Abstract
Magnetic Resonance Imaging is a fundamental tool in the evaluation of soft tissue sarcoma. Imaging features are relevant for the assessment of treatment strategies, surgical planning and also for patients’ prognosis prediction. Among soft tissue sarcoma and also other malignancies, the size of the mass is usually considered the prognostic key element in diagnostic imaging. Moreover, several other features should be obtained from MRI studies with prognostic implications in all type of soft tissue sarcoma: peritumoral enhancement, signs of necrosis, deep location, ill-defined borders/signs of infiltrations. Focusing on soft tissue sarcoma subtypes, some other magnetic resonance imaging features are more specific and related to prognosis. In myxofibrosarcoma the magnetic resonance imaging “tail sign” and a “water-like” appearance on fluid-sensitive sequences, due to rich myxoid matrix content, are both associated with higher risk of local recurrence after surgical excision; nevertheless, the “tail sign” is also related to a higher risk of distant metastases at diagnosis. The “tail sign” is associated with higher risk of local recurrence after surgical excision in undifferentiated pleomorphic sarcoma as well. In patients affected by synovial sarcoma, the “triple sign” identifiable in magnetic resonance imaging (T2w sequences) is associated with decreased disease-free survival and indicates the simultaneous presence of solid cellular elements (intermediate signal intensity), hemorrhage or necrosis (high signal intensity) and fibrotic regions (low signal intensity). In addition, absence of calcifications are associated with reduced disease-free survival in patients affected by synovial sarcoma. Signal heterogeneity is associated with worst prognosis in all type of soft tissue sarcoma, particularly in myxoid liposarcoma. In recent years, several new quantitative tools applied on magnetic resonance imaging have been proved to predict patients’ prognosis. Above all the new tools, radiomics seems to be one of the most promising, and, has been proved to have the capability in discriminating low-grade from high-grade soft tissue sarcomas. Therefore, magnetic resonance imaging studies in patients with soft tissue sarcoma should be accurately evaluated and their results should be taken into account for prognostic assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soft tissue sarcomas (STS) are a rare (about 1% of all adult malignancies) and heterogeneous group of ubiquitous tumors arising from the embryonic mesoderm [1], with male preponderance ratio of approximately 1.4:1 [2,3,4].
Etiology of the majority of STS is unknown, but several genetic syndromes, like Li-Fraumeni syndrome or Neurofibromatosis type 1, and also environmental risk factors, like ionizing radiation or chemical exhibitors, are related to an increased risk [5,6,7].
Anatomically, the extremities are the most common site for STS, with a 28% prevalence in the lower limb and 12% in the upper limb. In particular, the thigh is the most common site in the body (44% of all extremity-located STS) [8]. One-third of STS are superficially located, above the fascial plane [9].
According to multidisciplinary and multimodality treatment and histological subtype, 10–20% of these tumors recur locally and distant metastases develop in about 30% of the patients [1].
It is of recognized importance to assess the prognosis of a tumoral mass. Imaging plays a key role at this purpose. For STS, MRI is above all the most appropriate imaging tool at this aim. Although large size and high histological grade of the lesions are the two most known prognostic factors for STS, MRI can offer a wide range of information in terms of prognosis.
The aim of this review article is to summarize the most important MRI features associated with patients’ prognosis generally and, in the main STS subtypes.
Subtypes and main features
About two thirds of the sarcomas are histologically classified as liposarcoma, leiomyosarcoma, synovial sarcoma, myxofibrosarcoma, undifferentiated pleomorphic sarcoma, rhabdomyosarcoma, and malignant peripheral nerve sheath tumors (Table 1) [10].
Liposarcomas and leiomyosarcomas are the two most common STS subtypes, accounting both for up to 25% of all newly diagnosed STS [11].
Liposarcoma shows adipocytic differentiation and can potentially occur anywhere in the body, even if 65–75% of cases are located in the extremities [12]. In the recent 2020 update of World Health Organization (WHO) Classification of Soft Tissue Tumors, soft tissue liposarcomas are divided into five distinct histologic subtypes: atypical lipomatous tumors/well-differentiated, dedifferentiated, myxoid, pleomorphic and myxoid pleomorphic [13].
Leiomyosarcoma arises from the smooth muscle cells or from the mesenchymal cells that are committed to become smooth muscle cells in future [14]; they are sub-classified into uterine leiomyosarcomas, soft tissue leiomyosarcomas (cutaneous, major vessel, and deep soft tissue leiomyosarcomas, and bone leiomyosarcomas). Deep soft tissue leiomyosarcomas are sub-divided into retroperitoneal and somatic leiomyosarcomas, which include leiomyosarcomas arising in the extremities and trunk [14,15,16]. Rhabdomyosarcoma is an aggressive STS of striated muscle that derives from undifferentiated mesenchymal cell. It is the most common STS in children, accounting for about 40% of cases, and it rarely occurs in adults, where it usually affects the extremities [17,18,19].
Synovial sarcoma is the fourth most common type of STS, accounting for 2.5–10.5% of all primary soft tissue malignancies, and it’s a mesenchymal neoplasm with prevalent onset in children and young adults. Despite its aggressive nature with high metastatic potential, it is a slow-growing tumor, which presents as a deep mass that sometimes causes painful symptoms in patients [20].
Undifferentiated pleomorphic sarcoma, previously known as malignant fibrous histiocytoma, was the most common type of STS until the classification system has become more restrictive and it currently accounts for 10–20% of STS in adults. Undifferentiated pleomorphic sarcoma can occur in anybody district, but they have a predilection for extremities (lower and upper extremities are 50% and 20% respectively); most undifferentiated pleomorphic sarcomas are of high-grade neoplasm and up to 50% show metastasis at diagnosis.
Myxofibrosarcoma is one of the most frequent STS in the elderly, usually located in the extremities and characterized by a very high rate of local recurrence, and relatively low risk of distant metastases [21].
Magnetic resonance imaging related to soft tissue sarcoma
According to American College of Radiology (ACR) appropriateness Criteria guideline, MRI is the most appropriate imaging technique to detect and evaluate STS, especially with the use of contrast medium, that highlights pathologic enhancement [22, 23].
Advanced imaging sequences can improve standard MRI protocols, including functional MRI with dynamic contrast enhancement (DCE) and diffusion-weighted imaging (DWI). These tools increase sensitivity and specificity especially for local recurrence detection [23].
MRI represents the gold standard technique for the preoperative evaluation of STS, for its intrinsic high-spatial resolution that allows optimal evaluation of tumor extension and its relationship with adjacent structures [24].
MRI signs highly suggestive of malignancy are deep-seated large mass, heterogeneous appearance, surrounding edema and cortical bone erosion.
Moreover, MRI is essential for tumor staging and should be performed before biopsy, since it ensures that other compartments are not contaminated and that image interpretation is not affected by post biopsy edema or hemorrhage [25]. MRI is extremely valuable in locating neurovascular structures and defining specific muscular compartments being affected, as well as showing the spread of individual muscles by the tumor. These factors play an essential role in determining the tumor’s resectability and the surgical margins preliminary evaluation [24].
Different types and subtypes of STS have different features on MRI. Liposarcomas reduce fat-component with progressive dedifferentiation. Well-differentiated liposarcomas demonstrate an adipose mass (high signal intensity with T1- and T2w, suppression with fat–sat technique) representing over 75% of the lesion and components of low signal intensity, that distinguish it to lipoma [26], as thick septa (> 2 mm) with irregular aspect and gadolinium-enhancement or nodular foci [27]; pleomorphic liposarcoma is characterized by a relatively well-defined (even if infiltrative margins may also be seen on adjacent soft tissues) non-adipocytic mass, peritumoral edema, internal necrosis, and/or internal hemorrhage; dedifferentiated liposarcoma appears as fat-containing masses with a prominent soft tissue component, thick septations, intense heterogeneous enhancement, necrosis, and edema; myxoid liposarcoma looks like a well-delineated lobules located in the intermuscular space, of low signal intensity with T1w and marked high signal intensity with T2w, in relation to the adjacent muscle. The mixed-type liposarcoma represents a combination of two histologic subtypes of liposarcomas within the same tumor [27].
Rhabdomyosarcoma is generally hypo to isointense on non-contrast T1w images, with marked heterogeneous enhancement after gadolinium injection, and iso- to hyperintense (necrotic areas) on T2w sequences. Unlike other extremity sarcomas, they may be associated with regional and distant lymph-node metastases mimicking lymphoma and epithelial malignancies [28].
Synovial sarcoma typically appears on T2w as heterogeneous (for the presence of areas of low, intermediate and high signal intensity) multi-lobed soft tissue mass; calcifications are present in about 33% of cases and better detected on Computed Tomography (CT) than MRI [29]. After gadolinium injection, synovial sarcoma shows an intense and heterogeneous contrast enhancement [30].
Undifferentiated pleomorphic sarcoma is typically a large and well-circumscribed mass, located within or in proximity to muscles, that produces a compressive effect on adjacent structures. It shows a low to intermediated signal on T1w (similar to adjacent muscle) and intermediate to high signal on T2w; gadolinium injection shows an enhancement of solid component; it could show “tail sign” such as myxofibrosarcoma [31].
Myxofibrosarcoma usually presents low signal intensity on T1w (lower than normal muscle) and variable high signal intensity on water-sensitive sequences, depending on the content of myxoid matrix (a watery and gelatinous substance) that is a predominant component of myxofibrosarcoma and other malignant soft tissue neoplasm (e.g., round cells/myxoid liposarcoma) or benign lesions like ganglion cysts or myxoma; unlike simple fluid collection or ganglion cyst, however, myxofibrosarcoma presents significant enhancement after gadolinium injection [32].
General MRI features related to prognosis
Various prognostic systems are in use, most of which are based on combinations of tumor size, histological malignancy grade, necrosis, and vascular invasion [33].
MRI studies can help in assessing some of the above-mentioned prognostic factors (e.g., tumor size, necrosis, location) and, according to the recent literature in this regard, should offer several additional prognostic information [9, 11].
Tumor size
Tumor measurement is one of the key elements of oncologic imaging evaluation [34]. First of all, staging is usually influenced by radiologic measurements, and the results directly influence diagnostic and treatment algorithms. Nevertheless, tumor size is an independent prognostic factor for several malignancies including STS [34].
Tumor size is defined as the largest diameter of the tumor and can be considered a tumor-related mortality predictor when a cut off value of 5 cm is considered (P < 0.001) [34,35,36,37]. It is one of the most consistently reported prognostic factors also for disease-free survival and metastatic recurrence, being significant if greater than 5 cm [34]. Unlike metastatic recurrence, the relation between tumor size and local control is still controversial, some studies didn’t demonstrate an increased risk of local recurrence related to tumor size [32, 34], showing, instead, a close relation with histological subtypes [35,36,37].
Tumor location
Soft tissue sarcoma can potentially occur at any anatomical site within the human body. According to the last AJCC 8th edition staging, different classification is considered depending on the location of primary tumor [38], particularly four tumor locations are described: extremity and trunk, retroperitoneum, head and neck, and visceral sites. Majority of these tumors originate in the extremities (about 32% in the lower and 13% in the upper). Nearly one third is retroperitoneal or intra-abdominal. Thoracic and head and neck sites are rare [6]. Several studies show that location is an important prognostic factor for mortality/overall survival but not for local recurrence risk prediction [6].
One of the imaging findings that are strongly associated with the diagnosis of high-grade sarcoma include proximal distribution, with worst prognosis compared to distal ones [21, 39,40,41]. Tateishi reported in his study, conducted in a cohort of 30 patients diagnosed with synovial sarcoma, a higher disease-free survival rate at 5 years after diagnosis in patients with distal localization than in those with proximal ones (89% and 23% respectively) [42]. Moreover, retroperitoneal liposarcoma showed worst prognosis compared to other locations. Nowadays the role of tumor depth as a prognostic factor is a matter of debate. In fact, in the AJCC (American Joint Committee on cancer) 8th edition staging system one of the major changes concerns tumor depth (superficial or deep from the investing fascia), which is no longer considered a staging factor [43]. The difference as a prognostic factor between superficial and deep lesions may be considered related to the easier diagnosis of superficial ones when they are still small sized [38]. Despite this new classification system, several recent studies still found that deep localization is a worst prognostic factor, and this could be also related to a more difficult surgical approach. In fact, recent series focused on myxofibrosarcoma, revealed that deep localization, beneath the fascial plane, is associated with poorer sarcoma specific survival and increased risk of distant metastases at diagnosis compared to superficial ones [21, 41,42,43,44]. In addition to this, superficially located leiomiosarcomas have a better prognosis than deep located lesions [45, 46].
Compartmentalization is defined as whether or not the tumor is located in a well-defined fascial compartment. Tumors growing infiltratively into more than one compartment or also involving superficial tissue are considered extra-compartmental; this is also factor related to a possible worst prognosis [38].
Peritumoral enhancement
Among the different MRI imaging features that can help to discriminate high-grade sarcomas from low-grade lesions, the presence of peritumoral contrast enhancement is a characteristic of high-grade varieties [41,42,43,44].
Peritumoral enhancement, defined as contrast enhancement at T1-w imaging after gadolinium injection beyond the apparent tumor borders without mass effects, is associated with infiltration [44].
A recent large series by Crombé et al. [44] focused on different subtypes of STS, revealed that MRI peritumoral enhancement is the factor that most correlate with high histological grade (odds ratio, 3.4; P = 0.003), followed by signs of necrosis (odds ratio, 2.4; P = 0.03) and heterogeneous signal intensity on T2w sequences (odds ratio, 2.3; P = 0.04). The presence of at least two of these MRI features was an independent predictor of metastasis-free survival (hazard ratio, 4.5; P = 0.01) and overall survival (hazard ratio, 4.2; P = 0.04) [44].
Moreover, in a series of 157 STS, patients with abnormal MRI peritumoral flow voids showed poorer overall survival (P = 0.039) and metastases-free survival (P = 0.014) compared with ones with normal flow-voids [47].
The MRI evaluation of surrounding tissue in STS is also relevant for the assessment of neoadjuvant chemotherapy response, as highlighted by Crombé et al. in a study of 57 patients with newly diagnosed high-grade STS of trunk wall or extremities, without metastasis. They analyzed the features of MRI images at baseline and after therapy, demonstrating that both stable or increased oedema and contrast-enhanced oedema in surrounding tissue are predictors of poor response (OR = 6.87, p = 0.011 and OR = 8.06, p = 0.008, respectively) [48].
Tumor necrosis
Necrosis degree in STS is correlated with survival and it is the most applied assessment of response available to clinicians treating solid tumors with neoadjuvant therapy [47]. Macdermed et al. reported the analysis of potential prognostic factors considering a cohort of 34 patients with STS, finding a significant association between freedom from distant metastasis and treatment-induced necrosis. According to their results, higher degrees of necrosis (> 90%) after chemotherapies are associated with increased distant metastases-free survival (p = 0.029) [49]. Monsky et al. [50] showed that this parameter is better evaluated with semi-automated volumetric segmentation of MRI images of necrotic tumor that allows non-invasive depiction of the entire lesion and can be performed early in the course of therapy and serially throughout therapy.
Definition of tumor margin and peripheral growth pattern
On non-enhanced images high-grade tumors usually have a poorly defined margin, while low-grade tumors have a well-defined margin [44] In order to support these statements, Zhao et al. [51] demonstrated a comprehensive evaluation of the accuracy of all available MRI features images, taking into account 95 patients with STS’s diagnosis. They established that a poorly or partly defined tumor margin on non-enhanced and contrast-enhanced T1-weighted images, (P < 0.01) indicates tumor’s cell infiltration of surrounding tissues, proving the invasive and aggressive nature of the lesion [51].
MRI features related to prognosis in specific histotypes
Some specific subtypes of STS present peculiar MRI signs related to patients’ prognosis. The most known and recognized above all, is the so-called “tail sign” that can be found particularly in myxofibrosarcoma and less frequently in undifferentiated pleomorphic sarcoma and can be considered a sign of infiltration [52].
“Tail sign” of undifferentiated pleomorphic sarcoma and myxofibrosarcoma
“Tail sign” is represented by a curvilinear tumor spread, usually along the fascial plane, or just by a thickened enhancing fascia; the presence of “tail sign” is associated with a higher risk of local recurrence after excision and possibly distant metastases at diagnosis [31, 52].
In the largest analysis involving 150 patients affected by myxofibrosarcoma (mean follow-up 16 ± 28.3 months), Spinnato et al. [41] found that the “tail sign” is the second independent predictor, after tumor size, of local recurrence after excision (P = 0.045). Moreover, in the same series, the presence of “tail sign” was also strongly associated with distant metastases at diagnosis (P < 0.001) [41].
Fascial invasion on MRI is an independent prognostic factor for worse prognosis; the deep peripheral fascia represents the deep limit of the subcutaneous compartment and constitutes a natural barrier to tumor spread. The tendency of a soft tissue tumor to cross the fascia may express aggressiveness, and therefore, might be associated with more aggressive tumor biology and metastatic potential [8, 51].
“Water-like” appearance of myxofibrosarcoma
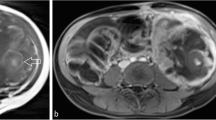
Myxofibrosarcoma presents a variable content of myxoid matrix and the more is the intratumoral myxoid content the higher is the signal appearance on MRI fluid-sensitive sequences. This very high water content of myxoid matrix (about 85%) is responsible of this “water-like” appearance of myxofibrosarcoma with a very high signal on fluid-sensitive sequences and very low signal on T1w sequences just as the water (Fig. 1) [37, 38]. In the largest series of myxofibrosarcoma involving 150 patients, “water like” appearance of the lesion was semiquantitatively classified in 4 grades (0–3), and the grade 3 (water-like signal in > 75% of tumor volume) was statistically associated with increased risk of local recurrence after excision compared to grade 0 (water-like signal < 25% of tumor volume) (P = 0.0493) [41].
“Triple sign” of synovial sarcoma
In T2-weighted sequences, synovial sarcoma typically appears as a prominently heterogeneous multi-lobed soft tissue mass, feature that was first described by Jones et al. [29, 30] with the name of “triple sign.”
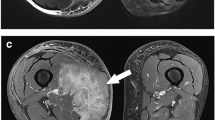
“Triple sign” indicates the simultaneous presence, on T2w sequences, of areas of low, intermediate and high signal intensity, result of solid cellular elements (intermediate signal intensity), hemorrhage with fluid levels or necrosis (high signal intensity) and fibrotic components (low signal intensity) combination (Fig. 2).
MRI of a synovial sarcoma of the right forearm showed three different signal intensity on T2w sequences with fat saturation reflecting the simultaneous presence of solid cellular elements, hemorrhage or necrosis and fibrotic regions: high signal intensity (hemorrhage, asterisk), intermediate signal intensity (cellular elements, arrow), low signal intensity (fibrotic component, arrowhead)
The presence of this sign is proven to be associated with high-grade synovial sarcomas and with a worst patients’ prognosis [42]. Tateishi et al. [42] in a retrospective analysis of 30 patients (median age 27 years) with synovial sarcoma (mean follow-up 32 months), found out that presence of “triple sign” is significantly associated with reduction of disease-free survival (P < 0.05).
Absence of calcifications in synovial sarcoma
Intratumoral calcifications or ossifications are seen in about 30–40% of synovial sarcoma [50]. They are detectable on plain radiograph or in more detail with CT [53]. MRI is able to show the presence of calcifications as areas of hypointense signal in all sequences [54, 55].
Tateishi et al. [42] in their analyses of 30 synovial sarcomas found a significant association with reduced disease-free survival and absence of intratumoral calcifications (P < 0.01).
Signal heterogeneity in myxoid liposarcoma
Signal heterogeneity on T2w MRI sequences is generally considered negative prognostic factor for all STS [44]. Anyway, this feature seems to be particularly relevant for the prognosis of myxoid liposarcoma [56, 57].
Myxoid liposarcoma is the second most common subtype of liposarcoma. Even if larger sizes (> 10 cm of maximum diameter) is the most important prognostic factor in myxoid liposarcoma, other MRI features can provide prognostic information. Above all, it is well known that a signal heterogeneity on MRI in this particular liposarcoma is associated with high-grade lesions and subsequently with poorer prognosis [56, 57]. Gimber et al. [56] in a series of 31 myxoid liposarcoma found out that there were trends toward more heterogeneous T1-weighted and T2-weighted signal in high-grade tumors (p = 0.1 and 0.07, respectively).
New imaging tools and quantitative imaging
Among the imaging tools recently introduced hybrid positron emission tomography (PET)/magnetic resonance imaging (MRI) has emerged as a useful technique that combines the optimal tissue contrast and anatomical detail of MRI, fundamental for STS assessment, with them metabolic imaging of PET useful for prognosis prediction [58].
Quantitative parameters derived by PET (SUV) and DWI in MRI (related to water movement in tissue and reformat in ADC map) and their combination increase the predicting the therapy response of STS and could improve the pre-therapeutic plan as well as monitoring of neoadjuvant treatment strategies of STS [59].
Another new promising tool is radiomics: a quantitative approach to imaging, enhancing the existing data available, by means of advanced mathematical analysis. Radiomics applied to MRI, have been proved to be able to discriminate low-grade from high-grade STS. This distinction, fundamental to assess patients prognosis, has been proved in a large recent series of 180 miscellaneous STS [60]. Moreover, in a study of 35 myxoid/round cell liposarcoma, radiomics analyses focused on tumor shape and heterogeneity, was able to offer prognostic information and predict metastatic relapse [61].
Discussion and conclusion
MRI is a fundamental tool for the assessment of STS and is considered the gold standard imaging technique. The main general and well-established prognostic factors for STS are: histological grade, lesion size, high level of tumor necrosis and metastases at diagnosis. MRI can accurately assess size, signs of necrosis and some features that can relate to the histological grade. Moreover, recent studies suggest that some specific MRI features are independent prognostic factors for all of STS and others features for some specific STS subtypes (Table 2).
The size of the lesions remains the key element that influence prognosis and treatment strategies in all STS as well as in other different malignancies and, due to this, it should be accurately assessed [34]. Lesions larger than > 10 cm are usually associated with worst sarcoma specific survival but not always with a higher risk of local recurrence [33]. The exact size of the tumor should be accurately assessed considering the maximum diameter, not only in the axial plane, and distinguishing exactly tumor boundaries from perilesional oedema [34].
In a large recent series of miscellaneous STS, Crombé et al. [44] found out that peritumoral enhancement was the most important MRI feature related to poor prognosis, followed by signs of necrosis and signal heterogeneity. Due to this, radiologist should accurately evaluate not only tumoral mass but also peritumoral regions.
Regarding specific features in STS subtypes it is well-established that the so-called “tail sign,” a curvilinear projection along the fascial plane arising from the tumor, is related with an increased risk of local recurrence in undifferentiated pleomorphic sarcoma and in myxofibrosarcoma and also with an higher incidence of distant metastases at diagnosis in myxofibrosarcoma [41, 52]. It is also known that a higher content of myxoid matrix in myxofibrosarcoma (“water like” appearance on fluid-sensitive sequences) is associated with an increased risk of local recurrence [32, 41].
In general, MRI signal heterogeneity of STS is a negative prognostic factor and, particularly in synovial sarcoma the recognition of the so-called “triple sign” is important in baseline MRI images for prediction of prognosis [42]. At the same way signal heterogeneity is a recognized negative prognostic factor especially for myxoid liposarcoma [56].
MRI features should be accurately evaluated and taken into account for decision making, surgery planning and prognostic assessment in patient with STS.
References
Trovik CS, Bauer HC, Alvegård TA, Anderson H, Blomqvist C, Berlin O, Gustafson P, Saeter G, Wallöe A (2000) Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian sarcoma group register. Eur J Cancer 36(6):710–716. https://doi.org/10.1016/s0959-8049(99)00287-7
Hui JY (2016) Epidemiology and etiology of sarcomas. Surg Clin North Am 96(5):901–914. https://doi.org/10.1016/j.suc.2016.05.005
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. https://doi.org/10.3322/caac.20107
Bolia IK, Savvidou OD, Kang HP, Chatzichristodoulou N, Megaloikonomos PD, Mitsiokapa E, Mavrogenis AF, Papagelopoulos PJ (2021) Cross-cultural adaptation and validation of the musculoskeletal tumor society (MSTS) scoring system and Toronto extremity salvage score (TESS) for musculoskeletal sarcoma patients in Greece. Eur J Orthop Surg Traumatol. https://doi.org/10.1007/s00590-021-02921-5
Honoré C, Méeus P, Stoeckle E, Bonvalot S (2015) Soft tissue sarcoma in France in 2015: epidemiology, classification and organization of clinical care. J Visc Surg 152(4):223–230. https://doi.org/10.1016/j.jviscsurg.2015.05.001
Farid M, Ngeow J (2016) Sarcomas associated with genetic cancer predisposition syndromes: a review. Oncologist 21(8):1002–1013. https://doi.org/10.1634/theoncologist.2016-0079
Lahat G, Lazar A, Lev D (2008) Sarcoma epidemiology and etiology: potential environmental and genetic factors. Surg Clin North Am 88(3):451–481. https://doi.org/10.1016/j.suc.2008.03.006
Choong PF, Gustafson P, Willén H, Akerman M, Baldetrop B, Fernö M, Alvegård T, Rydholm A (1995) Prognosis following locally recurrent soft-tissue sarcoma. A staging system based on primary and recurrent tumour characteristics. Int J Cancer 60(1):33–7. https://doi.org/10.1002/ijc.2910600104
Lee JH, Kim Y, Yoo HJ, Kim HS, Cho HS, Han I (2020) Prognoses of superficial soft tissue sarcoma: the importance of fascia-tumor relationship on MRI. Eur J Surg Oncol 46(2):282–287. https://doi.org/10.1016/j.ejso.2019.10.003
Oda Y, Yamamoto H, Kohashi K, Yamada Y, Iura K, Ishii T, Maekawa A, Bekki H (2017) Soft tissue sarcomas: from a morphological to a molecular biological approach. Pathol Int 67(9):435–446. https://doi.org/10.1111/pin.12565
Guo X, Jo VY, Mills AM, Zhu SX, Lee CH, Espinosa I, Nucci MR, Varma S, Forgó E, Hastie T, Anderson S, Ganjoo K, Beck AH, West RB, Fletcher CD, van de Rijn M (2015) Clinically relevant molecular subtypes in leiomyosarcoma. Clin Cancer Res 21(15):3501–3511
Wortman JR, Tirumani SH, Jagannathan JP, Tirumani H, Shinagare AB, Hornick JL, Ramaiya NH (2016) Primary extremity liposarcoma: MRI features, histopathology, and clinical outcomes. J Comput Assist Tomogr 40(5):791–798. https://doi.org/10.1097/RCT.0000000000000431
WHO classification of tumours editorial board (2020) WHO classification of tumours of soft tissue and bone, 5th edn. IARC Press, Lyon
Martin-Liberal J (2013) Leiomyosarcoma: principles of management. Intractable Rare Dis Res 2(4):127–129. https://doi.org/10.5582/irdr.2013.v2.4.127
Harati K, Daigeler A, Lange K, Niggemann H, Stricker I, Steinau HU, Lehnhardt M, Goertz O (2017) Somatic leiomyosarcoma of the soft tissues: a single-institutional analysis of factors predictive of survival in 164 patients. World J Surg 41(6):1534–1541. https://doi.org/10.1007/s00268-017-3899-5
Grossmann AH, Layfield LJ, Randall RL (2012) Classification, molecular characterization, and the significance of pten alteration in leiomyosarcoma. Sarcoma 2012:380896. https://doi.org/10.1155/2012/380896
Ferrari A, Dileo P, Casanova M et al (2003) Rhabdo-myosarcoma in adults: a retrospective analysis of 171 patients treated at a single institution. Cancer 98:571–580
Sambri A, Bianchi G, Cucurnia I, Gambarotti M, Donati DM (2018) Pediatric soft tissue sarcoma of the limbs: clinical outcome of 97 patients. Eur J Orthop Surg Traumatol 28(1):1–7. https://doi.org/10.1007/s00590-017-2019-4
Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galin-do C, Ferrari A (2009) Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemi-ology and end results program, 1973–2005: an analysis of 2600 patients. J Clin Oncol 27:3391–3397
Thway K, Fisher C (2014) Synovial sarcoma: defining features and diagnostic evolution. Ann Diagn Pathol 18(6):369–380. https://doi.org/10.1016/j.anndiagpath.2014.09.002
Spinnato P, Sambri A, Fujiwara T et al (2020) Myxofibrosarcoma: clinical and prognostic value of MRI features. Curr Med Imaging. https://doi.org/10.2174/1573405616999200729152135 (Published online ahead of print, 2020 Jul 29)
Roberts CC, Kransdorf MJ, Beaman FD, Adler RS, Amini B, Appel M et al (2016) ACR appropriateness criteria follow-up of malignant or aggressive musculoskeletal tumors. J Am Coll Radiol 13(4):389–400
Del Grande F, Subhawong T, Weber K, Aro M, Mugera C, Fayad LM (2014) Detection of soft-tissue sarcoma recurrence: added value of functional MR imaging techniques at 3.0 T. Radiology 271(2):499–511. https://doi.org/10.1148/radiol.13130844
Hanna SL, Fletcher BD (1995) MR imaging of malignant soft-tissue tumors. Magn Reson Imaging Clin N Am 3(4):629–650
Gerrand C, Athanasou N, Brennan B, Grimer R, Judson I, Morland B, Peake D, Seddon B, Whelan J (2016) UK guidelines for the management of bone sarcomas. Clin Sarcoma Res 6:7. https://doi.org/10.1186/s13569-016-0047-1
Jaovisidha S, Suvikapakornkul Y, Woratanarat P, Subhadrabandhu T, Nartthanarung A, Siriwongpairat P (2010) MR imaging of fat-containing tumours: the distinction between lipoma and liposarcoma. Singap Med J 51(5):418–423
El Ouni F, Jemni H, Trabelsi A, Ben Maitig M, Arifa N, Ben Rhouma K, Ben Ayech M, Tlili K (2010) Liposarcoma of the extremities: MR imaging features and their correlation with pathologic data. Orthop Traumatol Surg Res 96(8):876–883. https://doi.org/10.1016/j.otsr.2010.05.010
Allen SD, Moskovic EC, Fisher C, Thomas JM (2007) Adult rhabdomyosarcoma: cross-sectional imaging findings including histopathologic correlation. AJR 189:371–377
Jones BC, Sundaram M, Kransdorf MJ (1993) Synovial sarcoma: MR imaging findings in 34 patients. AJR Am J Roentgenol 161(4):827–830. https://doi.org/10.2214/ajr.161.4.8396848
van Rijswijk CSP, Hogendoorn PCW, Taminiau AHM, Bloem JL (2001) Synovial sarcoma: dynamic contrast-enhanced MR imaging features. Skelet radiology 30(1):25–30. https://doi.org/10.1007/s002560000295
Yoo HJ, Hong SH, Kang Y et al (2014) MR imaging of myxofibrosarcoma and undifferentiated sarcoma with emphasis on tail sign; diagnostic and prognostic value. Eur Radiol 24(8):1749–1757. https://doi.org/10.1007/s00330-014-3181-2
Sambri A, Spinnato P, Bazzocchi A, Tuzzato GM, Donati D, Bianchi G (2019) Does pre-operative MRI predict the risk of local recurrence in primary myxofibrosarcoma of the extremities? Asia Pac J Clin Oncol 15(5):e181–e186. https://doi.org/10.1111/ajco.13161
Gustafson P, Akerman M, Alvegård TA, Coindre JM, Fletcher CD, Rydholm A, Willén H (2003) Prognostic information in soft tissue sarcoma using tumour size, vascular invasion and microscopic tumour necrosis-the SIN-system. Eur J Cancer 39(11):1568–1576. https://doi.org/10.1016/s0959-8049(03)00369-1
Spinnato P (2021) The Importance of accurate tumor measurements and staging in oncologic imaging: impact on patients’ health. Acad Radiol. https://doi.org/10.1016/j.acra.2021.01.012 (Published online ahead of print, 2021 Jan 16)
Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS, Evans HL (2003) Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 97(10):2530–2543. https://doi.org/10.1002/cncr.11365
Stojadinovic A, Leung DH, Allen P, Lewis JJ, Jaques DP, Brennan MF (2002) Primary adult soft tissue sarcoma: time-dependent influence of prognostic variables. J Clin Oncol 20(21):4344–4352. https://doi.org/10.1200/JCO.2002.07.154
Maretty-Nielsen K (2014) Prognostic factors in soft tissue sarcoma. Dan Med J 61(11):B4957
Tanaka K, Ozaki T (2019) New TNM classification (AJCC eighth edition) of bone and soft tissue sarcomas: JCOG bone and soft tissue tumor study group. Jpn J Clin Oncol 49(2):103–107. https://doi.org/10.1093/jjco/hyy157
Yoon S, Maki RG, Asare EA, Cooper K, Hornick JL, Lazar AJ et al (2017) Soft tissue sarcoma of the trunk and extremities. In: Amin M (ed) AJCC cancer staging manual, 8th edn. Springer, New York, pp 507–515
Eilber FC, Rosen G, Nelson SD, Selch M, Dorey F, Eckardt J, Eilber FR (2003) High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg 237(2):218–226. https://doi.org/10.1097/01.SLA.0000048448.56448.70
Spinnato P, Clinca R, Vara G et al (2020) MRI features as prognostic factors in myxofibrosarcoma: proposal of MRI grading system. Acad Radiol. https://doi.org/10.1016/j.acra.2020.08.018 (published online ahead of print, 2020 Sep 11)
Tateishi U, Hasegawa T, Beppu Y, Satake M, Moriyama N (2004) Synovial sarcoma of the soft tissues. J Comput Assist Tomogr 28(1):140–148. https://doi.org/10.1097/00004728-200401000-00024
Cates JMM (2018) The AJCC 8th edition staging system for soft tissue sarcoma of the extremities or trunk: a cohort study of the SEER database. J Natl Compr Canc Netw 16(2):144–152. https://doi.org/10.6004/jnccn.2017.7042
Crombé A, Marcellin PJ, Buy X et al (2019) Soft-tissue sarcomas: assessment of MRI features correlating with histologic grade and patient outcome. Radiology 291(3):710–721. https://doi.org/10.1148/radiol.2019181659
O’Sullivan PJ, Harris AC, Munk PL (2008) Radiological imaging features of non-uterine leiomyosarcoma. Br J Radiol 81(961):73–81. https://doi.org/10.1259/bjr/18595145
Gordon RW, Tirumani SH, Kurra V, Shinagare AB, Jagannathan JP, Hornick JL, Ramaiya NH (2014) MRI, MDCT features, and clinical outcome of extremity leiomyosarcomas: experience in 47 patients. Skelet Radiol 43(5):615–622. https://doi.org/10.1007/s00256-014-1823-8
Ledoux P, Kind M, Le Loarer F et al (2019) Abnormal vascularization of soft-tissue sarcomas on conventional MRI: diagnostic and prognostic values. Eur J Radiol 117:112–119. https://doi.org/10.1016/j.ejrad.2019.06.007
Crombé A, Le Loarer F, Stoeckle E, Cousin S, Michot A, Italiano A, Buy X, Kind M (2018) MRI assessment of surrounding tissues in soft-tissue sarcoma during neoadjuvant chemotherapy can help predicting response and prognosis. Eur J Radiol 109:178–187. https://doi.org/10.1016/j.ejrad.2018.11.004
MacDermed DM, Miller LL, Peabody TD, Simon MA, Luu HH, Haydon RC, Montag AG, Undevia SD, Connell PP (2010) Primary tumor necrosis predicts distant control in locally advanced soft-tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys 76(4):1147–53. https://doi.org/10.1016/j.ijrobp.2009.03.015
Monsky WL, Jin B, Molloy C, Canter RJ, Li CS, Lin TC, Borys D, Mack W, Kim I, Buonocore MH, Chaudhari AJ (2012) Semi-automated volumetric quantification of tumor necrosis in soft tissue sarcoma using contrast-enhanced MRI. Anticancer Res 32(11):4951–4961
Zhao F, Ahlawat S, Farahani SJ, Weber KL, Montgomery EA, Carrino JA, Fayad LM (2014) Can MR imaging be used to predict tumor grade in soft-tissue sarcoma? Radiology 272(1):192–201. https://doi.org/10.1148/radiol.14131871
Spinnato P, Clinca R (2021) MRI tail sign in soft-tissue sarcoma. Radiology 16:203877. https://doi.org/10.1148/radiol.2021203877
Wilkerson BW, Crim JR, Hung M, Layfield LJ (2012) Characterization of synovial sarcoma calcification. AJR Am J Roentgenol 199(6):W730–W734. https://doi.org/10.2214/AJR.11.7342
Liang C, Mao H, Tan J, Ji Y, Sun F, Dou W, Wang H, Wang H, Gao J (2015) Synovial sarcoma: magnetic resonance and computed tomography imaging features and differential diagnostic considerations. Oncol Lett 9(2):661–666. https://doi.org/10.3892/ol.2014.2774
Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA (2006) From the archives of the AFIP: imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics 26(5):1543–1565. https://doi.org/10.1148/rg.265065084
Gimber LH, Montgomery EA, Morris CD, Krupinski EA, Fayad LM (2017) MRI characteristics associated with high-grade myxoid liposarcoma. Clin Radiol 72(7):613.e1-613.e6. https://doi.org/10.1016/j.crad.2017.01.016
Barile A, Zugaro L, Catalucci A et al (2002) Soft tissue liposarcoma: histological subtypes MRI and CT findings. Radiol Med 104(3):140–149
Qi J, Thakrar PD, Browning MB, Vo N, Kumbhar SS (2021) Clinical utilization of whole-body PET/MRI in childhood sarcoma. Pediatr Radiol 51(3):471–479. https://doi.org/10.1007/s00247-020-04834-7
Chodyla M, Demircioglu A, Schaarschmidt BM, Bertram S, Bruckmann NM, Haferkamp J, Li Y, Bauer S, Podleska L, Rischpler C, Forsting M, Herrmann K, Umutlu L, Grueneisen J (2021) Evaluation of 18F-FDG PET and DWI datasets for predicting therapy response of soft-tissue sarcomas under neoadjuvant isolated limb perfusion. J Nucl Med 62(3):348–353. https://doi.org/10.2967/jnumed.120.248260
Yan R, Hao D, Li J, Liu J, Hou F, Chen H, Duan L, Huang C, Wang H, Yu T (2021) Magnetic resonance imaging-based radiomics nomogram for prediction of the histopathological grade of soft tissue sarcomas: a two-center study. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27532
Crombé A, Le Loarer F, Sitbon M, Italiano A, Stoeckle E, Buy X, Kind M (2020) Can radiomics improve the prediction of metastatic relapse of myxoid/round cell liposarcomas? Eur Radiol 30(5):2413–2424. https://doi.org/10.1007/s00330-019-06562-5
Funding
No funding was received for conducting this study. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The idea for the article was by Paolo Spinnato, literature search was performed by Anna Parmeggiani, Claudia Martella and Giulia Scalas, while Roberta Clinca, Gianmarco Tuzzato, Giancarlo Facchini and Giuseppe Bianchi critically revised the work. The first draft of the paper was written by Giulia Scalas and Paolo Spinnato. All authors read and approved the final manuscript. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Scalas, G., Parmeggiani, A., Martella, C. et al. Magnetic resonance imaging of soft tissue sarcoma: features related to prognosis. Eur J Orthop Surg Traumatol 31, 1567–1575 (2021). https://doi.org/10.1007/s00590-021-03003-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-021-03003-2