Abstract
Background
Somatic leiomyosarcoma (LMS) is an aggressive soft tissue sarcoma entity with a high metastatic potential. The purpose of this study was to identify prognostic indicators of survival in patients with somatic LMS of the soft tissues.
Methods
We retrospectively assessed the relationship between local recurrence-free survival (LRFS), disease-specific survival (DSS), overall survival (OS) and potential prognostic factors in 164 patients who were suitable for surgical treatment in curative intent. Patients with soft tissue LMS of the extremities, the truncal wall and the head and neck area were included. The median follow-up time was 4.9 years.
Results
In the entire cohort, the 5-year estimate of the DSS, OS and LRFS rate were 74.5% (95% confidence interval [CI] 65.0–81.8), 70.6% (95% CI: 60.9–78.3) and 63.4% (95% CI 53.4–71.9), respectively. Thirty-eight patients (23.2%) developed distant metastases with a median survival time of 1.5 years after diagnosis of metastasis. Surgical margins attained at the initial oncologic resection and eventual re-excisions did not influence DSS, OS and LRFS significantly. Within the R0 subgroup, close and wide negative margins led to similar outcomes. High histologic grade (P < 0.001), size >5 cm (P = 0.002) and subfascial localisation (P = 0.002) were associated with significantly diminished DSS in univariate analysis. In multivariate analysis, only histologic grade was found to be an independent prognostic factor of DSS.
Conclusions
The data from this study could not determine a prognostic significance of surgical margins suggesting that tumour characteristics other than margin status are important. Tumour biology reflected by the histologic grade dictates the final outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leiomyosarcoma (LMS) is a relatively common subentity within the rare and heterogeneous group of soft tissue sarcomas. It originates from smooth muscle cells and may occur throughout the body. Depending on the localisation of the primary tumour, LMS can be classified into uterine, gastrointestinal, retroperitoneal and somatic subsets [1]. These LMS subtypes exhibit completely different clinical behaviours and, therefore, should be considered separately [2, 3].
Somatic LMS of the soft tissues accounts for approximately 7.6 to 16.1% of all non-visceral soft tissue sarcomas [4,5,6]. In comparison with other sarcoma subtypes, it represents a relatively aggressive subset with a high risk of metastasis [7,8,9]. In previous studies, rates of distant metastasis ranged from 29.4 to 44.7% [1, 9,10,11]. Reflecting the regular approach for the treatment of soft tissue sarcomas, the therapy of choice involves surgical resection with negative margins [12,13,14]. Due to its overall rarity, there have been only few studies that have analysed prognostic factors in patients with somatic LMS occurring in the extremities, the truncal wall and the head and neck area [1, 9, 10, 15, 16]. Among these factors, histologic grade and tumour size are the most significant for overall survival (OS). Notably, the achievement of negative surgical margins has been determined to be an important factor for improving local recurrence-free survival (LRFS), but none of the retrospective analyses could establish an association between the quality of surgical margins and OS [9, 10, 15]. This intriguing finding raises the question whether wide resections with clear margins at any price or more conservative, function-sparing resections should be performed in patients with somatic LMS, considering that only local control but not overall survival would depend on quality of surgery. Hence, the role of radical surgery, even in this aggressive sarcoma subtype, remains controversial as it is in soft tissue sarcomas in general [14, 17,18,19].
These findings challenged our own previous treatment policy and inspired us to review our institutional experience. The aim of this study was to identify prognostic indicators of recurrence and survival in patients with somatic LMS who underwent surgical resection. In particular, we focused on the effect of surgical margins on disease outcome.
Patients and methods
Patients
Between April 1996 and November 2015, 164 patients with somatic LMS of the soft tissues were treated surgically at our institution. Only patients with primary soft tissue LMS of the extremities, the truncal wall and the head and neck area were included. Patients with simultaneous distant metastases or with cutaneous LMS that were only confined to the dermis were excluded. We restricted analyses to 164 participants with full information available on the outcome, histology and surgical margins at the initial procedure. Patient follow-up was obtained from our database and patient correspondence. The study was approved by the local ethics committee.
Treatment
Preoperatively, contrast-enhanced magnetic resonance images of the tumour site were routinely obtained. The goal of surgical treatment for all patients was resection of the tumour with negative margins. A lateral clear margin of two centimetres of normal tissue was intended wherever feasible. In epifascial lesions, a deep clear margin of one fascial layer was intended. Several patients received adjuvant radiation and/or chemotherapy. The indication for adjuvant radiation or chemotherapy was given at the discretion of the interdisciplinary tumour board of either our institution or the referring institutions.
Histopathological classification
All tumours were diagnosed and classified using the guidelines of the French Federation of Cancer Centres and the World Health Organisation [20, 21]. All pathology slides were analysed or reviewed for consensus diagnosis by experienced soft tissue pathologists of our institution.
Statistical analysis
All patients were retrospectively analysed regarding possible prognostic factors influencing survival. Overall survival (OS) was defined as the time period from the date of surgery for primary disease to the date of death from any cause or the date of last follow-up assessment in living patients. For the measurement of disease-specific survival (DSS), patients who died from other causes were excluded. Local recurrence-free survival (LRFS) was calculated from the date of surgery for primary disease to the date of first recurrence or the date of last follow-up assessment in recurrence-free patients. Survival rates were estimated according to the Kaplan–Meier method with respective 95% confidence intervals (CIs) and were compared using the log-rank test. Multivariate analyses were performed using the Cox proportional hazards model and the Wald test. Variables associated with P < 0.1 in the univariate analysis were included in the multivariate regression to assess independent prognostic factors for DSS. The data analysis was performed using the statistical program Stata (Version 11.2, StataCorp, College Station, USA).
Results
Follow-up and patient characteristics
The median follow-up after primary diagnosis was 4.9 years (95% CI 3.6–6.4). The median age at the time of primary diagnosis was 63.9 years (range 19.4–88.1). There were 72 females (43.9%) and 92 males (56.1%) individuals. The distribution of the histologic grading was G1 in 30 cases (18.3%), G2 in 58 (35.4%) and G3 in 76 (46.3%). In total, 43 patients (26.2%) had at least one local recurrence, whereas 18 patients (10.1%) had two or more local recurrences (range 2–6). Over time, 38 patients (23.2%) developed distant metastases.
In the entire series, the 5-year estimates of the DSS and OS rate were 74.5% (95% CI 65.0–81.8) and 70.6% (95% CI 60.9–78.3). The median survival time after diagnosis of initial local recurrence and distant metastasis were 1.9 and 1.5 years, respectively.
Treatment characteristics
Surgical treatment in one or two steps led to microscopically negative margins (R0) only in 147 patients (89.6%), whereas 14 patients (8.5%) were left with microscopically positive margins (R1) and three (1.8%) with macroscopically positive margins (R2). In those patients with R1 or R2 margins, the tumours were too advanced and widespread for complete resection. Before definitive surgical treatment at our institution, 79 patients (48.1%) underwent previous whoops procedures with intralesional margins at the referring hospitals. All these patients underwent a re-resection in our institution. In 78 of the 79 patients (98.7%), negative margins could be attained.
A total of 58 patients (35.4%) received adjuvant radiotherapy after resection of their primary tumour, with a median overall dose of 60.0 Gray (range: 30.0–78.0). Of these 58 patients, 14 (24.1%) developed local recurrences in the further course of disease. Thirteen out of these 14 patients had previously undergone a R0 resection of their primary tumour while one patient had been left with R1 margins.
All 43 patients with local recurrences could undergo further resection of their initial local recurrence. Here, negative margins could be obtained in 33 of the 43 patients (76.7%), while five patients (11.6%) were left with R1 margins and another five patients (11.6%) with R2 margins.
Univariate analysis of LRFS
The 5-year rate of LRFS was 63.4% (95% CI 53.4–71.9) for the entire cohort. Patients treated with adjuvant radiation tended to have a prolonged LRFS compared with patients whose primary tumours were not treated with radiation (5-year LRFS: 69.6% [53.8–80.9] vs. 59.4% [45.9–70.5]), but the difference was not statistically significant in the univariate analysis (P = 0.124). Adjuvant chemotherapy also failed to alter LRFS (5-year LRFS: 62.5 [22.4–86.1] vs. 63.4% [52.9–72.1]; P = 0.726). The margin status had no impact on LRFS (5-year LRFS: R0 63.6 [53.2–72.2] vs. R1 44.4% [6.0–79.1]; P = 0.900). Previous whoops surgery did not affect the further local outcome (5-year LRFS: 63.8 [49.7–74.9] vs. 61.9 [46.2–74.2]; P = 0.962).
Univariate analysis of DSS
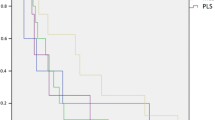
Histologic grade, tumour size and depth were the only factors that had a prognostic significance on DSS in univariate analysis (Table 1, Fig. 1). Patients with G1 tumours had more favourable prognoses than did patients with intermediate G2 or G3 lesions (5-year DSS: G1 100.0% [-] vs. G2 86.2% [69.5–94.1] vs. G3 56.1% [41.0–68.8]; P < 0.001) (Fig. 1). Primary tumours >5 cm at the initial presentation were associated with a significantly diminished outcome when compared with smaller tumours (5-year DSS: 58.0% [42.6–70.7] vs. 89.1% [77.1–95.0]; P = 0.002). Subfascial localisation was also associated with a significantly worse DSS compared with epifascial lesions (5-year DSS: 55.6% [36.8–70.8] vs. 82.9% [72.0–89.9]; P = 0.002).
In the univariate analysis, the surgical margin status failed to reach a prognostic significance (Table 2). Within the R0 subgroup, the clear margin width did not influence DSS significantly. Similar to findings for LRFS, adjuvant radiation and chemotherapy did not alter DSS. Adjuvant treatment of the primary tumour also did not influence DSS in patients that developed metastases during the course of disease. (results not shown). Intralesional whoops surgery before definitive surgical treatment had no significant effect on DSS (5-year DSS: 77.3 [63.0–86.6] vs. 72.0 [58.0–82.1]; P = 0.265).
Regression analysis of non-categorised surgical margin width
To evaluate the prognostic significance of non-categorised clear margin widths within the R0 subgroup more accurately, we performed a Cox regression analysis. The closest negative margin width could be assessed histologically in our institution for 115 patients with R0-resected tumours. Here, the closest surgical margin width did not influence OS significantly. In the Wald test, the hazard ratio (HR) for death was 0.66 (95% CI: 0.31–1.36) for wide margins which failed to reach statistical significance (P = 0.258). Thus, close and wide negative margins led to a similar DSS.
Multivariate analysis of DSS
Histologic grade, tumour size and depth were associated with P < 0.1 in the univariate analysis and, therefore, included in the Cox model to assess independent prognostic factors for DSS. Interestingly, only the histologic grade was found to be an independent prognostic factor for DSS (Table 3). This is due to the fact that size and depth were strongly dependent on the histologic grade. High-grade tumours were usually large and subfascially localised, and vice versa. The Spearman’s correlation coefficient for non-categorised tumour size and histologic grade was significant for a positive correlation between these two factors (ρ = 0.55; P < 0.001). When comparing the categorised tumour size (>5 cm, ≤5 cm) with the histologic grade, the Fisher’s exact test revealed also a significant dependency (P < 0.001). It could also demonstrate a dependency between tumour depth and histologic grade (P < 0.001).
Furthermore, we have determined the prognostic significance of the time of metastasis occurrence in patients with distant metastases. Here, late metastasis was associated with an better DSS than early metastasis (hazard ratio for dead of disease: 0.73 (95-CI 0.57–0.95; P = 0.014).
Discussion
Somatic LMS of the soft tissues represents an aggressive soft tissue sarcoma entity with a high metastatic potential. In the present study, 23.3% of all patients developed distant metastases during the course of disease. Previous analyses from other institutions reported even higher metastasis rates ranging from 29.4 to 44.7% [1, 9,10,11]. In our series, the median survival time after diagnosis of distant metastasis was only 1.5 years (95% CI 0.8–2.8). Histologic grade, tumour size and depth emerged as the only factors that influenced DSS significantly in univariate analysis. However, histologic grade was the only independent prognostic factor for DSS in multivariate analysis, while size and depth showed a dependency towards histologic grade. In our series, high-grade tumours were usually large and subfascially localised. Similar observations were also made by two largest, well-characterised studies that assessed the impact of surgical margins on patients with somatic LMS: In a single-institutional analysis of 115 patients from Brigham and Women’s Hospital (BWH), Abraham et al. [15] identified the histologic grade and tumour depth as independent factors predicting OS in multivariate analysis. In a multi-institutional analysis by the Scandinavian Sarcoma Group (SSG), Svarvar et al. [9] determined the surgical outcome of 225 patients with somatic LMS and found that grade, size and depth correlated significantly with OS in univariate analysis. However, in accordance with our findings, size and depth could not be revealed as independent predictors of OS in multivariate analysis. Only the histologic grade appeared to be a statistically independent prognostic factor. Hence, the histologic grade as a marker for the inherent biological aggressiveness was the most important factor that dictated OS in this multi-centre study. Several other studies could confirm the prognostic significance of the histologic grade as well, although they involved smaller subsets of patients with somatic LMS [1, 11, 22].
Interestingly, surgical margins attained at the primary resection and eventual re-excision did not influence LRFS, DSS and OS in our patient population. In accordance, both aforementioned studies from BWH and the SSG demonstrated that positive margins adversely affected the local outcome but did not influence OS. Thus, the quality of surgical margins had only an impact on local control. These findings are in line with the results of a retrospective analysis by the Memorial Sloan-Kettering Cancer Center which also included abdominal and retroperitoneal LMS. Here, positive margins were associated with a diminished LRFS, but did not have an adverse impact on OS [10]. Hence, none of the large studies that assessed the prognostic influence of surgery on somatic LMS could establish an association between the quality of surgical margins and OS.
To date, analyses with the emphasis on somatic LMS are sparse. The overall rarity of somatic LMS poses epidemiological challenges and impedes a reliable assessment of the surgical outcome through larger studies. In previous studies on non-visceral soft tissue sarcomas in general, somatic LMSs were merely assessed as a subgroup and not separately. However, the influence of surgical margins on LRFS and OS has also been discussed controversially in those studies. Several retrospective studies on extremity soft tissue sarcomas presented similar results and could not reveal a prognostic significance of negative margins on survival [23, 24]. Nevertheless, these findings are in contradiction to several large studies as well that determined a beneficial prognostic impact of negative margins on LRFS and OS [17, 18, 25]. However, a large study by Gronchi et al. [14] from the Instituto Nazionale Tumori in Milan analysed the outcome of 911 patients with extremity STS presenting a long-term median follow-up of 8.9 years. Notably, microscopic margin status failed to reach statistical significance as an independent predictive factor for OS in this long-term survival analysis. Finally, Kandel et al. [26] presented a meta-analysis including 32 retrospective and prospective studies in 2013. Here, most studies failed to establish a strong correlation between surgical margins and OS suggesting that tumour characteristics other than margin status are important.
However, although positive margins had no prognostic impact in the current and previous studies on somatic LMS, the same conclusions might be drawn as for other soft tissue sarcomas. Most of the recently published studies suggested a less radical surgical approach with limb- and function-sparing resections when feasible without leaving microscopic positive margins [14, 26]. As indicated by the data of BWH and the SSG, negative margins are associated with a higher rate of local control, but radical surgery with the goal of clear margins at any price cannot be justified by the presented findings in order to improve DSS.
Regarding adjuvant treatment modalities, radiation did not significantly improve LRFS and DSS in our series. In accordance, the other studies on somatic LMS were also not able to detect any beneficial prognostic effects of radiation or did not assess its impact [9, 10, 15]. Nevertheless, these findings have to be interpreted with caution because of the relatively small number of patients treated with adjuvant radiation. However, adjuvant radiation has been determined to control local disease in non-visceral soft tissue sarcomas in general. A recently published randomised, prospective study conducted by the National Cancer Institute in Bethesda included 141 patients with extremity STS and revealed that patients who underwent limb-sparing surgery with adjuvant radiation had an improved LRFS when compared with patients who underwent surgery without radiation [27]. Notably, overall survival was not improved in the radiation treatment group. However, there are also several Surveillance, Epidemiology and End Results (SEER) Database analyses that could find a significant association between radiation treatment and overall survival in the subgroup of patients with high-grade STS [28,29,30]. It therefore seems reasonable to include adjuvant radiation in all cases of high-grade STS, no matter whether negative or positive margins were attained.
Finally, the current study has several limitations which have to be stated. Although being one of the largest analyses on somatic LMS, the assessed subgroups are still relatively small and some findings have to be interpreted with caution. Due to the small subset of patients with positive margins, we cannot conclude that there is no association between margins and outcome at all. The present study indeed demonstrated that tumour characteristics such as histologic grade have a greater influence on the outcome than treatment-related factors such as surgical margins, but otherwise it cannot refute a potential influence of treatment-related factors which might become clearer when more patients are analysed. Another limitation of our study implies a study selection bias which has to be acknowledged. We only included patients with STS that were suitable for further surgical treatment with curative intent. Patients with extensive tumours that could not be approached surgically because of rapid disease progression and therefore with less favourable outcome were not assessed in this study. Hence, our findings are only applicable to the selected group of patients where further surgical treatment was possible and not to all patients with somatic LMS.
In conclusion, this study provides data that may help clinicians estimate the prognosis of patients with somatic LMS. Adverse prognostic features included high histologic grade, large tumour size and subfascial localisation, although these factors were depended of each other. The data from this study could not underscore the benefit of negative margins achieved at the resection of the primary tumour or eventual re-excisions. Neither local control nor DSS correlated significantly with the surgical margin status. With respect to the current available data from the present and previous studies on somatic LMS, surgical efforts should aim at limb- and function-sparing resections when feasible with negative margins to improve local control. When the goal of achieving clear margins will require major functional impairment or amputation, the postoperative consequences should be clearly discussed with each patient, as this can be highly subjective. The ultimate decision should be made in each case based on the histologic grade and progression of the tumour, the health status of the patient and, last but not least, the decision of the informed patient.
References
Farshid G, Pradhan M, Goldblum J et al (2002) Leiomyosarcoma of somatic soft tissues: a tumor of vascular origin with multivariate analysis of outcome in 42 cases. Am J Surg Pathol 26:14–24
Miettinen M, Fetsch JF (2006) Evaluation of biological potential of smooth muscle tumours. Histopathology 48:97–105
Pijpe J, Broers GH, Plaat BE et al (2002) The relation between histological, tumor-biological and clinical parameters in deep and superficial leiomyosarcoma and leiomyoma. Sarcoma 6:105–110
Hung GY, Yen CC, Horng JL et al (2015) Incidences of primary soft tissue sarcoma diagnosed on extremities and trunk wall: a population-based study in Taiwan. Medicine (Baltimore) 94:e1696
Gustafson P (1994) Soft tissue sarcoma: epidemiology and prognosis in 508 patients. Acta Orthop Scand Suppl259:1–31
Mastrangelo G, Coindre JM, Ducimetiere F et al (2012) Incidence of soft tissue sarcoma and beyond: a population-based prospective study in 3 European regions. Cancer 118:5339–5348
Coindre JM, Terrier P, Guillou L et al (2001) Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 91:1914–1926
Pisters PW, Leung DH, Woodruff J et al (1996) Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 14:1679–1689
Svarvar C, Bohling T, Berlin O et al (2007) Clinical course of nonvisceral soft tissue leiomyosarcoma in 225 patients from the Scandinavian Sarcoma Group. Cancer 109:282–291
Gladdy RA, Qin LX, Moraco N et al (2013) Predictors of survival and recurrence in primary leiomyosarcoma. Ann Surg Oncol 20:1851–1857
Mankin HJ, Casas-Ganem J, Kim JI et al (2004) Leiomyosarcoma of somatic soft tissues. Clin Orthop Relat Res 421:225–231
Kaushal A, Citrin D (2008) The role of radiation therapy in the management of sarcomas. Surg Clin North Am 88:629–646
Singer S, Demetri GD, Baldini EH et al (2000) Management of soft-tissue sarcomas: an overview and update. Lancet Oncol 1:75–85
Gronchi A, Casali PG, Mariani L et al (2005) Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: a series of patients treated at a single institution. J Clin Oncol 23:96–104
Abraham JA, Weaver MJ, Hornick JL et al (2012) Outcomes and prognostic factors for a consecutive case series of 115 patients with somatic leiomyosarcoma. J Bone Joint Surg Am 94:736–744
Miyajima K, Oda Y, Oshiro Y et al (2002) Clinicopathological prognostic factors in soft tissue leiomyosarcoma: a multivariate analysis. Histopathology 40:353–359
Novais EN, Demiralp B, Alderete J et al (2010) Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin Orthop Relat Res 468:3003–3011
Stojadinovic A, Leung DH, Hoos A et al (2002) Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg 235:424–434
Trovik CS, Bauer HC, Alvegard TA et al (2000) Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer 36:710–716
Coindre JM (2006) Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med 130:1448–1453
Fletcher CD (2014) The evolving classification of soft tissue tumours–an update based on the new 2013 WHO classification. Histopathology 64:2–11
Radkowski CA, Dodd LG, Johnson JL et al (2012) Leiomyosarcoma of the somatic soft tissues. J Surg Orthop Adv 21:96–101
Willeumier J, Fiocco M, Nout R et al (2015) High-grade soft tissue sarcomas of the extremities: surgical margins influence only local recurrence not overall survival. Int Orthop 39:935–941
McKee MD, Liu DF, Brooks JJ et al (2004) The prognostic significance of margin width for extremity and trunk sarcoma. J Surg Oncol 85:68–76
Potter BK, Hwang PF, Forsberg JA et al (2013) Impact of margin status and local recurrence on soft-tissue sarcoma outcomes. J Bone Joint Surg Am 95:e151
Kandel R, Coakley N, Werier J et al (2013) Surgical margins and handling of soft-tissue sarcoma in extremities: a clinical practice guideline. Curr Oncol 20:e247–254
Beane JD, Yang JC, White D et al (2014) Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol 21:2484–2489
Schreiber D, Rineer J, Katsoulakis E et al (2012) Impact of postoperative radiation on survival for high-grade soft tissue sarcoma of the extremities after limb sparing radical resection. Am J Clin Oncol 35:13–17
Kachare SD, Brinkley J, Vohra NA et al (2015) Radiotherapy associated with improved survival for high-grade sarcoma of the extremity. J Surg Oncol 112:338–343
Koshy M, Rich SE, Mohiuddin MM (2010) Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: a SEER analysis. Int J Radiat Oncol Biol Phys 77:203–209
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Harati, K., Daigeler, A., Lange, K. et al. Somatic Leiomyosarcoma of the Soft Tissues: A Single-Institutional Analysis of Factors Predictive of Survival in 164 Patients. World J Surg 41, 1534–1541 (2017). https://doi.org/10.1007/s00268-017-3899-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-3899-5