.7% were female, and mean disease duration was 20 ± 1 years, with an average fasting C-peptide of 1.81 ± 0.15 ng/mL. Most patients were on concomitant treatment with insulin and one or more OADs/non-insulin injectables such as metformin (47%), sulfonylureas (14%), DPP4-inhibitors (5.4%), GLP-1 RA (10%), and SGLT2 inhibitors (11%).
C-peptide levels showed a significant inverse correlation with the duration of diabetes (r = -0.24, p = 0.03; Fig. 1), but not with HbA1c and fasting glycaemia (Supplemental Fig. 1 A and B, respectively). Interestingly, fasting C-peptide levels were significantly higher in patients presenting nephropathy compared to those who did not (2.53 ± 0.38 ng/mL and 1.55 ± 0.13 ng/mL, respectively; P = 0.027, Supplemental Fig. 2) and trended higher in the uppermost quartiles of kidney failure stages (n of trials, Mean ± SE; Stage I 19, 1.58 ± 0.2 ng/mL; Stage II 34, 1.37 ± 0.2 ng/mL; Stage III 30, 2.1 ± 0.3 ng/mL; Stage IV 7, 2,4 ± 0.5 ng/mL, respectively; p = 0.06; Supplemental Fig. 3). A linear multivariate analysis showed that triglycerides (βs 0.828 95% CI (0.503–1.152) P < 0.0001, Supplemental Fig. 4) and duration of T2D (βs −0.055 95% CI (−0.088–0.022) P < 0.035, Supplemental Fig. 4) were the only significant markers of C-peptide concentration, even after adjustment for HDL, total cholesterol, kidney function, insulin requirement, HbA1c, BMI and age (see also Supplemental Fig. 4). HDL showed a trend increase with the rise of C-peptide concentration (βs 95% CI 0,387, (−0,0022–0,796), P = 0.063, Supplemental Fig. 4).
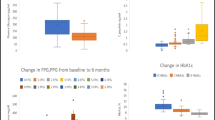
Despite a similar disease duration, participants belonging to the BB group displayed lower fasting C-peptide levels (1.51 ± 0.14 ng/mL vs.2.03 ± 0.22 ng/mL, respectively p < 0.005; Table 2, Fig. 2) and higher fasting glycemia (171 ± 8.71 mg/dL vs. 142 ± 6.73 mg/dL, respectively, P = 0.01; Table 2) compared to those in the B group. A receiver operating characteristic (ROC) curve showed that a C-peptide concentration below 1.09 ng/mL could predict the adoption of a basal–bolus treatment with a sensitivity of 45% and specificity of 81% (Area 0.64, SE 0.061, 95% CI 0.521–0.759, P = 0.038, Supplemental Fig. 5). Predictably, the insulin requirement for patients in the BB group was significantly higher compared to those in the B group (0.74 ± 0.06 IU/Kg vs. 0.08 ± 0.05 IU/Kg, p < 0.0001; Table 2). Moreover, the B group presented with an increased use of OADs (Table 2); although none of the patients in the B group was on SGLT2i therapy, while 10.8% of BB group was on this drug class (Table 2). Furthermore, the eGFR was significantly lower in the BB group compared to the B group (61.82 ± 3.01 ml/min vs. 72.53 ± 4.37 mL/min, respectively, P = 0.049; Table 2), with no difference observed regarding macrovascular complications, retinopathy, hypertension or dyslipidemia. Conversely, an increase in nephropathy and neuropathy among microvascular complications was observed in the BB group (p = 0.062 and 0.1, respectively; Supplemental Table 1).
Discussion
Nowadays, among other clinical features [20, 21], the clinical role of C-peptide is mainly that to exclude an absolute insulin deficiency at the time of diagnosis and despite its possible role in the T2D clinical management has been widely studied, still controversies remain. In addition, since basal insulin is usually the first step of insulin-based therapies, herein for the first time, we explored to what extent the C-peptide level differs between patients on basal–bolus compared to those on basal insulin scheme. In a recent systematic review by Shields et al., it was found that a fasting C-peptide of 0.2 nmol/l (0.6 ng/mL) could differentiate insulin requirement among patients diagnosed with T2D [22]. Nevertheless, an open debate remains whether C-peptide measurement can be introduced in clinical practice to detect residual β-cells function in poorly controlled patients with diabetes mellitus. In this respect, some studies evaluating conditions influencing the efficacy of non-insulin therapies and insulin treatment reported significant associations with fasting C-peptide [18, 23]. Similarly, stimulated C-peptide levels were found to predict a successful response to oral antidiabetic therapy after discontinuation of insulin [24]. A previous study observed that prandial insulin injection had a greater impact on glucose response in patients with a lower level of endogenous insulin (as defined by a fasting C-peptide < 0.29 nmol/l), but was less effective in those with higher C-peptide levels, suggesting that other glucose-lowering agents, possibly acting on insulin resistance in combination with basal insulin, might be considered for this subgroup of patients [25].
We report, as expected, that C-peptide correlated with the duration of diabetes. This finding is consistent with previous reports [18] and reflects the progressive decline of β-cells function (secondary failure) that characterizes the natural course of the disease. A 10-years-disease duration seems to be the threshold after which β-cell loss becomes permanent and difficult to restore when achieving normoglycemia [26]. Interestingly, our patients had a mean disease duration of 20 years and yet had detectable C-peptide, including those on a basal–bolus regimen. It could be therefore postulated that the onset of secondary failure may take place much later than previously reported. Our findings may have potential implications for the clinical management of T2D. ADA/EASD guidelines currently suggest adding insulin therapy based primarily on HbA1c levels, starting by adding basal insulin treatment when HbA1c levels are above target despite maximal doses of combined OADs/injectable non-insulin drugs, and further transition into a basal–bolus regimen in case the target is not reached [19]. As a result, insulin regimens are often improperly prescribed at an early stage of the disease when β-cell reserve is still present, unnecessarily exposing patients to unwanted side effects such as weight gain and hypoglycemia. Taking into account previous studies, we assume that, in our cohorts, fasting C-peptide levels may reflect a certain degree of residual β-cells function that might open the way for effective alternative therapies, when not contraindicated. Although there is evidence that insulin therapy improves β-cell function reducing glucotoxicity [27], a large body of preclinical and clinical data demonstrated that some glucose lowering agents, including dipeptidyl peptidase 4 (DPP-4) inhibitors [28], GLP1-RAs [29] and SGLT-2i [30], could be effective in slowing down β-cell loss, thereby improving the natural course and prognosis of the disease. The introduction of these cytoprotective medications could possibly be the reason why we found detectable C-peptide in patients with such a long-standing T2D, conversely to what observed in older studies. It should also be noted that most patients advancing toward poor glycemic control, and therefore being switched to insulin treatment, are often older adults, where the possible simultaneous presence of poor eyesight might predispose to dosing mistakes posing at increased risk of severe adverse consequences. Alternatives to insulin treatment should therefore be considered for the more favorable safety profile. Thus, in addition to the HbA1c evaluation, it may be critical to assess the residual β-cell function in order to identify who cannot avoid insulin treatment, currently considered the last resort of diabetes treatment. Moreover, we propose a C-peptide level cut-off of 1.09 ng/mL, under which the introduction of insulin boluses on top of basal insulin may be advisable.
Increasing evidences from other studies are revealing that C-peptide is associated with microvascular complications [31, 32]. In our cohort, no significant differences both in macrovascular and microvascular complications were found between the subgroups. However, fasting C-peptide levels showed significantly higher concentrations in subjects with nephropathy compared to those who did not. It is important that fasting C-peptide be interpreted with caution in case of renal impairment. In fact, C-peptide is partially removed by urine excretion, therefore its level can be falsely elevated in the case of renal failure [33]. In this study, the median value of eGFR was more than 60 mL/min in both B and BB groups, confirming a globally maintained renal function, but we are unable to exclude partial filtration impairment as a possible cause of the observed finding.
Intriguingly, triglycerides and disease duration were independently associated with C-peptide levels after adjustment for several possible confounders. The finding might be interpreted in light of the fact that serum triglycerides represent indirect markers of liver fat deposition and more generally of ectopic deposition of lipids. It is, in fact, well established that this is a crucial step in the development of insulin resistance, which is the main condition inducing increased insulin secretion [34]. Disease duration from the other side is well-known to lead to progressive cell failure, and the finding comes therefore not unexpected.
This study presents several limitations. The cross-sectional design prevented a longitudinal evaluation to predict C-peptide influence on the natural history of glycemic control, the possibility to delay insulin treatment and the development of complications. The small sample size is also a major limitation. Moreover, adult autoimmune forms based on the presence of anti-GAD65, anti-IA2, and anti-ZnT8 antibodies were not examined in our trials, and the inclusion of patients affected by these could have potential distortion on the study as a result of their different characteristics and clinical history. However, as the prevalence is very low among those with a classical disease progression, the risk of bias is very little. C-peptide levels from patients with impaired kidney function may have influenced the results; however, there was no significant different in C-peptide levels among the 4 stages of kidney failure. Besides, the small number of subjects on oral antidiabetic medications in the BB group did not allow for proper stratification to explore the role of oral anti diabetic medications on C-peptide levels between the two groups.
In conclusion, assessment of β-cell function may be useful in providing additional information on patients’ beta cells-status and in guiding toward effective therapeutic strategies both in patients incurring treatment failure with oral hypoglycemic agents and in those with long duration of disease. The use of an easy-to-measure and inexpensive marker such as fasting c-peptide should be therefore reconsidered in light of recent evidence.
Data availability
The data are available from the corresponding author on reasonable request.
References
World Health Organization. Health topics. Obesity. https://www.who.int/topics/obesity/en/. https://www.who.int/topics/obesity/en/. 2020
Watanabe M, Risi R, De Giorgi F et al (2020) Obesity treatment within the Italian national healthcare system tertiary care centers: what can we learn? Eat Weight Disord. https://doi.org/10.1007/s40519-020-00936-1
Hu FB (2011) Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34(6):1249–1257. https://doi.org/10.2337/dc11-0442
Basciani S, Camajani E, Contini S et al (2020) Very-low-calorie ketogenic diets with whey, vegetable or animal protein in patients with obesity: a randomized pilot study. J Clin Endocrinol Metab 105:336. https://doi.org/10.1210/clinem/dgaa336
Basciani S, Costantini D, Contini S et al (2015) Safety and efficacy of a multiphase dietetic protocol with meal replacements including a step with very low calorie diet. Endocrine 48(3):863–870. https://doi.org/10.1007/s12020-014-0355-2
Bruci A, Tuccinardi D, Tozzi R et al (2020) Very low-calorie ketogenic diet: a safe and effective tool for weight loss in patients with obesity and mild kidney failure. Nutrients. https://doi.org/10.3390/nu12020333
Soare A, Khazrai YM, Del Toro R et al (2014) The effect of the macrobiotic Ma-Pi 2 diet versus the recommended diet in the management of type 2 diabetes: the randomized controlled MADIAB trial. Nutr Metab (Lond). https://doi.org/10.1186/1743-7075-11-39
Look ARG, Pi-Sunyer X, Blackburn G et al (2007) Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 30(6):1374–1383. https://doi.org/10.2337/dc07-0048
Watanabe M, Gangitano E, Francomano D et al (2018) Mangosteen extract shows a potent insulin sensitizing effect in obese female patients: a prospective randomized controlled pilot study. Nutrients. https://doi.org/10.3390/nu10050586
Yilmaz Z, Piracha F, Anderson L, Mazzola N (2017) Supplements for diabetes mellitus: a review of the literature. J Pharm Pract 30(6):631–638. https://doi.org/10.1177/0897190016663070
Soare A, Del Toro R, Khazrai YM et al (2016) A 6-months follow-up study of the randomized controlled Ma–Pi macrobiotic dietary intervention (MADIAB trial) in type 2 diabetes. Nutr Diabetes 6(8):e222. https://doi.org/10.1038/nutd.2016.29
Watanabe M, Tuccinardi D, Ernesti I et al (2020) Scientific evidence underlying contraindications to the ketogenic diet: an update. Obes Rev. https://doi.org/10.1111/obr.13053
Tuccinardi D, Farr OM, Upadhyay J et al (2019) Lorcaserin treatment decreases body weight and reduces cardiometabolic risk factors in obese adults: a six-month, randomized, placebo-controlled, double-blind clinical trial. Diabetes Obes Metab 21(6):1487–1492. https://doi.org/10.1111/dom.13655
American Diabetes A (2019) 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care 42:S90–S102. https://doi.org/10.2337/dc19-S009
Bretzel RG, Eckhard M, Landgraf W, Owens DR, Linn T (2009) Initiating insulin therapy in type 2 diabetic patients failing on oral hypoglycemic agents: basal or prandial insulin? The APOLLO trial and beyond. Diabetes Care 32(Suppl 2):S260-265. https://doi.org/10.2337/dc09-S319
Centers for Disease Control and Prevention. National Diabetes Statistics Report (2020) Centers for disease control and prevention. US Department of Health and Human Services, Atlanta, GA
Swinnen SG, Hoekstra JB, DeVries JH (2009) Insulin therapy for type 2 diabetes. Diabetes Care 32(Suppl 2):S253-259. https://doi.org/10.2337/dc09-S318
Landin-Olsson M, Nilsson KO, Lernmark A, Sundkvist G (1990) Islet cell antibodies and fasting C-peptide predict insulin requirement at diagnosis of diabetes mellitus. Diabetologia 33(9):561–568. https://doi.org/10.1007/BF00404145
American Diabetes A (2020) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 43:S14–S31. https://doi.org/10.2337/dc20-S002
Sabbah E, Savola K, Ebeling T et al (2000) Genetic, autoimmune, and clinical characteristics of childhood- and adult-onset type 1 diabetes. Diabetes Care 23(9):1326–1332. https://doi.org/10.2337/diacare.23.9.1326
Di Stasio E, Maggi D, Berardesca E et al (2011) Blue eyes as a risk factor for type 1 diabetes. Diabetes Metab Res Rev 27(6):609–613. https://doi.org/10.1002/dmrr.1214
Shields BM, Peters JL, Cooper C et al (2015) Can clinical features be used to differentiate type 1 from type 2 diabetes? a systematic review of the literature. BMJ Open 5(11):e009088. https://doi.org/10.1136/bmjopen-2015-009088
Jones AG, McDonald TJ, Shields BM et al (2016) Markers of beta-cell failure predict poor glycemic response to glp-1 receptor agonist therapy in type 2 diabetes. Diabetes Care 39(2):250–257. https://doi.org/10.2337/dc15-0258
Lee A, Morley J (1999) Classification of type 2 diabetes by clinical response to metformin-troglitazone combination and C-Peptide criteria. Endocr Pract 5(6):305–313. https://doi.org/10.4158/EP.5.6.305
Jones AG, Besser RE, Shields BM, McDonald TJ, Hope SV, Knight BA, Hattersley AT (2012) Assessment of endogenous insulin secretion in insulin treated diabetes predicts postprandial glucose and treatment response to prandial insulin. BMC Endocr Disord. https://doi.org/10.1186/1472-6823-12-6
White MG, Shaw JA, Taylor R (2016) Type 2 diabetes: the pathologic basis of reversible beta-cell dysfunction. Diabetes Care 39(11):2080–2088. https://doi.org/10.2337/dc16-0619
Pieralice S, Pozzilli P (2018) Latent autoimmune diabetes in adults: a review on clinical implications and management. Diabetes Metab J 42(6):451–464. https://doi.org/10.4093/dmj.2018.0190
Foley JE, Bunck MC, Moller-Goede DL et al (2011) Beta cell function following 1 year vildagliptin or placebo treatment and after 12 week washout in drug-naive patients with type 2 diabetes and mild hyperglycaemia: a randomised controlled trial. Diabetologia 54(8):1985–1991. https://doi.org/10.1007/s00125-011-2167-8
Chon S, Gautier JF (2016) An update on the effect of incretin-based therapies on beta-cell function and mass. Diabetes Metab J 40(2):99–114. https://doi.org/10.4093/dmj.2016.40.2.99
Kaneto H, Obata A, Kimura T et al (2017) Beneficial effects of sodium-glucose cotransporter 2 inhibitors for preservation of pancreatic beta-cell function and reduction of insulin resistance. J Diabetes 9(3):219–225. https://doi.org/10.1111/1753-0407.12494
Leighton E, Sainsbury CA, Jones GC (2017) A Practical review of C-peptide testing in diabetes. Diabetes Ther 8(3):475–487. https://doi.org/10.1007/s13300-017-0265-4
Alves MT, Ortiz MMO, Dos Reis G et al (2019) The dual effect of C-peptide on cellular activation and atherosclerosis: protective or not? Diabetes Metab Res Rev 35(1):e3071. https://doi.org/10.1002/dmrr.3071
Covic AM, Schelling JR, Constantiner M, Iyengar SK, Sedor JR (2000) Serum C-peptide concentrations poorly phenotype type 2 diabetic end-stage renal disease patients. Kidney Int 58(4):1742–1750. https://doi.org/10.1046/j.1523-1755.2000.00335.x
Haffner SM, Miettinen H, Stern MP (1997) Are risk factors for conversion to NIDDM similar in high and low risk populations? Diabetologia 40(1):62–66. https://doi.org/10.1007/s001250050643
Acknowledgements
We are grateful to the study participants and the nurse Milena Rosati who contributed to patients’ management.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
PP SM DT RG designed the study. RG, DT, DM, AP, GD, SP, EF conducted research. DT and MW performed analyses. DT, SP, RG, MW, PP and SM wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethical approval
The study has been approved by the University Campus Bio-Medico Ethics Committee # 54/20 OSS ComEt CBM.
Informed consent
All patients signed an informed consent.
Consent for publication
The manuscript received the consent to be published.
Additional information
Managed by Antonio Secchi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dario, T., Riccardo, G., Silvia, P. et al. The utility of assessing C-peptide in patients with insulin-treated type 2 diabetes: a cross-sectional study. Acta Diabetol 58, 411–417 (2021). https://doi.org/10.1007/s00592-020-01634-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-020-01634-1