Abstract
We investigated roots of 107 medicinal and aromatic plants (MAPs) in the Western Ghats region of Southern India for arbuscular mycorrhizal (AM) and dark septate endophyte (DSE) associations. Of the 107 MAPs belonging to 98 genera in 52 families examined, 79 were AM and 38 harbored a DSE association. Typical Arum- and Paris-type mycorrhizas are first reported in the presumed nonmycorrhizal family Amaranthaceae. Similarly, DSE associations are recorded for the first time in nine plant families and 37 plant species. Thirty MAPs had both AM and DSE associations. The number of MAPs having Arum-type mycorrhiza was greater than those having Paris-type. This was more prominent among herbaceous plants than in trees where the Paris-type was predominant. Similarly, the Arum-type was more prevalent in annuals than in perennials. DSE associations were more frequent in herbs and perennials compared to other MAPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants roots are colonized by numerous species of fungi with extensive, symptomless nonpathogenic endophytic or biotrophic phases in their life cycles (Sieber 2002). These include associations formed by mycorrhizal and several nonmycorrhizal fungi. In addition to the widely studied arbuscular mycorrhizal (AM) fungi (Smith and Read 1997), increased attention has recently been given to a ubiquitous group of miscellaneous fungi designated as dark septate endophytes (DSE) and characterized by melanized septate hyphae and microsclerotia. These fungi are frequent root colonizers of trees, shrubs, terrestrial orchids, and a broad range of plants in temperate and tropical habitats (Jumpponen and Trappe 1998).
AM morphology is distinguished into Arum-type and Paris-type. The Arum-type association is characterized by intercellular hyphal growth in the root cortex, with short lateral branches into cortical cells forming arbuscules (Smith and Smith 1997). Intracellular hyphal coils frequently having intercalary arbuscules spreading cell to cell in the cortex characterize the Paris-type association. The Arum-type has been reported to be abundant in agricultural crops, whereas, the Paris-type has been found to be more frequent in plants in natural ecosystems (Smith and Smith 1997; Yamato and Iwasaki 2002; Ahulu et al. 2005; Tsuyuzaki et al. 2005). Though physiological or functional disparity between Arum-type and Paris-types has not yet been fully elucidated, it has been reported that the development of Arum-type is faster than that of Paris-type (Brundrett and Kendrick 1990b; Cavagnaro et al. 2001). Anatomical characters of host roots are thought to influence the AM morphology (Brundrett and Kendrick 1988, 1990a,b). However, Kubato et al. (2005) indicated that the morphology of AM type is the result of the interaction between both the plant and the fungal species. It is therefore necessary to examine a wider range of plants growing in different habitats to fully understand the control of Arum- and Paris-type.
In contrast to the assumption that DSE associations are more frequent in cold, nutrient stressed environments, recent reports indicate their prevalence in arid and semiarid regions (Barrow et al. 1997; Miller et al. 1999; Muthukumar and Udaiyan 2002a). However, the ecological role of DSE fungi is currently unresolved and recent studies do indicate their potential to function as plant growth promoters under stressed environments (Newsham 1999; Barrow and Osuna 2002; Jumpponen 2001). Jumpponen and Trappe (1998) summarized the incidence of DSE associations in nearly 6,000 plants species representing about 320 genera and 100 families, but only 59 were tropical plant species (Jumpponen and Trappe 1998).
Around 35,000 to 70,000 medicinal and aromatic plants (MAPs) are used in various systems of medicine in different parts of the globe (Farnsworth and Soejarto 1991). Cultivation of MAPs is being currently carried out to meet the increasing demand for herbal drugs. In addition to conventional cultivation of MAPs, recent emphasis is on exploiting useful and appropriate soil microorganisms present in the rhizosphere of medicinal plants (Sen 1998). Although researchers (Udea et al. 1992; Muthukumar and Udaiyan 2001; Khade et al. 2002; Bukhari et al. 2003) have reported the wide spread occurrence of AM fungal associations in MAPs, there exists no information on the distribution of AM morphological types in MAPs nor on the occurrence of DSE associations in MAPs. In view of this, we analyzed the distribution of AM morphological types and the incidence of DSE associations in certain MAPs occurring in the Western Ghats region of Southern India, as well as the occurrence of AM types and DSE associations in function of plant growth forms (herbaceous, shrubs, trees, etc.).
Materials and methods
Sample collection
Roots and soil samples were collected from five individuals for 107 MAPs belonging to 98 genera in 52 families at different stages of growth (vegetative and reproductive) between December 2004 and February 2005 from different vegetation types in the Western Ghats region, Southern India (Tables 1 and 2). Care was taken during collection that roots of shrubs and tree species were positively identified. Roots were washed and stained within 24 h or preserved in FAA (formalin: glacial acetic acid: 70% ethanol, 5:5:90) up to processing. Rhizosphere soils shaken from roots of different individuals of a species were collected and were mixed to form a composite sample. These composite soil samples were used for chemical analyses and enumeration of AM fungal spores.
Determination of soil characteristics
Soil pH was determined in 1:1, soil: water (v:v), total nitrogen (N), and total phosphorus (P) were determined according to Jakson (1971), exchangeable potassium (K) was determined after extraction with ammonium acetate (Davis 1962), and soil organic matter was assessed according to Piper (1950).
Estimates of AM and DSE colonization
FAA fixed roots were washed, cleared in 2.5% KOH at 90°C (Koske and Gemma 1989), acidified with 5N HCl and stained with trypan blue (0.05% in lactoglycerol). Roots that remained dark after clearing were bleached with 3% H2O2 before staining. The roots were left overnight in trypan blue-lactoglycerol for staining. For observation of DSE, cleared roots were observed either directly or placed in lactoglycerol containing 0.05% trypan blue and left overnight. Fifty 1-cm long stained or unstained root samples were mounted on microscope slides in lactoglycerol and examined for AM fungal structures, melanized hyphae, and microsclerotia. Only species in which arbuscules were found were considered to be arbuscular mycorrhizal. Root length colonization by AM fungi or DSE was estimated according to the intersect method of McGonigle et al. (1990).
Enumeration and isolation of AM fungal spores
One hundred gram of soil was dispersed in 1 l water and decanted through a series of 710- to 38-μm sieves. Residues were filtered through gridded filter papers and all intact spores (noncollapsed spores with cytoplasmic contents, free from parasitic attack) were counted using a dissection microscope at ×40 magnification. Sporocarps and spore clusters were considered as one unit. Intact AM fungal spores were mounted in polyvinyl alcohol–lactoglycerol with or without Melzers reagent for identification using keys of Schenck and Perez (1990) and INVAM (http://www.invam.caf.wvu.edu). Because of the generally poor state of field material, and the low abundance of certain morphotypes, species identification was performed only with sufficient spores (minimum 25) in good condition (no sign of degradation or parasitism). Some could be identified only up to the genus.
Life history attributes and plant nomenclature
Each plant species recorded during the survey was categorized for life-form attributes as determined from the literature (Prain 1981a,b; Nair and Henry 1983; Henry et al. 1987, 1989) or field observations. Nomenclature and authorities are as used by Nair and Henry (1983), Henry et al. (1987, 1989) for angiosperms, and Dixit (1984) and Satija and Bir (1985) for pteridophytes.
Results
Soil and AM fungal characteristics of the study sites
The soils in the study sites were slightly alkaline and low in available nutrients (Table 1). A total of twenty-three AM fungal spore morphotypes could be confidently distinguished on the basis of spore morphology, but only spores of eleven morphotypes could be identified to the species level. These included one species in Acaulospora (Acaulospora scrobiculata) and Gigaspora (Gigaspora gigantea), six in Glomus (Glomus aggregatum, Glomus sinuosa, Glomus taiwanensis, Glomus mosseae, Glomus geosporum, and Glomus viscosum) and three in Scutellospora (Scutellospora calospora, Scutellospora heterogama, Scutellospora persica).
Incidence of AM and DSE associations in plant species
In the present study, 79 out of 107 MAP species were colonized by AM fungi (Table 2). AM were not observed in Crossandra infundibuliformis, Strobilanthes asperrimus (Acanthaceae), Adiantum capillus-veneris (Adiantaceae), Aerva lanata, Achyranthes aspera, Celosia cristata, Gomphrena globosa, Gomphrena serrata (Amaranthaceae), Artabotrys hexapetalus, (Annonaceae), Catharanthus roseus (Apocynaceae), Trichodesma indicum (Boraginaceae), Salacia chinensis (Celastraceae), Commelina bengahlensis (Commelinaceae), Cyperus rotundus (Cyperaceae), Mentha arvensis (Labiatae), Artocarpus heterophyllus, Ficus benghalensis (Moraceae), Brachiaria ramosus, Cynodon dactylon, Cymbopogan caesius, Vetiveria zizanoides (Poaceae), Pleopeltis macrocarpa, Nephrolepis cordifolia (Nephrolepidaceae), Thecagonum pteritum (Rubiaceae), Murraya koenigii (Rutaceae), Solanum nigrum (Solanaceae), Lantana camara and Vitex negundo (Verbenaceae).
DSE were recorded in 38 of the 107 MAP species examined: Acalypha indica, A. capillus-veneris, Allium cepa, Aloe vera, Amaranthus viridis, Asparagus racemosus, Bambusa arundinacea, Cardiospermum halicacabum, C. cristata, Chrysanthemum cinerariifolium, Cissus quadrangularis, C. infundibuliformis, Curcuma longa, C. caesius, Cyperus rotundus, Euphorbia hirta, Gloriosa superba, Jasminum sambac, Justica adhatoda, Michelia champaca, Mimosa pudica, Moringa pterygosperma, Musa paradisiaca, N. cordifolia, Nyctanthes arbor-tristis, Ocimum tenuiflorum, Ophioglasum reticulatum, Oxalis corymbosa, Pergularia daemia, Perotis indica, Peperomia thomsonii, P. macrocarpa, Pseudarthira viscida, Punica granatum, Rostellularia procumbens, Solanum melongena, V. zizanoides, and Zingiber officinale.
Distribution of AM and DSE associations in plant families
It was possible to discriminate between Arum- and Paris-type AM at family levels only in certain cases. These were analyzed for families where three or more plant species were available (Table 2). Arum-type was found in Acanthaceae and Euphorbiaceae (except Phyllanthus emblica) and Paris-type was found in Oxalidaceae. Both Paris- and Arum-types were present in Amaranthaceae, Asclepiadaceae, Compositae, Lilliaceae, and Piperaceae. Similarly, both Arum- and intermediate types were present in Solanaceae and Zingiberaceae. Five plant species Hibiscus rosa-sinensis (Malvaceae), Scoparia dulcis (Scrophulariaceae), Solanum surattense (Solanaceae), Centella asiatica (Umbelliferae) and Z. officinale (Zingiberaceae), had both intercellular hyphae, intracellular hyphal coils, inter- or intracellular vesicles, arbusculate structures or Arum-type arbuscules in their cortex indicating an intermediate between the Arum- and Paris-type morphologies. Plant families where AM morphology was previously unknown were: Paris-type in Oxalidaceae, Caricaceae, Convolvulaceae, Musaceae, Nyctanthaceae, Santalaceae, Punicaceae, Moringaceae, Arum-type in Acanthaceae, and Arum- and Paris-types in Piperaceae.
Of the 52 plant families examined, DSE associations were found in 27 MAP families (Table 2), which included Acanthaceae, Adiantaceae, Amaranthaceae, Asclepiadaceae, Asparagaceae, Compositae, Cyperaceae, Euphorbiaceae, Labiatae, Liliaceae, Magnoliaceae, Mimosaceae, Moringaceae, Musaceae, Nephrolepidaceae, Nyctanthaceae, Oleaceae, Ophioglosaceae, Oxalidaceae, Papilionaceae, Piperaceae, Poaceae, Polypodiaceae, Punicaceae, Sapindaceae, Solanaceae, and Zingiberaceae.
Extent and morphology of AM and DSE associations
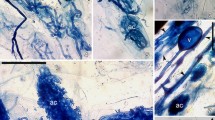
There were large differences between patterns of colonization in MAPs (Fig. 1), and in the colonized root lengths occupied by hyphal coils, intercellular, intracellular hyphae, arbusculate coils, arbuscules, and vesicles (Table 2). The root entry by AM fungi was characterized by the formation of an appressorium (Fig. 1a). The Arum-type mycorrhizas were characterized by the presence of intercellular hyphae, arbuscules, and vesicles (Fig. 1b–d). Intracellular hyphal coils, arbusculate structures, and intracellular vesicles characterized the Paris-type (Fig. 1e,f). Intracellular hyphal coils as well as intercellular hyphae, arbuscules, and vesicles, characterized the intermediate types. The extent of colonization ranged between 49% (Sphaeranthus indicus, Compositae; Mirabilis jalapa, Nyctaginaceae) to 84% (Santalum album, Santalaceae).
Light and phase contrast micrographs showing arbuscular mycorrhizal types in medicinal and aromatic plants. a Appressorium (ap) on the root surface of Nyctanthes arbor-tristis. b–d Arum-type mycorrhizas in Hibiscus rosa-sinensis (b), Amaranthus spinosus (c), Datura metel (d). Paris-type mycorrhizas in Gloriosa superba (e) and Santalum album (f) (a arbuscule, at arbuscular trunk, v vesicle; Scale bar = 50 μm)
The extent of DSE colonization ranged between 27% (A. indica, Euphorbiaceae) and 56% (A. racemosus, Asparagaceae). The pattern of DSE colonization was similar in roots of different MAPs. Among the MAP species examined, the majority (65%) had root colonization levels ranging between of 40 and 50%. Root entry by DSE was characterized by the presence of an appressorium (Fig. 2a). DSE were frequently characterized by narrow, septate, runner hyphae (2–4 μm wide) commonly occurring on the root surface and typically running parallel to the long axis of roots (Fig. 2b). Individual hyphae sometimes grew along the grooves between adjacent epidermal cells and colonized roots intercellularly. Runner hyphae on the root surface were infrequently branched at a 90° angle to the main hyphae, and occasionally bore swollen tips. The hyphae penetrated the epidermis and tended to coil in cortical cells of the outer or inner layers (Fig. 2c). Penetration through root hair was not observed. Once in the epidermis, hyphae grew from cell to cell within the epidermis parallel to the main axis of the host root, usually causing no distortion of host root. At regions the hyphae penetrated the cortical cells filling each cell with distinct sclerotia (Fig. 2d). There was no morphological distinction in intraradical and extramatrical hyphae. The root stele was not colonized in any of the plant species that had DSE fungal association and there was no evidence of damage to host root tissues arising from fungal colonization.
Dark septate endophytic fungal association in medicinal and aromatic plants. a Appressorium (ap) on root surface in Euphorbia hirta. b Runner hyphae (rh) and extraradical hyphae in Perotis indica. c Intraradical hyphae in Asparagus racemosus. d Microsclerotia within root cortical cells of Curcuma longa. (Scale bar: a, b = 100 μm, c, d = 50 μm)
Extent of AM and DSE colonization in plant growth forms
The AM association was present in 78% of herbs, 58% of shrubs/undershrubs, and 89% of tree species. The extent of colonization ranged between 43%(Sida acuta, Malvaceae) to 83% (S. dulcis, Scrophulariaceae; S. melongena, Solanaceae) in herbs, 55% (Lawsonia inermis, Lythraceae; C. quadrangularis, Vitaceae) to 83% (H. rosa-sinensis, Malvaceae) in shrubs/undershrubs and 50% (M. pterygosperma, Moringaceae) to 84% (S. album, Santalaceae) in trees.
The DSE association was present in 43% of herbs, 29% of shrubs/undershrubs, and 11% of tree species. The extent of DSE fungal colonization was similar, with mean colonization levels of 43% in herbs, 41% in shrubs or undershrub, and 45% in tree species. The extent of DSE colonization ranged between 27% (A. indica, Euphorbiaceae) to 56% (A. racemosus, Asparagaceae) in herbs, 32% (O. tenuiflorum, Labiatae) to 43% (P. viscida, Papilionaceae; P. granatum, Punicaceae; C. quadrangularis, Vitaceae) in shrubs, and 42% (M. champaca, Magnoliaceae) to 49% (N. arbor-tristis, Nyctanchaceae) in trees.
Plant growth forms and AM morphology
Distribution of Arum- and Paris-type AM in MAPs could be linked to plant growth characters. Arum-type was found in 56% of herbs, 67% of shrubs/undershrubs, and 38% of trees. Paris-type was found in 62% of trees, 37% of herbs, and 25% of shrubs/undershrubs. The intermediate type was not found in trees whereas 8% of herbs and shrubs/undershrubs had intermediate AM types. Arum-type was more prevalent in annuals than in perennials. In contrast, Paris- and intermediate-types were prevalent in perennial species (Fig. 3). Incidence of DSE associations was greater in perennials than in annuals.
Discussion
AM and DSE associations in this study was present in roots of, respectively, 75 and 36% of the MAPs examined. AM associations were found in Amaranthus spinosus, and A. viridis, which belong to the presumed nonmycorrhizal family Amaranthaceae (Tester et al. 1987). In the present study, frequency of mycorrhizas in different plant life forms were in the order of trees > herbs > shrubs/undershrubs. This is in accordance with the fact that trees are more mycorrhiza-dependent than other plant life forms. The high mycorrhizal dependence of tree species is thought to arise from the fact that trees commonly have low rooting densities in soil (Baylis 1975). With the exception of two families, species within 18 plant families had the same AM morphology as in earlier descriptions (Smith and Smith 1997; Yamato and Iwasaki 2002; Muthukumar et al. 2003; Ahulu et al. 2005), indicating that AM morphology is influenced by the identity of the host plant. Studies do indicate that the same AM fungus that formed Arum-type mycorrhiza in a host species also formed the Paris-type in a different host (Gerdemann 1965; Jacquelinet-Jeanmougin and Gianinazzi-Pearson 1983). The formation of the two AM types is thought to be related to the presence of continuous longitudinal air spaces in the root cortex, so that hyphae grow along the intercellular air spaces in the Arum-type, and intracellularly in their absence in the Paris-type (Brundrett and Kendrick 1988, 1990b). Intermediate morphological types tend to occur in species with discontinuous intercellular air spaces (Smith and Smith 1997). However, Imhof and Weber (1997, 2000) noted that Voyria obconica formed Paris-type AM inspite of presence of clear intercellular spaces in the root cortex. In the present study, AM morphology of individual MAPs of the same species was consistent. This indicates that genetic factors pertaining to the host plant influence AM morphology, as more than one AM fungal species can colonize the roots of an individual plant (Van Tuinen et al. 1998; Helgason et al. 1999). Species of Oxalidaceae, Caricaceae, Amaranthaceae, Convolvulaceae, Musaceae, Acanthaceae, Nyctaginaceae, Piperaceae, Santalaceae, Punicaceae, and Moringaceae (Vijayalakshmi and Rao 1988; Neeraj et al. 1991; Muthukumar and Udaiyan 2000) have been reported to be AM but their AM morphology had not been described. In this study, it was found that Oxalidaceae, Caricaceae, Convolvulaceae, Musaceae, Nyctaginaceae, Santalaceae, Punicaceae, and Moringaceae had Paris-type; Acanthaceae and Sapindaceae had Arum-type and Amaranthaceae and Piperaceae had more than one type of AM morphology.
DSE associations were observed in roots of several plant families examined in the present study. DSE are reported for the first time in nine plant families (Amaranthaceae, Moringaceae, Punicaceae, Piperaceae, Nyctaginaceae, Zingiberaceae, Acanthaceae, Musaceae, and Asclepiadaceae) and 37 plant species. Among plant species harboring a DSE association in the present study, only DSE occurrence was known for C. rotundus (Cyperaceae) (Jumpponen and Trappe 1998; Muthukumar and Udaiyan 2002a). However, no DSE association was found in sixteen plant families for which this association has previously been reported. These families include Compositae, Rubiaceae, Convolvulaceae, Rutaceae, Piperaceae, Santalaceae, Rosaceae, Myrtaceae, Anacardiaceae, Poaceae, Scrophulariaceae, Vitaceae, Apocynaceae, Boraginaceae, Caesalpiniaceae, and Cycadaceae (Jumpponen and Trappe 1998; Muthukumar and Udaiyan 2002a). Similarly four plant species Citrus limon (Rutaceae), S. nigrum (Solanaceae), Cycas circinalis (Cycadaceae) and Psidium sp. (Myrtaceae) reported to form DSE associations (Jumpponen and Trappe 1998; Muthukumar and Udaiyan 2002b), lacked the association in the present study.
The frequency of Arum-type mycorrhiza in each plant growth form was shrubs/undershrubs > herbs > trees, whereas it was the inverse for Paris-type trees > herbs > shrubs/undershrubs. Yamato and Iwasaki (2002) found Paris-type in nine out of 10 herbaceous understory plants of some Japanese deciduous forests, and noted that Paris-type was more frequent than Arum-type in each level of plant taxonomy from species to family. A majority of herbs examined by Ahulu et al. (2005) formed Arum-type and there were more Paris-type than Arum-type plant families in a mixed pine forest on a sand dune in central Hosshu, Japan. Also in the present study, only Paris-type mycorrhiza were found in 12 plant families compared to the Arum-type, which was found in eight plant families. O’Connor et al. (2001) found Arum-type mycorrhiza in 21 herbaceous plant species growing in an Australian desert, whereas, in the present study, both Arum- and Paris-type occurred in 56% and 37% of herbs, respectively. These differences in distribution of Arum- and Paris-types in different studies could be the result of differences in plant species composition and environmental factors operating at different sites.
DSE associations were most prevalent in herbs compared to shrubs and trees. This is in accordance with several previous studies where DSE were frequently found in herbs and infrequently in trees species (Ahlich and Sieber 1996; Ruotsalainen et al. 2002; Urcelay 2002; Barrow 2003). Only three tree species N. arbor-tristis (Nyctanthaceae), M. pterygosperma (Moringaceae), and M. champaca (Magnoliaceae) in the present study had DSE associations. Microsclerotia were observed in root cortical cells and their frequency of occurrence varied with the plant species. No fungal structures were found in the root stelar region of any of the plants examined which contrasts with reports by Yu et al. (2001) of occasional penetration of the vascular cylinder by P. frontii hyphae, and by Barrow (2003) of DSE fungi within sieve elements of Bouteloua spp. Root colonization by DSE has been observed simultaneously with AM or ectomycorrhizal fungi (Jumpponen and Trappe 1998). We observed such simultaneous occurrence of AM fungi and DSE in 30 plant species. This type of dual colonization by different root-associated fungi reflects a dynamic nature of the root-colonizing fungal community. DSE are known to frequently colonize older parts of the root system (Jumpponen and Trappe 1998), suggesting that they prefer ageing root tissue or that they are recycling nutrients from the senescent or dead root cells. In the present study, care was taken not to include old or dead roots for assessment and DSE was present in young roots suggesting a concurrent colonization with AM fungi. It has been proposed that DSE enhance root functions of native plants in arid ecosystems, where they are chronically exposed to very dry soils (Barrow 2003). Low soil moisture in the tropics likewise imposes problems like root desiccation, reduced mineralization, uptake of nutrients, and maintenance of adequate water relationships for plant survival. The widespread occurrence of DSE in tropical soils, as indicated in this study, emphasizes their potential to function as mutualistic fungi along with mycorrhizal fungi (Jumpponen 2001; Barrow and Osuna 2002). Further studies are ongoing to characterize the AM fungi and DSE involved and to examine their potential for promoting the growth of MAPs.
References
Ahlich K, Sieber TN (1996) The profusion of dark septate endophytic fungi in non-ectomycorrhizal fine roots of forest trees and shrubs. New Phytol 132:259–270
Ahulu EM, Nakata M, Nonaka M (2005) Arum- and Paris-type arbuscular mycorrhizas in a mixed pine forest on sand dune soil in Niigata Pretecture, central Honashu, Japan. Mycorrhiza 15:129–136
Barrow JR (2003) A typical morphology of dark-septate fungal root endophytes of Bouteloua in arid southwestern USA rangelands. Mycorrhiza 13:239–247
Barrow JR, Havsrtad KM, McCaslin BD (1997) Fungal root endophytes in fourwing saltbush, Atriplex canescens on arid rangelands of southwestern USA. Arid Soil Res Rehabil 11:177–185
Barrow JR, Osuna P (2002) Phosphorus solubilization and uptake by dark septate fungi in fourwing saltbush, Atriplex carescens (Pursh) Nutt. J Arid Environ 51:449–459
Baylis GTS (1975) The magnoloid mycorrhiza and mycotrophy in root systems derived from it. In: Sanders FE (ed) Endomycorrhizas. Academic, New York, pp 373–389
Brundrett MC, Kendrick B (1988) The mycorrhizal status, root anatomy and phenology of plants in sugar maple forest. Can J Bot 66:1153–1173
Brundrett MC, Kendrick B (1990a) The roots and mycorrhizas of herbaceous woodland plants. I. Quantitative aspects of morphology. New Phytol 114:457–468
Brundrett MC, Kendrick B (1990b) The roots and mycorrhizas of herbaceous woodland plants. II. Structural aspects of morphology. New Phytol 114:469–479
Bukhari MJ, Khade SW, Jaiswal V, Gaonkar UC, Rodrigues BF (2003) Arbuscular mycorrhizal (AM) status of tropical medicinal plants: a field survey of arbuscular mycorrhizal fungal association in herbs. Plant Arch 3:167–174
Cavagnaro TR, Gao L-L, Smith FA, Smith SE (2001) Morphology of arbuscular mycorrhizas is influenced by fungal identity. New Phytol 151:469–475
Davis DJ (1962) Emission and absorption spectrochemical methods. In: Peach K, Tracey MV (eds) Modern methods of plant analysis. Springer, Berlin Heidelberg New York, pp 1–25
Dixit RD (1984) (ed) A census of the Indian pteridophytes. Botanical Survey of India, New Delhi, India
Farnsworth NR, Soejarto DD (1991) Global importance of medicinal plants. In: Akerele O, Heywood V, Synge H (eds) The conservation of medicinal plants. Cambridge University Press, Cambridge, UK, pp 25–51
Gerdemann JW (1965) Vesicular-arbuscular mycorrhizas formed on maize and tulip tree by Endogone fasciculata. Mycologia 57:562–575
Helgason T, Fitter AH, Young JPW (1999) Molecular diversity of arbuscular mycorrhizal fungi colonizing Hyacinthoides non-scripta (blue bell) in a seminatural woodland. Mol Ecol 8:659–666
Henry AN, Kumari GR, Chitra V (eds) (1987) Flora of Tamil Nadu, India, vol 2. Botanical Survey of India, Coimbatore, India
Henry AN, Chitra V, Balakrishnan NP (eds) (1989) Flora of Tamil Nadu, India, vol 3. Botanical Survey of India, Coimbatore, India
Imhof S, Weber HC (1997) Root anatomy and mycotrophy (AM) of the achlorophyllous Voyria truncata (Standley) Standely & Steyermark (Gentianaceae). Acta Bot 110:127–134
Imhof S, Weber HC (2000) Root structures and mycorrhiza of the achlorophyllus Voyria obconica Progel (Gentianaceae). Symbiosis 29:209–211
Jacquelinet-Jeanmougin S, Gianinazzi-Pearson V (1983) Endomycorrhizas in the Gentianaceae. I. The fungus associated with Gentiana lutea L. New Phytol 95:663–666
Jakson ML (ed) (1971) Soil chemical analysis. Prentice Hall, New Delhi
Jumpponen A (2001) Dark septate endophytes—are they mycorrhizal? Mycorrhiza 11:207–211
Jumpponen A, Trappe JM (1998) Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytol 140:295–310
Khade SW, Bukhari MJ, Jaiswal V, Gaonkar UC, Rodeigues BF (2002) Arbuscular mycorrhizal status of medicinal plants: a field survey of AM fungal association in shrubs and trees. J Econ Taxon Bot 26:571–578
Koske RE, Gemma JN (1989) A modified procedure of for staining roots to detect VA mycorrhizas. Mycol Res 92:486–488
Kubato M, McGonigle TP, Hyakumachi M (2005) Co-occurrence of Arum- and Paris-type morphologies of arbuscular mycorrhizae in cucumber and tomato. Mycorrhiza 15:73–77
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi. New Phytol 115:495–501
Miller RM, Smith CI, Jastrow JD, Bever VD (1999) Mycorrhizal status of the genus Carex (Cyperaceae). Am J Bot 86:547–553
Muthukumar T, Udaiyan K (2000) Arbuscular mycorrhizas of plants growing in the Western Ghats region, Southern India. Mycorrhiza 9:297–313
Muthukumar T, Udaiyan K (2001) Vesicular arbuscular mycorrhizal association in medicinal plants of Maruthamalai Hills, Western Ghats, Southern India. J Mycol Plant Pathol 31:180–184
Muthukumar T, Udaiyan K (2002a) Seasonality of vesicular–arbuscular mycorrhizae in sedges in a semi-arid tropical grassland. Acta Oecol 23:337–347
Muthukumar T, Udaiyan K (2002b) Arbuscular mycorrhizas in cycads of Southern India. Mycorrhiza 12:213–217
Muthukumar T, Sha L, Yang X, Cao M, Tang J, Zheng Z (2003) Mycorrhiza of plants in different vegetation types in tropical ecosystems of Xishuanbanna, southwest China. Mycorrhiza 13:289–297
Nair NC, Henry AN (eds) (1983) Flora of Tamil Nadu, India, vol 1. Botanical Survey of India, Coimbatore, India
Neeraj Shanker A, Mathew J, Varma A (1991) Occurrence of vesicular–arbuscular mycorrhizae with Amaranthaceae in soils of the Indian semi-arid region. Biol Fertil Soils 11:140–144
Newsham KK (1999) Phialophora graminicola, a dark septate fungus, is a beneficial associate of the grass Vulpiaciliata ssp. ambigua. New Phytol 144:517–524
O’Connor PJ, Smith SE, Smith FA (2001) Arbuscular mycorrhizal associations in the Southern Simpson Desert. Aust J Bot 49:493–499
Piper CS (ed) (1950) Soil and plant analysis. Interscience, New York, USA
Prain D (ed) (1981a) Bengal plants, vol 1. Bishen Singh Mahendra Pal Singh, Dehradun, India
Prain D (ed) (1981b) Bengal plants, vol 2. Bishen Singh Mahendra Pal Singh, Dehradun, India
Ruotsalainen AL, Väre H, Vesterg M (2002) Seasonality of root fungal colonization in low-alpine herbs. Mycorrhiza 12:29–36
Satija CK, Bir SS (eds) (1985) Polypodiaceous ferns of India. Today and Tomorrow’s Printers and Publishers, New Delhi, India
Schenck NC, Perez Y (eds) (1990) Manual for the identification of VA mycorrhizal fungi. Synergistic, Gainesville, FL
Sen AN (1998) Harnessing of soil microorganisms for the benefit of medicinal plants. In: Gautam PL, Raina L, Srivastava U, Rayachaudhuri SP, Singh BB (eds) Prospects of medicinal plants. Indian Society of Plant Genetic Resources, New Delhi, pp 275–280
Sieber TN (2002) Fungal root endophytes. In: Waisel Y, Amram E, Kafkafi U (eds) Plant roots: the hidden half. Marcel Dekker Inc, New York, USA, pp 887–917
Smith SE, Read DJ (eds) (1997) Mycorrhizal symbiosis. Academic, San Diego, CA
Smith FA, Smith SE (1997) Structural diversity in (vesicular)–arbuscular mycorrhizal symbioses. New Phytol 137:373–388
Tester M, Smith SE, Smith FA (1987) The phenomenon of non-mycorrhizal plants. Can J Bot 65:419–431
Tsuyuzaki S, Hase A, Niinuma H (2005) Distribution of different mycorrhizal classes on Mount Koma, northern Japan. Mycorrhiza 15:93–100
Udea T, Husoe T, Kubo S, Nakawashi I (1992) Vesicular arbuscular mycorrhizal fungi (Glomales) in Japan II: a field survey of vesicular arbuscular mycorrhizal association with medicinal plants in Japan. Trans Mycol Soc Japan 33:77–86
Urcelay C (2002) Co-occurrence of three fungal root symbionts in Gaultheria poeppigii DC in Central Argentina. Mycorrhiza 12:89–92
Van Tuinen D, Jacquot E, Zhao B, Gollotte A, Gianazzi-Pearson V (1998) Characterization of root colonization profiles by microcosm community of arbuscular mycorrhizal fungi using 25S r DNA targeted nested PCR. Mol Ecol 7:879–887
Vijayalakshmi M, Rao AS (1988) Vesicular–arbuscular mycorrhizal associations of some Asteraceae and Amaranthaceae. Acta Bot Indica 16:168–174
Yamato M, Iwasaki M (2002) Morphological types of arbuscular mycorrhizal fungi in roots of understorey plants in Japanese deciduous broadleaved forests. Mycorrhiza 12:291–296
Yu T, Nassuth A, Peterson RL (2001) Characterization of the interaction between dark septate fungus Philaocephala fortinni and Asparagus officinalis roots. Can J Microbiol 47:741–753
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muthukumar, T., Senthilkumar, M., Rajangam, M. et al. Arbuscular mycorrhizal morphology and dark septate fungal associations in medicinal and aromatic plants of Western Ghats, Southern India. Mycorrhiza 17, 11–24 (2006). https://doi.org/10.1007/s00572-006-0077-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-006-0077-2