Abstract
Background and aims
Type 2 diabetes mellitus (T2DM) is characterized by progressive metabolic deterioration due to a decline in β-cell function. The relation between glucagon and insulin secretion in the setting of T2DM is still not clear. The present study evaluated relationship between glucagon level, glycemic status, and β-cell indices in newly diagnosed Indian T2DM patients.
Methods
Patients with HbA1c > 8.5% and/or fasting plasma glucose > 200 mg/dl were treated with insulin for 6 months (May 2017 to December 2018). Metabolic parameters and hormonal tests (insulin, glucagon, C-peptide in the fasting state, and 2-h meal stimulated C-peptide) were assessed at baseline and after 6 months of insulin treatment.
Results
Seventy-five newly diagnosed T2DM patients were enrolled in the study, of which 56 completed the study. At baseline a significant correlation was observed between fasting plasma glucagon and plasma glucose (r = 0.33, p = 0.04), insulin level (r = − 0.38, p = 0.004), and HOMA B (r = − 0.347, p = 0.009). After 6 months of insulin treatment, a significant (p = 0.001) change in glycemic status (− 35.28%), fasting glucagon level (− 45.84%), and an increase in fasting and meal stimulated C-peptide (144.44%) was observed. A significant inverse correlation between percentage change of plasma glucagon and plasma insulin was noted (r = − 0.282, p = 0.035).
Conclusion
Among newly diagnosed T2DM patients with moderate to severe hyperglycemia fasting glucagon correlated positively with glycemic status and negatively with insulin levels at baseline. Early and intensive insulin treatment was associated with good glycemic control and improvement in glucagon and insulin levels. A modest reciprocal correlation was noted between α- and β-cell function in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterized by chronic hyperglycemia as a result of insulin resistance and a progressive decline in β-cell mass and function, rendering most of the patients insulin-dependent with time [1, 2]. The normal relationship between β-cell function and insulin sensitivity is already disturbed by the time patients are diagnosed with hyperglycemia [3]. Severe hyperglycemia causes “glucotoxicity” which is a state of high and sustained glucose levels that paradoxically worsens both insulin secretion and insulin resistance [4]. The role of glucagon as a counter regulatory hormone in the glucose metabolism has been highlighted in Unger's glucagon−centric hypothesis of diabetes [5]. Elevated glucagon levels and impaired incretin axis contribute to the poor glycemic status in type 2 diabetes [6, 7]. Hyperglucagonemia among newly diagnosed T2DM correlates with measures of abdominal obesity [8]. Islets of Langerhans by modulating the activity of α−cells and β−cells control glucose production, triglyceride deposition, and protein synthesis. Thus, glucagon and insulin bipolar axis regulates the intermediary metabolic fuel balance [9, 10]. Early intensive glycemic control may prolong β−cell lifespan and function as well as reduce long−term vascular outcomes [10, 11]. Few studies reported the effect of insulin treatment on α−cells in newly diagnosed T2DM [12, 13]. Here we report results from a 24−week insulin treatment study, designed to explore the relationship between glucagon level, glycemic status, and β−cell indices in newly diagnosed Indian T2DM patients with moderate and severe hyperglycemia.
Materials and methods
Study design
This was a single-center, prospective, observational follow-up study conducted between May 2017 and December 2018 at the Department of Endocrinology of a medical college hospital, in southern India. Treatment naïve outpatients with newly diagnosed T2DM, between 18 and 65 years of age, with hemoglobin A1c (HbA1c), > 8.5% and < 12.5% and/or fasting plasma glucose (FPG) > 200 mg/dl and willing to give signed informed consent for study participation, were enrolled in this study. The sample size was calculated as below: A high prevalence of type 2DM has been reported in India, especially in the urban areas to the tune of 12.6% especially in the state of Andhra Pradesh [14, 15]. No statistical data on the prevalence of newly diagnosed type 2 DM is available on extensive literature search. Hence, on retrospective analysis at our center, we found the prevalence of type2 DM to be 12% and the newly diagnosed type 2 DM to be 4%, and at a confidence interval of 95% and a margin of error of 5%, a population proportion of 4%; we arrived at a sample size of 60 (n = 60) which is significant enough to analyze the outcome of the clinical study. Though the sample size is small, the power of the study is 0.8, as the insulin treatment is highly effective in controlling the sugars and the probability of error decreases, when detecting difference between post treatment and baseline when the true difference is high.
Patients with pregnancy, ketonuria or diabetic ketoacidosis (DKA), type 1 diabetes mellitus (T1DM), pancreatic diabetes, other cause of secondary diabetes, comorbidities, ongoing usage of steroids, immunomodulatory drugs, and with a history of smoking and alcohol consumption were also excluded. Baseline blood samples were obtained for FPG, 2-h postprandial glucose (PPG), HbA1C, lipid profile, blood urea, creatinine, liver function tests (LFT), and thyroid-stimulating hormone (TSH). Plasma was separated for assessment of fasting plasma insulin, C-peptide, and glucagon. Homeostasis model assessment of β-cell function (HOMA-β) was calculated with FPG, and insulin with the formula: 360 × fasting insulin (μU/mL) / (fasting glucose (mg/dL) − 63.
All patients were advised on appropriate medical nutrition treatment and lifestyle changes. Multiple insulin doses (MDI) were initiated at 0.5 U/kg/ day in divided doses which were titrated according to FPG and 2-h PPG to maintain glycemic targets as per ADA guidelines. The total duration of the study was 6 months. Patients were followed -up initially at days 15 and 30 and later monthly for 6 months. Fasting plasma glucose, PPG was done at every visit, HbA1c at 3 and 6 months. Plasma was separated from the blood sample after cold centrifugation at 2 °C for 10 min at 2000 rpm and immediately stored at –20 °C for analyzing insulin, C-peptide, and at –70 °C for glucagon. Plasma glucose was measured by the hexokinase method. HbA1c levels were measured using ion-exchange high-performance liquid chromatography technique (Bio-Rad Laboratories, Hercules, CA, USA) with an intra- and inter-assay coefficient of variation of 0.4% and 1.6%, respectively. C-peptide levels were determined by ELISA (Calbiotech Inc) with a cross reactivity less than 10%. Currently, most of the commercial and research kits have cross reactivity less than 10%. The analytical sensitivity of the test was 0.01 ng/ml, within-run precision expressed as the percentage coefficient with an intra- and inter-assay coefficient of variation of < 3.3% and 7.8%, respectively. Plasma glucagon levels were determined by a human GC ELISA kit [16]. The analytical sensitivity of the test was 2.40 pg/ml, and within-run precision was expressed as the percentage coefficient with an intra- and inter-assay coefficient of variation of < 8%and < 10%, respectively.
Ethical clearance
As we planned to study the relation between glucagon and insulin at diagnosis and the effect of insulin treatment after 6 months in these patients, we reasoned that OAD such as SU’s are insulin secretory agents, and DPP4 inhibitors have glucose-dependent insulin tropic effect and suppress endogenous glucagon; thus, both can affect the assay test results C-peptide and glucagon levels. Furthermore, insulin treatment is an anabolic physiologic replacement therapy and not known to affect the assay test results of endogenous beta cell secretory index C-peptide and glucagon assays. Hence, we chose insulin as first line of intervention in the newly diagnosed diabetic patients in our study. The study protocol and the informed consent form were reviewed and approved by the institutional ethics committee of Andhra Medical College, Visakhapatnam, Andhra Pradesh, India.
Statistical analysis
Qualitative and quantitative variables are presented using descriptive statistics. Quantitative variables were evaluated using a paired t-test at a 5% level of significance and the corresponding p value is presented. The correlation between variables was tested with the Pearson coefficient. p value < 0.05 was considered significant. Data were analyzed using SPSS® statistics software, version 25.0 (IBM Corp., Armonk, NY, USA).
Results
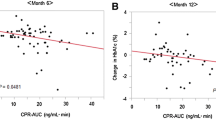
A total of 75 patients were enrolled in the study, out of which 56 patients (Male: Female— 30:26) with a mean (SD) age of 41.24 (5.64) years completed the study. The demographic and baseline characteristics of 56 patients are summarized in Table 1. At baseline a significant correlation was observed between fasting plasma glucagon and FPG (r = 0.323, p = 0.04), insulin level (r = − 0.379, p = 0.004), and HOMA B (r = − 0.347, p = 0.009) (Table 2). However, there was no correlation between fasting plasma glucagon and PPG (r = − 0.132 p = 0.332) and stimulated C-peptide (r = − 0.075 p = 0.582).
Changes in clinical and biochemical parameters of the study population after 6 months of insulin treatment are presented in Table 2. Six months after the initiation of insulin therapy, a significant improvement (p = < 0.001) in the glycemic and hormonal status was seen as indicated by a reduction of FPG (–35.28%), 2-h PPG (–46.84%), HbA1c (–27.43%), and fasting plasma glucagon (–45.84%), compared to baseline. Additionally, there was a significant increase (p = < 0.001) in fasting plasma insulin (43.86%), fasting C-peptide levels (78.04%), and meal-stimulated C-peptide levels (144.44%), from baseline to 6-month post-initiation of insulin treatment (Fig. 1).
At the end of the 6 months, there was a significant increase (p = < 0.001) in the BMI (2.77%) of the study population from as compared to the baseline. Insulin therapy also resulted in a significant decrease (p = < 0.005) in total cholesterol (–10.84%), low-density lipoprotein cholesterol (LDL-C) (–15.05%), triglyceride (–19.86%) levels, and a significant increase (p = < 0.005) in high-density lipoprotein cholesterol (HDL-C) (16.04%), levels.
At the end of 6 months of therapy, there was a significant correlation between the percentage reduction in glucagon and FPG (r = − 0.287, p = 0.032). The percentage change in stimulated C-peptide over 6 months also correlated significantly with FPG (r = − 0.271, p = 0.043), PPG (r = − 0.421, p = 0.001), and HbA1c (r = − 0.428, p = 0.001). There was a significant inverse correlation between the percentage change of plasma glucagon and the percentage change of plasma insulin (r = − 0.282, p = 0.035). However, no such relation was seen between percentage changes in PPG and stimulated C-peptides (Fig. 2).
Discussion
In the newly diagnosed T2DM patients α- and β-cells dysfunction contributes to hyperglycemia and glucotoxicity, either independently or in combination [17, 18]. The normal fasting plasma glucagon levels range from 50 to 100 pg/mL and may vary with different laboratory methods [19].
Kumpatla et al. demonstrated the progressively increasing levels of glucagon from normal glucose tolerance to impaired glucose tolerance in prediabetic and newly diagnosed diabetic individuals [20]. Zheng et al. also reported elevated mean fasting glucagon levels (105.2 pg/ml) in newly diagnosed T2DM patients [13]. With baseline fasting glucagon levels of 197.68 pg/ml, the results from our study are in line with the results reported earlier. At baseline, our observation of a positive correlation between fasting plasma glucagon and fasting glucose suggests a relation between hyperglucagonemia and fasting hyperglycemia. Similarly, a significant inverse correlation between glucagon with fasting insulin and HOMA-B demonstrated the reciprocal relation between α- and β-cell activity in uncontrolled newly detected T2DM patients in our study.
The effect of short-term insulin therapy on abnormal α-cell function in diabetes has been studied by Raskin et al., and the authors demonstrated a significant reduction in the immunoreactive glucagon concentrations (p < 0.02) with improved glucose control in comparison with the “uncontrolled” period [21]. Kramer et al. studied the effect of 4-week intensive insulin therapy on glucagon level and glycemic status among 62 patients with T2DM of 3–5 years duration. They observed a significant reduction in fasting hyperglucagonemia and glycemic variability at the end of treatment [22].
Likewise, in our study, 6-month multiple subcutaneous intensive insulin regimen in newly diagnosed T2DM patients resulted in a significant reduction (p = < 0.001) in the glycemic parameters and plasma glucagon. Moreover, there was a significant correlation between percentage reduction in glucagon and FPG (p = 0.032) following treatment, suggesting a relation between glycaemic status and α-cell activity. A review of the studies targeting β-cell function early in the course of therapy for T2DM indicated that short-term intensive insulin therapy preserves endogenous insulin secretion and promotes metabolic control [17].
Weng et al. in a multicenter randomized study of 382 newly diagnosed T2DM patients, aged 25–70 years, reported that compared to oral hypoglycaemic agents, early intensive insulin therapy (MDI vs CSII) facilitates recovery and maintenance of β-cell function and induces protracted glycemic remission at 1-year after diagnosis. The authors concluded that the shorter period of prior glucotoxicity is more likely to result in the restoration of β-cell function with early insulin therapy [23]. However the standard policy at our institute is to offer insulin treatment to all newly diagnosed type 2 diabetic patients with severe hyperglycemia Hba1c > 10%, to reduce glucotoxicity and improve metabolism, But for this study purpose, we have devised a protocol to initiate insulin therapy for newly diagnosed patients with moderate to severe hyperglycemia. Our study demonstrated that the insulin treatment over 6 months resulted in a significant improvement in the glycaemic status with a 43.86% increase in fasting plasma insulin compared to baseline. Similarly, fasting and meal-stimulated C-peptide levels showed significant improvement of 78.04% and 144.4% respectively from baseline (p = < 0.001). In addition, a significant correlation between the percentage change in stimulated C-peptide over 6 months and glycemic parameters FPG (p = 0.043), PPG (p = 0.001), and HbA1c (p 0.001), was demonstrated in our study suggesting clear relation between glucotoxicity and β-cell function. No relation between improvement in insulin and C-peptide levels and weight gain was seen at the end of the treatment.
Physiologically, insulin and glucagon may work in tandem to maintain normal metabolic fuel balance. The relative glucagon and insulin concentrations modulated by α- and β-cell activity control endogenous glucose production, triacylglycerol deposition, and protein synthesis [9]. A low insulin:glucagon ratio (IGR) as is characteristic of T2DM stimulates the mobilization of stored nutrients, and glycogenolysis, promoting the breakdown of adipose tissue and gluconeogenesis. Biosynthesis of proteins, inhibition of glucose production, and reduction of free fatty acid release are seen normally with a high IGR state [24].
Previous studies focusing on the interrelation between α-cell activity, glycemic status, and insulin secretion markers in newly diagnosed diabetes treated with insulin, demonstrated improvement in individual parameters with glycemic control. However, no association could be documented among them. Kramer et al. performed an oral glucose tolerance test after 4 weeks of intensive insulin therapy in early T2DM patients and demonstrated improvement in the area under the curve (AUC) of glucagon and insulin sensitivity index [22]. However, no relation was seen between the improvement in α-cell function and β-cell functioning indices. Zeng et al. demonstrated similar findings in the 3-month study with intensive insulin therapy [12].
In contrast to these findings, our study demonstrated with good glycemic control, there is a moderate correlation between percentage reduction in plasma glucagon and percentage increment in plasma insulin indicating a relationship between β-cell function and glucagon levels among newly diagnosed T2DM patients treated with insulin. The difference in observations obtained in our work from previous studies could be because we analyzed percentage changes in FPG, fasting glucagon, fasting insulin, and stimulated C-peptide over 6 months, whereas the previous studies analyzed AUC of glucose and meal stimulated values of glucagon respectively. The area under the curve (AUC) is the measure of the ability of a classifier to distinguish between classes and is used as a summary of the ROC curve.
Percentage change equals the change in value divided by the absolute value of the original value, multiplied by 100. Since we compared 6 months post insulin treatment values with the baseline, we used percentage change in order to depict the magnitude of the change.
Though a significant improvement in fasting, insulin, and C-peptide levels and BMI are seen at 6 months compared to baseline, the quantum increase in percentage change of plasma insulin is huge (43.86%), while the percent change in BM1 is only 2.77% which, even otherwise is expected with insulin treatment in diabetic patients. Furthermore, the calculated HOMA IR values as derived from the baseline mean plasma insulin and fasting glucose values as projected in Table 2 at baseline and at 6 months are 2.5 and 1.90 respectively indicating improvement in insulin sensitivity at 6 months. Hence, the weight gain does not seem to have contributed to the insulin resistance.
Our study documented evidence that chronic hyperglycemia has a negative influence on β-cell function and increases α-cell activity, suggesting dysfunction of both at baseline. Besides that the early and effective insulin treatment in newly diagnosed T2DM patients has a beneficial effect on these parameters suggesting improved combined α- and β-cell function at 6-months after therapy. In addition, a significant reduction in mean total daily insulin dose requirement was also seen at 6 months.
The limitations of the study are its open-label observational method rather than a randomized pattern, lack of comparative treatment arm, and fewer patients. Dynamic testing of glucagon and insulin secretion was not done. We have not done hyper insulinemic euglycemic clamp to study insulin sensitivity as we primarily focused on insulin secretion indices and its relation to glucagon levels at baseline and after insulin treatment. The gold standard Mixed Meal Tolerance Test (MMTT) could not be used in our study, as the liquid mixed meals used in major research trials (e.g. Sustacal™ and Boost™) are not easily obtainable in many countries, although it is likely that preparations with broadly similar nutritional content will be interchangeable. [25]. Hence, we did a mixed meal test modified to local cuisine with a calorie and protein, and fat nutrient constitution to provide calories of 350 cal per meal.
The strength of the study is the prospective treatment-related follow-up for 24 weeks and well-documented correlative data between baseline and 6 months. These observations highlight the relation between glycemic status, β-cell function, and glucagon level in newly diagnosed T2DM patients, treated with insulin. The observations from our study also provide supportive evidence for an early treatment-related benefit from a pathophysiologic perspective. However, further detailed evaluation of functional indices of α- and β-cells would be beneficial in understanding islet dysfunction in newly diagnosed T2DM patients.
Conclusion
Among the newly diagnosed T2DM patients with moderate to severe hyperglycemia fasting glucagon correlated positively with glycemic status and negatively with insulin levels at baseline. Early and intensive insulin treatment in these patients was associated with good glycemic control and improvement in glucagon and insulin levels. A modest reciprocal correlation was noted between α- and β-cell function in these patients. The observations from our data provide strength to the recommendation of early insulin initiation in the newly diagnosed type 2 diabetes especially in those with moderate to severe hyperglycemia.
References
Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. https://doi.org/10.1007/s00125-002-1009-0.
Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, et al. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37:1751–8.
Kahn SE. The importance of the beta-cell in the pathogenesis of type 2 diabetes mellitus. Am J Med. 2000;108(Suppl):6a:2S–8S. https://doi.org/10.1016/s0002-9343(00)00336-3.
Kaiser N, Leibowitz G, Nesher R. Glucotoxicity and beta-cell failure in type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2003;16:5–22.
Unger RH, Orci L. Role of glucagon in diabetes. Arch Intern Med. 1977Apr;137(4):482–91 (PMID: 403869).
D’Alessio D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes Metab. 2011;13(Suppl 1):126–32.
Fujioka K. Pathophysiology of type 2 diabetes and the role of incretin hormones and beta- cell dysfunction. JAAPA. 2007;Suppl:3–8. https://doi.org/10.1097/01720610-200712000-00001.
Anoop S, Misra A, Bhatt SP, Gulati S, Mahajan H, Prabakaran G. High plasma glucagon levels correlate with waist-to-hip ratio, suprailiac skinfold thickness, and deep subcutaneous abdominal and intraperitoneal adipose tissue depots in nonobese Asian Indian males with type 2 diabetes in North India. J Diabetes Res. 2017;2017:2376016. https://doi.org/10.1155/2017/2376016.
Unger RH. Glucoregulatory hormones in health and disease. A teleologic model Diabetes. 1966;15:500–6.
Kalra S, Gupta Y. The insulin:glucagon ratio and the choice of glucose-lowering drugs. Diabetes Ther. 2016;7:1–9.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Vilsbøll T, Knop FK, Krarup T, Johansen A, Madsbad S, Larsen S, et al. The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide-regardless of etiology and phenotype. J Clin Endocrinol Metab. 2003;88:4897–903.
Zheng HL, Xing Y, Li F, Ding W, Ye SD. Effect of short-term intensive insulin therapy on α-cell function in patients with newly diagnosed type 2 diabetes. Medicine (Baltimore). 2020;99: e19685. https://doi.org/10.1097/MD.0000000000019685.
Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research–INdia DIABetes (ICMR–INDIAB) study. Diabetologia. 2011;54:3022–7.
Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narnia K, Das HK, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–96.
Chernecky C, Berger B. Glucagon - plasma In Laboratory Tests and Diagnostic Procedures. 6th ed. p. 580–1. St Loius: Elsevier Saunders; 2013.
Kramer CK, Zinman B, Retnakaran R. Short-term intensive insulin therapy in type 2 diabetes mellitus: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013;1:28–34.
Walker JN, Ramracheya R, Zhang Q, Johnson PR, Braun M, Rorsman P. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes Metab. 2011;13(Suppl 1):95–105.
Hædersdal S, Lund A, Knop FK, Vilsbøll T. The role of glucagon in the pathophysiology and treatment of type 2 diabetes. Mayo Clin Proc. 2018;93:217–39.
Kumpatla S, Parveen R, Murugan P, Juttada U, Devarajan A, Viswanathan V. Hyperglucagonemia and impaired insulin sensitivity are associated with development of prediabetes and type 2 diabetes - a study from South India. Diabetes Metab Syndr. 2021;15: 102199. https://doi.org/10.1016/j.dsx.2021.102199.
Raskin P, Unger RH. Effect of insulin therapy on the profiles of plasma immunoreactive glucagon in juvenile-type and adult-type diabetics. Diabetes. 1978;27:411–9.
Kramer CK, Zinman B, Choi H, Retnakaran R. Effect of short-term intensive insulin therapy on post-challenge hyperglucagonemia in early type 2 diabetes. J Clin Endocrinol Metab. 2015;100:2987–95.
Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–60.
Unger RH. Glucagon and the insulin: glucagon ratio in diabetes and other catabolic illnesses. Diabetes. 1971;20:834–8.
Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013Jul;30(7):803–17. https://doi.org/10.1111/dme.12159.PMID:23413806;PMCID:PMC3748788.
Acknowledgements
We thank AP RSSDI for supporting the novel study and Sai’s Institute of Endocrinology for providing Laboratory and Technical support.
Funding
Partial funding by the Andhra Pradesh state chapter of Research Society for Study of Diabetes in India [RSSDI].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study was conducted in conformity with the principles of the Declarations of Helsinki, International Council for Harmonization-Good Clinical Practices (ICH-GCP) guidelines, Indian Council of Medical Research, Indian GCP guidelines, and as per the approved protocol. Written informed consent was obtained from all study participants, before being examined for eligibility criteria.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Type 2 DM is characterized by elevated glucagon level and relative insulin deficiency.
• Fasting glucagon correlates positively with glycemic status and negatively with insulin levels in T2DM.
• Good glycemic control with early Insulin treatment improves insulin and glucagon level.
• Modest reciprocal correlation was noted between α- and β-cell function in these patients.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Makineni, A.C., Mudimela, M., CM, S.S. et al. Study of relationship between glucagon level, glycemic status, and β-cell function in newly diagnosed T2DM patients, treated with insulin. Int J Diabetes Dev Ctries 43, 765–771 (2023). https://doi.org/10.1007/s13410-022-01154-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-022-01154-4