Abstract
To investigate safety, compliance, and efficacy, on weight loss and cardiovascular risk factors of a multiphasic dietary intervention based on meal replacements, including a period of very low calorie diet (VLCD) in a population of obese patients. Anthropometric parameters, blood tests (including insulin), dual-energy-X-ray absorptiometry (DXA), and questionnaires for the assessment of safety and compliance before and after (phase I) a 30-day VLCD, 700 kcal/day, normoproteic, 50 g/day carbohydrate, four meal replacements; (phase II) a 30-day low calorie diet (LCD), 820 kcal/day, three meal replacements plus a protein plate; (phase III) 60-day LCD, 1,100 kcal/day, two meal replacements plus two protein plates and reintroduction of small amounts of carbohydrates; (phase IV) 60-day hypocaloric balanced diet (HBD), 1,200 kcal/day, one meal replacement, two protein plates and the reintroduction of carbohydrates. 24 patients (17 females, 7 males, mean BMI 33.8 ± 3.2 kg/m2, mean age 35.1 ± 10.2 years) completed the study. The average weight loss was 15.4 ± 6.7 %, with a significant reduction of fat mass (from 32.8 ± 4.7 to 26.1 ± 6.3 % p < 0.05) and a relative increase of lean mass (from 61.9 ± 4.8 to 67.1 ± 5.9 % p < 0.05). An improvement of metabolic parameters and no variations of the liver and kidney functions were found. A high safety profile and an excellent dietary compliance were seen. The VLCD dietary program and the replacement dietary system described here is an effective, safe, and well-tolerated treatment for weight control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a chronic disease. Treatment of obesity includes hypocaloric diet, exercise, lifestyle modifications, use of endoscopic device (e.g., intragastric balloon), drugs, and bariatric surgery [1–3]. The therapeutic benefit of all currently available anti-obesity interventions is often limited by their subjective efficacy, variable tolerability, safety profiles, and poor compliance, the latter being a hard limiting variable, especially when long-term treatments are needed. In addition to obesity, diets play a pivotal role in the clinical treatment of a bunch of health conditions, such as dyslipidemia, diabetes, cardiovascular diseases, hypertension, and many others [4].
Often, the poor efficacy in terms of adequate and prolonged weight loss is ascribable to difficulties in the adherence to the diets. Furthermore, most patients expect fast weight reduction with the least possible effort. Therefore, they often follow unsafe, although popular dieting programs, which may be also dangerous for their health [5]. A series of programs have been studied to guide the overweight-obese patients throughout a reduction of body weight and a long lasting maintenance of such reduction. Unfortunately, clinical experience and scientific data suggest that this target is difficult to achieve in a high percentage of cases [5, 6]. Furthermore, an effective program for weight reduction should be followed for long time [7]. Emerging evidences suggest that rapid initial weight loss results in better long-term weight loss maintenance [8]. On the other hand, one of the well-known obstacles to which nutritionists are familiar with is the so called “good start”, with adequate loss of weight in the beginning, followed by a shutdown of the weight descent soon after. This may depend on factors ranging from the metabolism rate of the patient to poor compliance. To overcome these obstacles and to give additional impulse to weight descent, the employment of alternatively balanced diets has been proposed [9–12]. A reduction of carbohydrates and lipids, associated with a correct consumption of protein, allows to reduce the mass of adipose tissue through the rebalancing of the insulin-glucagon ratio in favor of lipolysis [9, 13]. The very low calorie diets, (VLCDs) consist in a complete replacement of regular meals with food or formulations that provide 400–800 calories daily [14, 15]. They are commonly used under medical supervision in patients with BMI > 30 (> 27 < 30 in the presence of co-morbidities related to obesity), or in subjects that need a rapid weight loss. To preserve lean mass the VLCDs provide at least 0.8 g of protein per kg of ideal body weight per day in addition to the recommended daily allowances of vitamins, minerals, trace elements, and essential fatty acids; 10 g/die dietary fat to stimulate gallbladder contraction, 50 g/die of carbohydrates, to maintain a normal blood sugar levels, to prevent electrolytes leakage, and to control ketogenesis are also recommended [16, 17]. Recent data indicate that provision of meals and use of meal-replacement products promote greater weight loss [18]. In addition, to increase patient’s compliance, attention is now paid to the development of products with the highest possible degree of palatability [19, 20].
We evaluated safety, adherence, acceptability, and efficacy, on weight loss and cardio-metabolic risk factors of a commercially available multiphase, four-stage sequence, dietary intervention based on meal replacements, which also includes an initial period of VLCD followed by phases of dietary education based on a programed reintroduction of carbohydrates in a group of obese patients. The phases, over a 6 months period, were chosen to exploit the potential of a VLCD initial phase and to develop a progressive reintroduction of food to achieve a hypocaloric balanced diet (HBD). Weight loss, body composition, cardio-metabolic risk, and improvement of the insulin resistance were studied.
Methods and procedures
Selection of the population and study design
The protocol was 6 months, single-center (Center for the Study of Eating Disorders and Obesity, Department of Experimental Medicine, Section of Medical Pathophysiology, Food Science and Endocrinology of the Sapienza University of Rome, Italy), open-study including 27 obese out-patients. Inclusion criteria were: age between 18 and 50 years and body mass index (BMI) between 30 and 40 kg/m2 (Fig. 1). Exclusion criteria were: known hypersensitivity to one or more components used in the protocol products; history of renal, cardiac, cerebrovascular, or gastrointestinal diseases; psychiatric disturbances; diagnosis of insulin-dependent diabetes mellitus (IDDM), or non-IDDM (NIDDM); thyroid dysfunctions; pregnancy; lactation; vegetarian habit, and lack of informed consent.
Body weight, height, blood pressure (systolic and diastolic), heart rate, waist, and hip circumference were measured at baseline, every 2 weeks and at the end of the trial. Weight was measured using a balance-beam scale (Seca, UK), with the subject wearing undergarments and no shoes, and with an empty bladder. Height was measured in the standing position, without shoes and corrected to the closest 0.5 cm. BMI was calculated, as weight divided by squared height (kg/m2). Waist circumference was measured midway between the costal arch and the iliac crest, hip circumference was measured at the symphysis-trochanter femoris level to the closest 1.0 cm. All measurements were obtained by trained staff using calibrated equipments.
Blood and urine chemistry
Complete blood count (CBC), electrolytes (chloride, calcium, potassium, sodium, magnesium), fasting glucose and insulin, lipids (total and fractionated cholesterol and triglycerides), protein, blood urea nitrogen (BUN), uric acid, creatinine, alanine transferase (AST), aspartate transaminase (ALT) were determined at baseline and after the first, second, fourth, and sixth month. Urine tests, using Multistix Reagent strips and Ketostix strips (Bayer, Germany) were performed at baseline, every 2 weeks and at the end of the protocol.
Dual-energy-X-ray absorptiometry (DXA) measurement
Body composition, body fat mass (FM), and fat-free mass (FFM), were measured by DXA (Hologic 4500 RDR) at baseline and at the end of the trial. Trunk fat, a surrogate markers of visceral fat, was defined as the adipose tissue localized within the region below the chin, delineated by vertical lines within the left and right glenoid fossae bordering laterally to the ribs, and by the oblique lines that cross the femoral necks and converge below the pubic symphysis. All measurements for each parameter were gathered by the same investigator.
Treatment
The diet program was divided into four phases. In the first phase, 27 patients were given a 1 month period of normoproteic VLCD of 700 kcal/day, with carbohydrates intake of 50 g/day, including 4 or 5 meal replacement protein preparations, with low carbohydrates (Protiligne, Penta s.r.l., Cuneo, Italy), a habitual drink for breakfast, 1 serving of vegetables at lunch and dinner, without quantitative, but only qualitative limits (vegetables with low glycemic index) to avoid constipation. To ensure higher food palatability, the use of 1–2 tablespoons of extra virgin olive oil per day, wine vinegar, herbs and spices, according to personal taste, was permitted.
The second phase consisted, in a 1 month period, of low calorie diet (LCD) of 820 kcal, including three meal replacements and a protein dish.
In the third phase, which lasted 2 months, an LCD which granted a daily caloric intake of 1,100 kcal, including two meal replacements, two protein plates and the reintroduction of small amounts of carbohydrates (1 fruit, vegetables without quantitative and qualitative limits and 1 serving of low-fat dairy) were assigned. In the fourth phase, which lasted 2 months, patients were given a HBD of 1,250 kcal/day, with 1 meal replacement only, two protein plates, and the reintroduction of carbohydrates (bread and pasta).
Since at the beginning of the program consuming fruits was not permitted and the amount of vegetables was limited, the patients were instructed to assume a multi-vitamin and multi-saline supplement formulated to maintain the physiological acid/base balance (PentaCal, Penta, s.r.l., Cuneo, Italy). Furthermore, it was recommended to introduce an adequate sodium chloride supply, when possible, in order to prevent any drop in blood pressure and to drink not less than 1.5–2 l of water per day.
The meal replacement preparations were formulated to obtain a balanced protein content, equal to 15–18 g/product so that a daily protein intake equals to 1–1.2 g/kg of ideal body weight was granted. In addition, they also contained carbohydrates equivalent to 50 g/day, in combination with vegetables. All products had an average content of 18 g of proteins.
Medications containing sugar, syrups as well as various vitamins and supplements were prohibited. The patients were instructed to avoid the intake of sweets and/or sugar-free gums, soft drinks (even without added sugar), herbal teas containing pieces of fruits, preserved vegetables (often containing sugar), balsamic vinegar, barley, and onions.
All patients had scheduled visits at week 0 (baseline) and every 2 weeks thereafter. In addition, patients were given support and counseling to enhance their compliance. Eating habits, dietary compliance and side effects were checked by interviews through the use of appropriate questionnaires [21] at baseline and every 2 weeks, while more frequent contacts were offered if needed. All the information and paperwork necessary for the study, including the personal diary to identify possible problems associated with the intake of meal replacements, the questionnaires on compliance, for the assessment of side effects and about the food acceptability, were provided. The patients were also instructed to have moderate-intensity physical activity (e.g., 30 min walking every day) during the first month of the study.
At the end of the study a written “exit” questionnaire about ease and convenience to follow the protocol was requested from each subject. An overall opinion about the treatment, according to the following scheme referred to the degree of satisfaction was required: poor = 1; average = 2; moderate = 3; good = 4; excellent = 5.
Data management and statistical methods
The estimated sample size for one sample comparison of mean to hypothesized value was calculated [22]. The collection and processing of data were performed by the use of commercial softwares (Excel, SPSS Statistics 22.0). Data obtained are expressed as mean values ± SD and finally processed to ascertain statistical significance. The analysis of variance (ANOVA) at different times was used to evaluate efficacy and safety data. P values < 0.05 were considered statistically significant.
Ethical aspects
The protocol was approved by the local Ethic Committee (protocol n. 2555/13.12.2012). The study was conducted in accordance with the Declaration of Helsinki (http://www.wma.net/en/30publications/10policies/b3/). In addition, it was the responsibility of the investigators and the sponsor to ensure that the study was conducted in accordance with the requirements of Good Clinical Practice (Ministerial Decree of 15/07/97).
Each subject was informed about all the aspects related to the participation in the study, in terms of objectives and procedures of the study, possible benefits and potential risks associated with the voluntary participation. An informed consent was signed by both the investigator and the individual patient before the beginning of the study. The subjects were informed about the option to leave the study at any time, without penalty and/or loss of benefits.
Results
27 patients (20 females and 7 males) aged between 20 and 49 years (mean = 35.1 ± 10.2 years) were enrolled in the study. 3 female patients were voluntarily dropped-out, for reasons of not connected to the study. The number of patients eligible to the final evaluation was 24 (17 females and 7 males).
Anthropometric measures, body composition and blood pressure
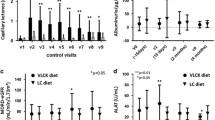
The differences of anthropometric measures, weight, and blood pressure at the beginning of the program and during the four phases are shown in Table 1. The multiphasic dietary intervention determined a profound reduction of the initial body weight (−14.7 ± 6.4 kg, range 8.0–35.7 kg). A significant reduction of BMI, waist circumference, and blood pressure was also seen. A detailed monthly analysis of weight changes at each phase, allowed us to identify differences depending on the type of diet. The mean weight loss was 15.4 % with an average descent rate of 2.45 kg/month. As expected, the pattern of weight loss was greater during the initial phases of dietary treatment. Figure 2 shows the modifications of body composition of the patients at the beginning and at the end of the observation period. A significant decrease in percentage of fat mass and trunk fat between pre- (T0) and post-treatment (T + 180) was found. Interestingly, an increase in the percentage of lean mass was constantly observed.
Blood tests and urine chemistry
Table 2 shows the clinical chemistry and blood count values. BUN, creatinine, uric acid, AST, ALT, HDL cholesterol, and mean CBC did not change significantly during the phases of the study. Glucose, insulin, triglycerides, and total cholesterol, showed a statistically significant progressive reduction. Electrolytes values did not vary significantly (data not shown). The reductions in fasting glucose and insulin, caused a significant improvement in the HOMA-IR.
Urinary pH values, showed variations within the normal reference intervals (not shown), consistent with the phases of the nutrition program that paralleled the amount of acid versus alkaline foods ingested. The urinary ketone body measurements increased significantly during the VLCD phase (19.6 ± 8.2 mg/dl at the end of the first phase of the program, 1 month from the beginning of the VLCD, vs absent urinary ketone body at baseline) and returned to the normal basal values in the subsequent steps.
Evaluation of the questionnaires
The questionnaires on compliance and for the assessment of safety were administered at the beginning and during the different phases of the study. A daily diary for the identification of potential problems associated with the intake of the preparations was also given. At the end of the study, a questionnaire on the ease in following program and a questionnaire for the overall evaluation were also administered.
The questionnaire on compliance showed 95 % adherence. It is noteworthy that the unanimity of patients considered the program as “easy to use”. On the contrary, much more variability, even in a negative sense, was obtained with the answers concerning the taste and palatability of individual products, given the large differences in anyone’s taste.
The questionnaire for the safety assessment showed a low incidence of side effects, with a prevalence of the adverse event “acetone breath” in the first and second steps (60.3 %) and a prevalence of “sense of hunger” (75 %) in the third and fourth steps (Fig 3).
The questionnaire on the ease in the following program showed that the diet program was evaluated “very easy” by 5 % of patients, “easy” by 4 % and “not easy” by 4 %.
Finally, all subjects who completed the study were asked to give a subjective evaluation on the overall aspects of the diet program they just ended, using an arbitrary score. 35 % of patients considered the program “excellent”, 35 % “good”, 21 % voted it as “moderate”, 4 % as “average” and no patient rated it as “poor”. 100 % of patients would recommend this diet program to other people.
Discussion
We evaluated safety, efficacy, and compliance of a 6 months multiphase dietetic protocol, based on sequential very low carbohydrate, low fat, normal protein meals, in a group of obese out-patients. The main difference between the dietary intervention used here and similar calorie restriction procedures consists in the progressive transition from VLCD to a HBD with a concomitant balance of the nutrients. Clinical examination showed the maintenance of a good general health status during the entire length of the study. Electrolyte concentrations, renal, and hepatic parameters showed no significant changes, indicating a high safety profile of the program. During the first 2 months, corresponding to the first and second phases of the program, the most frequent side effect was acetone breath, an event consistent with moderate to mild ketosis. In rare cases, headache was also present, which rapidly disappeared with small amounts of carbohydrates.
During the third and fourth phases (LCD and HBD) of the study, an increased number of patients felt hungry (+75 %) compared to the previous phases (+16.6 %, in the VLCD period). The gradual reintroduction of carbohydrates could explain these results. These data are in agreement with the hypothesis that a limited intake of carbs (50 g/day) and lipids (15 g/day) may reduce and normalize the levels of circulating insulin, while activating the β-oxidation process of lipids stored in the adipose tissue. A controlled ketogenesis, which induces a decrease in the sense of hunger and fatigue (anorectic and euphoric effect at the hypothalamic level), promoting patient’s adherence to the diet, is therefore obtained [23, 24]. Diet-induced weight loss is usually accompanied by compensatory changes which increase appetite and encourage weight regain, while there is evidence that ketogenic diets suppress appetite [8]. Indeed, a diet low in carbohydrates limits the feeling of hunger, influencing the activity of anorexigenic and orexigenic hormones and, therefore, adjusting the signals of satiety and appetite [23]. Such a dietary approach can reduce the circulating levels of postprandial ghrelin (a gastric peptide that stimulates appetite) and increase yy33-36 peptide, leptin sensitivity and GH production which participate to the satiety-promoting mechanism [24]. The data collected herein confirm previous studies, since in our patients the feeling of hunger that accompanies weight reduction during a LCD, was strongly mitigated by ketosis.
The mean weight loss was 15.4 % (14.7 ± 6.4 kg in 6 months) with an average descent rate of 2.45 kg/month and, in particular, −6.1 kg/month in the first phase, −3.1 kg/month during the second phase and −1.4 kg/month in the third and fourth phases. Being a multiphasic dietary intervention based on meal replacements, including a period of VLCD, it is difficult to compare the results obtained here with what observed in other studies. However, diets lasting for 6 months (including low carbohydrate/high-protein, classical low-fat, low carbohydrate diets) show a range of weight loss between 3.2 and 12.00 kg [25], confirming the efficacy of our dietary intervention. In addition, we recorded a great subjective variability, both in terms of weight loss and descent rate. Accordingly, patients who lost a total of 8 kg, with a mean global descent rate of 1.3 kg/month and patients who otherwise lost up to a total of 35.7 kg, with a global average rate of descent of about 6 kg/month were seen. At the same time, a 12 % change in waist circumference was observed. 57 % of the male patients and 65 % of the female patients experienced a reduction of waist circumference from > 102 to < 94 and from > 88 to < 80 cm, respectively. These results are important, being the decrease in waist circumference closely related to the reduction of cardiovascular risk. The evaluation of a surrogate markers of visceral fat such as trunk fat % confirmed the reduction of fat mass in these areas.
An average 5.2 % relative increase of patients’ lean body mass by DXA was seen. No clear explanation is apparent for the maintenance of lean body mass despite the reduction in caloric intake and the rapid weight reduction; however, the protective effect of the assumption of about 1.2 g/kg of ideal body weight of proteins might explain these findings [26].
A significant reduction of blood glucose, insulin, HOMA, and triglycerides was also found. Interestingly, the reduction in HOMA-IR up to the normalization of the index, both in patients with higher weight loss and in those in which the weight loss was less evident, indicates the involvement of an adjusting mechanism, at least partly uncoupled from weight reduction. An explanation could rely on diet composition since several healthy diets particularly, when restricted in calories and carbohydrates are consistently associated with a significant reduced risk of metabolic diseases [27, 28].
High level of general compliance, ease in following the program and overall appreciation by patients were observed. In this context, it is important to underline the difficulty to maintain a low calorie dietary regimen without meal replacements longer than a few days, without dropouts. Our patients followed a VLCD for an entire month, indicating that the low carbs content of the VLCD used herein and the meal replacements, reducing hunger are the key elements to the high compliance obtained here.
There are concerns on the use of VLCDs; they have been associated with adverse events like gallstones formation and even sudden deaths. A further concern is the weight regain, a common event in the current diet treatment methods of obesity [29–31]. However, recent studies reported a persistency of weight loss higher than 10 % of the initial weight after a VLCD at one year follow-up [32]. There were no dropouts due to side effects and the side effects recorded were transient and well tolerated.
In conclusion, the results of the present study, focused on a dietary multiphase program, emphasize that the program is an effective approach when a controlled body weight reduction is required. Weight descent has proved to be sufficiently fast during the first month of treatment that contributes in ensuring maximum adhesion to the program.
The ease in the handling and preparation of meal replacements was important to attain a good compliance. However, it is noteworthy not to underestimate the data concerning the palatability, which was quite variable and not widely accepted.
Therefore, this program may be reasonably considered as a valuable, safe, and well-tolerated tool for the treatment of weight excess and for the rehabilitation of obese patients toward a more appropriate lifestyle. After 1 month of VLCD, the slow, gradual reintroduction of carbohydrates, to re-accustom the body to manage incrementing levels of carbohydrates, up to the achievement of the correct quota provided an adequate food setting. This approach could maintain weight reduction also in the long-term follow-up, thereby overcoming the critical limitation of many diets that is the recovery of the lost weight within a short time [32].
The study has some limitations. It involves a unicentre source of patients that may introduce some selection bias and baseline differences. A more extended follow-up is required to evaluate the long-term efficacy of the intervention. Finally, we are aware that our results should be confirmed in larger cohorts.
References
D.W. Haslam, W.P. James, Obes. Lancet. 366, 1197–1209 (2005)
V.I. Kraak, B. Swinburn, M. Lawrence, P. Harrison, An accountability framework to promote healthy food environments. Public Health Nutr. 25, 1–17 (2014)
L.M. Donini, M. Cuzzolaro, L. Gnessi, C. Lubrano, S. Migliaccio, A. Aversa et al., Obesity treatment: results after 4 years of a Nutritional and Psycho-Physical Rehabilitation Program in an outpatient setting. Eat Weight Disord. 1 Mar (2014)
S. Desroches, A. Lapointe, S. Ratté, K. Gravel, F. Légaré, S. Turcotte, Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst. Rev. 2, CD008722 (2013). doi:10.1002/14651858
M.J. Munsters, W.H. Saris, Body weight regulation and obesity: dietary strategies to improve the metabolic profile. Annu. Rev. Food Sci. Technol. 5, 39–51 (2014)
S. Melotto, Clinical pharmacology of eating and not eating. Curr. Opin. Pharmacol. 14C, 1–5 (2014)
N. Vogels, P. Westerterp, Successful long-term weight maintenance: a 2-year follow-up. Obesity (Silver Spring) 15, 1258–1266 (2007)
C. Rolland, K.L. Johnston, S. Lula, I. Macdonald, J. Broom, Long-term weight loss maintenance and management following a VLCD: a 3-year outcome. Int. J. Clin. Pract. 68, 379–387 (2014)
S. Ramage, A. Farmer, K. Apps Eccles, L. McCargar, Healthy strategies for successful weight loss and weight maintenance: a systematic review. Appl. Physiol. Nutr. Metab. 39, 1–20 (2014)
R.U. Pliquett, D. Fuhrer, The effect of insulin on the central nervous system-focus on appetite regulation. Horm. Metab. Res. 38, 442–446 (2006)
D. Weigle, P. Breen, C. Matthys, A high protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 82, 41–48 (2005)
R. Vettor, R. Fabris, Neuroendocrine regulation of eating behavior. J. Endocrinol. Investig. 25, 836–854 (2002)
P. Björntorp, Obes. Lancet 350, 423–426 (1997)
S. Rössner, J.S. Torgerson, VLCD a safe and simple treatment of obesity. Lakartidningen 97, 3876–3879 (2000)
W.Y. Lin, C.H. Wu, N.F. Chu, Efficacy and safety of very-low-calorie diet in Taiwanese: a multicenter randomized, controlled trial. Nutrition 25, 1129–1136 (2009)
P. Dhindsa, A.R. Scott, R. Donnelly, Metabolic and cardiovascular effects of very-low-calorie-diet therapy in obese patients with type 2 diabetes in secondary failure: outcome after 1 year. Diabet. Med. 20, 319–324 (2003)
G.F. Cahill Jr, R.L. Veech, Ketoacidosis? Good medicine? Trans. Am. Clin. Climatol. Assoc. 114, 149–161 (2003)
K. Casazza, K.R. Fontaine, A. Astrup, L.L. Birch, A.W. Brown, M.M. Bohan Brown et al., Myths, presumptions, and facts about obesity. N. Engl. J. Med. 368, 446–454 (2013)
K.R. Ryttig, H. Flaten, S. Rössner, Long-term effects of a very low calorie diet (Nutrilett) in obesity treatment. A prospective, randomized, comparison between VLCD and a hypocaloric diet + behavior modification and their combination. Int. J. Obes. Relat. Metab. Disord. 21, 574–579 (1997)
J.L. Childs, M.D. Yates, M.A. Drake, Sensory properties of meal replacement bars and beverages made from whey and soy proteins. J. Food Sci. 72, S425–S434 (2007)
S. Sette, C. Le Donne, R. Piccinelli, L. Mistura, M. Ferrari, C. Leclercq, INRAN-SCAI 2005–06 study group. The third National Food Consumption Survey, INRAN-SCAI 2005–06: major dietary sources of nutrients in Italy. Int J Food Sci Nutr. 64, 1014–1021 (2013)
D. Machin, M.J. Campbell, S.B. Tan, S.H. Tan, Sample Size Tables for Clinical Studies, 3rd edn. (Wiley-Blackwell, Oxford, 2009)
P. Sumithran, L.A. Prendergast, E. Delbridge, Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur. J. Clin. Nutr. 67, 1–6 (2013)
Y. Gu, A. Zhao, F. Huang, Y. Zhang, J. Liu, C. Wang et al., Very low carbohydrate diet significantly alters the serum metabolic profiles in obese subjects. J. Proteome Res. 12, 5801–5811 (2013)
M. Hession, C. Rolland, U. Kulkarni, A. Wise, J. Broom, Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes. Rev. 10, 36–50 (2009)
A. Paoli, A. Rubini, J.S. Volek, K.A. Grimaldi, Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 67, 789–796 (2013)
K. Esposito, P. Chiodini, MI. Maiorino, G. Bellastella, D. Panagiotakos, D. Giugliano. Which diet for prevention of type 2 diabetes? A meta-analysis of prospective studies. Endocrine 18, (2014). doi:10.1007/s12020-014-0264-4
L. Velázquez-López, E. González-Figueroa, P. Medina-Bravo, I. Pineda-Del Aguila, L. Avila-Jiménez, R. Ramos-Hernández et al., Low calorie and carbohydrate diet: to improve the cardiovascular risk indicators in overweight or obese adults with prediabetes. Endocrine 43, 593–602 (2013)
N.B. Bueno, I.S. Vieira, S. Lima, T. Rocha, Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br. J. Nutr. 110, 1178–1187 (2013)
C.B. Ebbeling, J.F. Swain, H.A. Feldman, W.W. Wong, D.L. Hachey, E. Garcia-Lago et al., Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 307, 2627–2634 (2012)
S.G. Camps, S.P. Verhoef, K.R. Westerterp, Weight loss, weight maintenance, and adaptive thermogenesis. Am. J. Clin. Nutr. 97, 990–994 (2013)
B. Moreno, D. Bellido, I. Sajoux, A. Goday, D. Saavedra, A.B. Crujeiras, F.F. Casanueva, Comparison of a very low-calorie-ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine (2014). doi:10.1007/s12020-014-0192-3
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Basciani, S., Costantini, D., Contini, S. et al. Safety and efficacy of a multiphase dietetic protocol with meal replacements including a step with very low calorie diet. Endocrine 48, 863–870 (2015). https://doi.org/10.1007/s12020-014-0355-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0355-2