Abstract
Native grasses of semi-arid rangelands of the southwestern USA are more extensively colonized by dark septate endophytes (DSE) than by traditional mycorrhizal fungi. Roots of dominant grasses (Bouteloua sp.) native to arid southwestern USA rangelands were prepared and stained using stains specific for fungi (trypan blue) and for lipids (sudan IV). This revealed extensive internal colonization of physiologically active roots by atypical fungal structures that appear to function as protoplasts, without a distinguishable wall or with very thin hyaline walls that escape detection by methods staining specifically for fungal chitin. These structures were presumed to be active fungal stages that progressed to form stained or melanized septate hyphae and microsclerotia characteristic of DSE fungi within dormant roots. The most conspicuous characteristic of these fungi were the unique associations that formed within sieve elements and the accumulation of massive quantities of lipids. This interface suggests a biologically significant location for carbon transfer between the plant and fungus. The continuous intimate association with all sieve elements, cortical and epidermal cells as well as external extension on the root surface and into the soil indicates that they are systemic and considerably more prevalent than previously thought. A fungal network associated with a mucilaginous complex observed on the root surface and its potential role in root function in dry soil is discussed. It is suggested that those fungi that non-pathogenically and totally colonize plant cells be classed as systemic endophytic fungi (SEF). This would refine the broad designation of DSE fungi. The potential mutualistic benefit of SEF for native plants in arid ecosystems based on the extent of lipid accumulation and its apparent distribution is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The roots of all vascular plants are universally colonized by diverse groups of fungi. These plant fungal associations range from pathogenic to mutually beneficial. Root pathogens invade the vascular cylinder, plug or destroy the conductive tissues, and induce wilting and stunting. This results in reduced productivity or death of the host plants. On the other hand, mycorrhizal fungi have significant and well-documented ecological roles in the nutrition and survival of host plants in natural ecosystems. Mycorrhizal fungi are restricted to and grow both inter- and intracellularly in cortical and epidermal cells and on the root surface. Mycorrhizal associations enhance plant nutrition, water relationships and other parameters in exchange for photosynthetic carbon (Smith and Read 1997). Grasses of both managed and native grasslands are naturally infected by other fungal endophytes that enhance host vigor, competitive ability and resistance to pests (Clay 1990).
Plant roots are colonized also by numerous species of saprophytic fungi with extensive, symptomless endophytic or biotrophic phases in their life cycles that are not apparent to causal observers (Parbery 1996). Increased attention has been given to an ubiquitous group of miscellaneous ascomycetous fungi, designated as dark septate endophytes (DSE). Melanization of their septate hyphae and microsclerotia gives them their characteristic dark color. DSE fungi may function as pathogens or saprophytes, as well as forming mutualistic associations similar to mycorrhizas (Jumpponen 2001). DSE are found extensively in cold, nutrient-stressed environments where AM fungi do not proliferate (Kohn and Stasovski 1990). A higher incidence of DSE than AM fungi was found in Carex sp. in subarctic alpine regions (Haselwandter and Read 1982; Ruotsalainen et al. 2002). Barrow et al. (1997) found DSE to be more prevalent than conventional mycorrhizae in native grasses and shrubs in the warm semi-arid rangelands of the southwestern USA.
DSE are easily visible as stained or pigmented structures under low-power light microscopy. They are observed most frequently growing inter- and intracellularly within the cortex and the epidermis and on root surfaces. Hyaline hyphae that are continuous with stained or melanized structures have been reported by Barrow and Aaltonen (2001), Haselwandter and Read (1982), Newsham (1999) and Yu et al. (2001). Barrow and Aaltonen (2001) carefully examined histochemically stained roots of Atriplex canescens, an important chenopodaceous shrub indigenous to arid southwestern USA rangelands, with high magnification Nomarski differential interference contrast (DIC) microscopy. They observed active non-staining, non-pigmented fungal structures that non-destructively and predominantly colonized sieve elements and were also found frequently in cortical cells. Active forms consisted of hyphae with distinct non-staining, non-pigmented hyaline walls and presumed protoplasts where fungal walls were not microscopically distinguishable. These active structures escaped detection by conventional fungus staining and microscopic methods.
The ecological role of DSE fungi is currently unresolved. However, their widespread occurrence in cold or drought-stressed ecosystems, their potential to function as mycorrhizal fungi and the extensive internal colonization by active structures suggest that these endophytes are significant components of stressed ecosystems (Barrow and Osuna 2002; Haselwandter and Read 1982; Jumpponen 2001). The objective of this study was to analyze the roots of native grasses belonging to Bouteloua to determine the nature, location and extent of active forms of DSE fungi.
Materials and methods
Root collections
Roots were sampled weekly during 2001 from native populations of black grama, Bouteloua eriopida sp. (Torr.) Torr., and periodically from native populations of Bouteloua curtipendula Michx. A. Gray. These populations were located on the Jornada Experimental Range of the USDA Agricultural Research Service in southern New Mexico. Soil was chronically dry and soil moisture was generally less than 3% at most sampling times, except for brief periods following precipitation events, when the soil was nearly saturated. Blue grama, Bouteloua gracilis (Kunth) Lag. Ex Steud., from various northern and high elevations was also collected periodically. B. gracilis is adapted to more mesic, higher elevations than B. eropida on the Jornada Experimental Range. Some samples were taken from actively growing sites that received normal and above-normal precipitation during the growing season. Fungal colonization was determined to be consistent and expression was uniform in this and previous studies within the population at each sampling period. For this study, roots from 2–5 plants of each species were collected, bulked, sealed in a plastic bag and taken to the laboratory for preparation and analysis.
Root preparation and clearing
Methods developed by Bevege (1968), Brundrett et al. (1983), Kormanik et al. (1980), and Phillips and Hayman (1970) were modified for optimal visualization of fungi in native grass roots. Roots were washed in tap water to remove soil. From each bulked sample, healthy feeder roots of uniform maturity and appearance (approximately 0.25 mm in diameter) were randomly selected and cleared by placing in an autoclave in 2.5% KOH. The temperature was increased to121°C over 5 min, maintained for 3 min, and samples were removed from the autoclave after 8 min. Roots were rinsed in tap water, bleached in 10% alkaline H2O2 for 10–45 min to remove pigmentation, and placed in 1% HCl for 3 min. Decolorized roots were rinsed for 3 min in distilled water (dH2O) before staining with either trypan blue (TB), sudan IV (SIV) or both. To stain with TB, roots were placed in prepared TB stain (0.5 g trypan blue in 500 ml glycerol, 450 ml dH2O, 50 ml HCl), autoclaved at 121°C for 3 min and stored in acidic glycerol (500 ml glycerol, 450 ml H20, 50 ml HCl). For SIV staining, roots were placed in prepared SIV (3.0 g sudan IV in 740 ml 95% ETOH plus 240 ml dH2O), autoclaved at 121°C for 3 min and stored in acidic glycerol. For dual staining, roots were stained first in TB, autoclaved as above and destained 24 h in the acidic glycerol. Roots were then transferred to the SIV stain and autoclaved as above. Dual-stained roots were destained in dH2O for 3 min and stored in acidic glycerol until mounting. Chitin, a specific component of fungal walls, stained dark blue with TB. SIV stained both plant and fungal lipids bright red.
Root segments (10–12 samples 2 cm long) were placed on a microscope slide in several drops of permanent mounting medium. A cover slip was placed over the root sections and pressed firmly to facilitate analysis at high magnification. Analysis was done with a Zeiss Axiophot microscope using both conventional and DIC optics at ×1000 magnification.
Results
Lipid bodies of varied shapes and sizes were observed at times in all root cells of actively growing plants (Figs. 1, 2, 3). However, they were most prevalent in the sieve elements and frequently occupied their entire volume. Initially, most of the lipid bodies were presumed to be within plant vacuoles; however, examination of many samples showed them to have fungal coil-like shapes (Fig. 2A, D). As roots tended towards dormancy, TB-stained fibers in clumps were observed (Fig. 2B) in the same roots within a few millimeters; lipid bodies were observed forming within the clumps (Fig. 2C). Most frequently, lipid bodies in the sieve elements of physiologically active roots were not visibly associated with TB-stained tissue. However, as plants became dormant, considerable TB-stained tissue (chitin) was observed in sieve elements (Fig. 1E). When these roots were dual stained, the lipid bodies were found to be associated with the TB-stained fungal tissue (Fig. 1F). On the basis of these and continuous microscopic observation of roots sampled at varying times and conditions, it was concluded that most of the lipid bodies were associated with fungal structures. As plants became dormant, stained and melanized associations were increasingly evident, consistent with previous observations (Barrow and Aaltonen 2001). Native B. eropida roots stained with either SIV, TB or dual stained revealed a continuous non-pathogenic fungal colonization of sieve elements, cortical, epidermal cells, root hairs and the root surface. Non-staining, non-pigmented DSE fungal structures were observed at a substantially greater frequency and they differed from the commonly observed stained and pigmented hyphae and microsclerotia. Furthermore, they were uniquely different from typical mycorrhizal and pathogenic fungal structures. Protoplasts and generally hyaline structures were not evident using fungus-targeted staining and microscopy methods but required staining with SIV and careful examination at ×1000 magnification. In some root samples, both forms were found to be continuous and were regularly associated with either stained or pigmented hyphae or microsclerotia. The results indicate a high degree of polymorphism and a substantially greater incidence of internal colonization than when only stained or pigmented structures are considered. However, it will require further examination by electron microscopy to determine better the structure of these interfaces. In addition to extensive internal colonization, substantial colonization of the root surface and rhizosphere soil with morphologically similar fungal structures was also observed. Structural variability was dynamic and was likely influenced by differences in fungal taxa, the seasonal, climatic, edaphic and physiological state and the maturity of the roots.
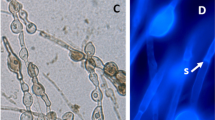
Morphological variability of dark septate endophyte (DSE) colonization in sieve elements. A Bouteloua eropida roots stained with sudan IV. Vacuoles in sieve elements of the vascular cylinder stained positively for lipids (arrow A). Cortical cells are not stained (arrow B); bar 250 µm. B Dual-stained Bouteloua curtipendula root. Sieve elements (SE) have no visible fungi; bar 10 µm. C Dual-stained B. eropida root. Sieve elements are filled with spherical and irregular-shaped, lipid-filled vacuoles stained with sudan IV (arrow); bar 10 µm. D Dual-stained B. eropida roots. Fine trypan blue-stained fibrils (arrow A) accumulating lipids (arrow B) in a sieve element; bar 3 µm. E Trypan blue-stained Bouteloua gracilis roots. Sieve elements stained only with trypan blue showing clear vesicles (arrow A) and the network staining positively for chitin; bar 10 µm. F Roots from same sample as E, dual stained showing the chitin network (stained blue) and the lipid filled vacuoles (stained red); bar 10 µm
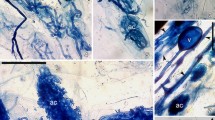
Morphological variability of DSE colonization observed in the root cortex. A Dual-stained B. gracilis roots showing fungal coils staining positively for lipids with sudan IV (arrow); bar 5 µm. There is no distinguishable fungal wall. B Dual-stained B. gracilis roots. Trypan blue-stained masses in cortical cells adjacent to vascular cylinder (arrows). The vascular cylinder (out of focus) stained for lipids; bar 5 µm. C Chitin structures (arrow A), in the same root sample as B, are associated with lipid-filled vacuoles (arrow B); bar 5 µm. D Dual-stained B. gracilis root. Intracellular lipid-filled coils with no distinguishable or stained fungal wall (arrow A). Spherical lipid vacuoles within a hyaline wall (arrow B) in an adjacent cortical cell; bar 5 µm. E Dual-stained B. gracilis root. Septate coils stained positively with trypan blue (arrow); bar 10 µm. F Dual-stained B. eropida root. Intercellular lipids forming a network around cortical cells (arrow A). Intracellular network stained positively for lipids (arrow B); bar 5 µm. G Dual-stained B. eropida root. Intercellular stained segments (arrow A). Intercellular hyaline hypha with segments staining with trypan blue; bar 10 µm. H Dual-stained B. gracilis root. Cortical cells adjacent to vascular cylinder filled with dense melanized microsclerotia and hyphae (arrow A). Underlying sieve elements are colonized with hyaline hyphae (arrow B); bar 10 µm. I Dual-stained B. gracilis root showing a unique pattern of intracellular melanized hyphae in cortical cells; bar 10 µm
Morphological variability of external colonization by DSE. A A mucilage complex (MC) on roots of a dual-stained B. eropida root. Note the vacuolated non-staining hyphae (arrows) connected to the mucilage; bar 5 µm. B Dual-stained B. curtipendula roots showing a branched network of external mucilaginous hyphae. Within these hyphae, a trypan blue-stained strand can be seen in many of the segments (arrows); bar 5 µm. C Dual-stained B. curtipendula root. Branched mucilaginous hyphae containing lipid vesicles; bar 5 µm. D Dual-stained B. eropida root showing mucilaginous hyphal extensions from the root surface that are similar in appearance to root hairs (arrow A); bar 5 µm. E Several different forms of external hyphae connected together. A trypan blue-stained network can be seen in the mucilaginous complex on the root surface and is continuous with other forms of hyphae (arrows A). The trypan blue strand continues through hyaline hyphae (arrows B) and connects to trypan blue-stained hyphae (arrow C) and melanized hyphae (arrows D); bar 10 µm. F Hyaline hyphae extending from the root to a sand grain (arrow A). A melanized segment is shown by arrow B; bar 3 µm
Colonization of sieve elements
The most surprising and prominent feature of active internal colonization by DSE fungi was the non-pathogenic inter- and intracellular colonization of all sieve elements. Figure 1 illustrates some of the variability and atypical fungal morphology observed in the sieve elements. In SIV-stained B. eriopida roots sampled during the spring, all sieve elements within the vascular cylinder had large, stained lipid bodies (Fig. 1A, arrow A). No visible fungal structures or lipids were observed in the cortex (arrow B). For brief periods during summer drought and when plants were dormant, fungal structures were not observed in sieve elements of dual-stained roots of B. curtipendula (Fig. 1B). For the most part, in more active roots, lipid bodies in sieve elements ranged from spherical to irregular in shape (Fig. 1C, arrows). In B. eriopida roots sampled following winter rains, fine fibrils of fungal tissue stained with TB were observed (Fig. 1D, arrow A) with small lipid bodies attached (arrow B). These lipid bodies were variable in shape and increased in size over time until they filled the entire volume of the sieve element (Fig. 1C). In some roots of actively growing plants sampled in the summer time from moist soil, as many as 20–40 intercellular hyphae were observed, 3–7 µm in diameter, with and without distinguishable hyaline walls and densely filled with lipid bodies. The quantity of fungal tissue and the lipid content were substantial, with fungal lipids occupying virtually the entire vascular cylinder (Fig. 1A, C). In different root preparations over the course of the study, these morphological types were found to be associated with stained or melanized septate fungal structures.
Roots of B. gracilis (Fig. 1E) were sampled as plants were entering fall dormancy near Heber, Ariz. (N 34° 37.913' W 110° 21.683) after abundant summer rainfall. Extensive TB-stained networks were observed in all sieve elements (Fig. 1E, arrows B). Within the network, clear non-staining areas were observed (arrows A). In dual-stained roots from the same sample, these areas stained positively for lipids and were attached to the fungal chitin network (Fig. 1F, arrows A). These structures are found in all sieve elements and differ from any known fungal structures. Intercellular lipid-filled hyphae, without distinguishable walls, were also observed (arrows B). These linear fungal protoplasts were traced regularly to hyphae with either hyaline, stained or pigmented walls and ranged from 3 to 7 µm in diameter. This high level of polymorphism expands the conceptual morphology of DSE fungi.
Microsclerotia were infrequently observed in sieve elements in physiologically active roots. As roots became senescent, segments of hyaline, stained, and melanized hyphae and microsclerotia were observed to form around the lipid bodies within a single sieve element.
Colonization of the cortex
The variability of fungal morphology within the cortex is illustrated in Fig. 2. Most frequently, colonization of cortical cells was not evident in physiologically active roots, while the sieve elements were densely colonized (Fig. 1A). At times, fungal structures similar to those in the sieve elements were observed growing both inter and intracellulary within the cortex. Fig. 2A (arrow) shows lipid-filled fungus coils with no visible wall in the cortex of B. eropida roots. Developing clusters of TB-stained chitin fibrils (arrows) were observed in the innermost layer of cortical cells adjacent to the vascular cylinder in B. gracilis roots (Fig. 2B). In some roots, these clusters were observed forming in outer layers of cells until they occupied all cortical cells. Underlying SIV-stained lipids in the sieve elements are evident but out of focus. At a different locus on the same root (Fig. 2C), large lipid bodies (arrow B) were seen in association with the TB-stained fibers (arrow A). These structures are similar to those observed in sieve elements (Fig. 1F). Figure 2D shows a large, linear lipid-filled fungal-like coil structure without a visible wall, which is presumed to be a protoplast (arrow A). In an adjoining cell, spherical lipid bodies can be seen inside a slightly visible hyaline wall (arrow B), showing structural differences from cell to cell. This could be attributed to morphological variability within a fungus species or differences between species. Septate coils were observed infrequently. However, in some B. gracilis and B. eriopida roots sampled following the summer monsoon season just prior to fall dormancy, coils were abundant in many of the lateral roots. Figure 2E shows TB-stained septate coils in B. gracilis roots that differed from aseptate AM coils because of the septa. Occasionally, a network staining only for lipids was observed in the intercellular spaces of all cortical cells (Fig. 2 F, arrow A). A very thin, fibrous branched intracellular network was also seen (arrow B) in B. eriopida roots. This suggests that lipid-bearing fungal protoplasts most likely grow in the apoplastic spaces, adjacent to the plant cell membrane. In some dual-stained dormant B. eriopida roots, lipids were not evident, but segments of chitin were observed forming in the intercellular spaces (Fig. 2G, arrows A). Chitin was also observed in segments of intercellular hyaline hyphae (arrow B), also indicating structural differences in the intercellular hyphae.
In dormant B. gracilis roots, dense melanized hyphae and microsclerotia were observed in the inner layer of cortical cells (Fig. 2H, arrow A). Only hyaline hyphae were seen in underlying sieve elements (Fig. 2H, arrow B). Figure 2I shows a unique pattern of melanized hyphae in cortical cells of B. gracilis roots. Microsclerotia developed only in sieve elements after roots became senescent. It is apparent that stained or pigmented microsclerotia develop from the active forms as physiological activity decreases. These observations illustrate an extensive and continuous polymorphic internal fungal network that is morphologically variable and connects sieve and cortical cells. This fungal network has been largely overlooked in the past.
Extraradical colonization
Internal hyphae grew from the intercellular regions of the epidermal cells and spread out on the root surface. External fungal structures at the root surface and rhizosphere had the same polymorphic variability observed in sieve and cortical cells. During the summer monsoons, plants were actively growing and the soil was generally moist in the root zone. Roots sampled during this period were coated with a mucilaginous gel that could also be observed microscopically. Figure 3A shows this gel as an amorphous mucilaginous complex (MC) on the surface of dual-stained B. eriopida roots. Looking at many samples, it was evident that a highly branched hyphal network occupied the MC. Figure 3A (arrows) shows non-stained hyaline vacuolated hyphae connected to and extending from the MC. Figure 3B shows a different hyphal form, not observed within root tissues, but regularly observed associated with the MC. A significant characteristic of these hyphae was a thin TB-stained internal fiber (Fig. 3B, E). These hyphae, termed mucilaginous hyphae, were common at the root surface following precipitation events; many extended 10–20 µm from the MC. One such hyphae that survived extraction from the soil and root preparation was over 300 µm long, indicating potential exploration of soil considerable distances from the root.
Mucilaginous hyphae also extended from the MC and formed root-hair-like appendages from the MC (Fig. 3C, D) in dual-stained B. eriopida roots sampled during the summer monsoon season. These differed from actual root hairs that originated from epidermal cells in that they were often branched (Fig. 3C, arrow). Figure 3D shows roots of the same plant connected by mucilaginous hyphae that also contain a linear arrangement of SIV-stained lipids, indicating the potential for carbon transport to the MC on the root surface. Figure 3E illustrates connections of several different hyphal forms of the external fungi. A fibrous network of TB-stained fibers can be seen in the MC and in the trypan blue-stained hypha (arrows A). A TB-stained fiber extends from the MC through hyaline hyphae (arrows B) and connects to a distinct TB-stained hypha (arrow C). Note that the TB-stained fiber is also connected to melanized hyphae (arrows D). Figure 3F shows a dual-stained B. eropida root also sampled during the summer monsoon. A mucilaginous hypha (arrow A) extends from the MC on the root surface and is attached to a sand grain that is also covered with MC. These results illustrate a continuous fungal network connecting the vascular cylinder to the root surface and rhizosphere.
Inter- and intracellular colonization of meristematic cells by lipid-containing protoplasts and hyaline and stained hyphae was consistently observed in developing lateral roots. Surface-sterilized roots plated on sterile PDA agar plates had fungi exiting from cut ends and scars where branch roots were removed. These results confirm the total colonization of root tissues. Fungal isolates were facultative endophytes and could be grown on a number of defined and undefined media. Preliminary identification showed that these endophytes represent several unrelated fungal species.
Discussion
The results indicate that the incidence of active DSE fungal structures is much greater than the stained and melanized hyphae and microsclerotia commonly observed with conventional root-staining methodology. This is in agreement with Yu et al. (2001), who concluded that DSE colonization may be underestimated because hyaline hyphae are difficult to detect. The discrepancy is exacerbated when most of the active structures lack walls. Decreased visibility was attributed to the absence of chitin, which prevented staining, and the natural pigment melanin, which gives DSE fungi their characteristic dark color. Chitin and melanin increase wall rigidity and reduce permeability, protecting the fungus as it invades harsh environments (Cousin 1996; Henson et al.1999; Money et al.1998). These internal structural differences suggest interesting aspects of the internal plant-fungus association. A fully developed fungal wall is probably not necessary for protection within plant tissue. Lack of rigidity would allow the fungus to be more flexible within or between plant cells and would, therefore, explain the observed atypical morphology. Increased permeability of the fungal wall-membrane complex would enhance the potential for resource exchange with the host. Lipid bodies were most extensive in non-staining, non-pigmented structures, suggesting considerable uptake of photosynthate by the fungus and its conversion to lipids, similar to AM fungi (Bago et al. 2002). Therefore, because of dynamic lipid accumulation and apparent transport, these non-staining, non-pigmented structures were considered to be active stages of the fungi and revealed a substantially greater incidence of DSE fungi than has been seen previously.
Yu et al. (2001) observed some colonization of the vascular cylinder by hyaline and melanized DSE hyphae. In this study, virtually all sieve elements were colonized by non-staining, non-pigmented structures. Colonization of the stele (Fig. 1) is a distinctive biological feature of active DSE fungi, differing from fungal pathogens that destroy vascular tissue or from mycorrhizal fungi that are restricted to cortical and epidermal cells and, to my knowledge, do not penetrate the stele.
Presently, the sites of carbon transfer between the host and mycorrhizal fungi are in question (Smith and Smith 1996). The staining with SIV revealed massive quantities of lipid bodies within sieve elements that were associated with atypical fungal structures. This would not only suggest a potential site for substantial carbon exchange between fungus and plant, but also represents a large surface area in the internal plant-fungus interface.
Fungal lipids were generally less apparent in cortical cells than in sieve elements, particularly in physiologically active roots. As roots became dormant, lipids became more evident in cortical cells. In the cortex, lipid bodies could serve as energy-rich carbon reserves to sustain plant cells during extended drought or for the development of melanized hyphae and microsclerotia (Fig. 2H, I). These results indicate that DSE fungi form a continuous integrated network that intimately interfaces with all sieve elements, cortical and epidermal cells. The consistent colonization of meristematic cells in developing lateral roots by stained and lipid-bearing hyphal protoplasts suggested that the fungus was distributed at cell division to all root cells. Yu et al. (2001) likewise observed occasional colonization of meristems with pigmented hyphae, but did not report non-staining active structures. This total integrated colonization suggests that these DSE fungi are systemic within the root. It is proposed that this internal fungal network enhances nutrient and water transport in roots exposed to very low water potentials and other environmental stresses. The accumulation of lipids by fungi in sieve elements in both photosynthetically active and non-photosynthetic dormant plants suggests the potential of bidirectional carbon transport. The continuous network extending from the vascular cylinder to the rhizosphere would support the potential of mycorrhizal function or the bidirectional transport of carbon and mineral nutrients between the soil and the plant (Jumpponen 2001).
It is proposed that the fungus enhances the functioning of native plant roots in arid ecosystems, where they are chronically exposed to very dry soils. Chronic drought imposes severe problems for root desiccation, mineralization, uptake of nutrients and maintaining adequate water relationships for plant survival. Root systems of desert cacti have rectifier-like properties that rapidly take up water when the soil is wet but lose very little as the soil dries. Such mechanisms would be expected in xeric plants adapted to desert conditions. North and Nobel (1994) found that soil sheaths composed of soil, root hairs and mucilage helped limit desiccation of young roots in dry soils. The soil sheath, referred to as the rhizosphere, is distinctly different from and wetter than adjacent bulk soil and provides a favorable micro-environment for root activity (Young 1995). This difference is ascribed to rhizodeposition of plant photoassimilates, which directly affects nutrient availability (Grayston 2000).
Mucopolysaccharides, generally referred to as mucilage, are polymers of simple carbohydrates and are products of rhizodeposition. Mucilage has unique and superior physical properties that make it an important component of the rhizosphere. Sutherland (1998) reported that one polysaccharide retained 1,500 times its weight in water. Polysaccharides in the rhizosphere function as water and carbon reservoirs, protect roots from dessication and facilitate nutrient uptake (Young 1995). Watt et al. (1994) found that the largest and most coherent rhizosheaths were formed in grass roots in drying soil. Read et al. (1999) found that root mucilage of Zea mays L. cv Freya had a major role in maintaining hydraulic continuity between the root and soil. Mucilage also positively influences soil aggregation, stability and water transport, as well as providing a favorable micro-environment and energy source for soil microflora (Czarnes et al. 2000, de Cano et al.1997; Gantar et al.1995). Similarly, soil bacteria and fungi live within a matrix largely composed of extracellular polysaccharides. These matrices protect soil microflora from drying and from fluctuations in water potential, allowing them to function in dry soil (Gantar et al. 1995; Roberson and Firestone 1992). Such characteristics would be advantageous to native plants in an arid ecosystem.
The volume of lipids accumulated by fungi in the sieve elements and in intercellular hyphae is substantial (Fig. 1A, C, F). Likewise the mucilage complex and lipid-filled vacuoles (Fig. 3A, E) in hyphae on the root surface suggest that there are appreciable quantities of polysaccharides in the rhizosphere. From these observations, it is proposed that DSE fungi access and transport carbon from the sieve elements to the root surface, where it is converted to polysaccharides that protect roots, store water and enhance mineral uptake. Carbon drain from the host by these fungi is apparently sizable and raises the question whether the association is mutualistic or parasitic (Smith and Smith 1996). In arid ecosystems, conditions favoring photosynthetic activity are brief and supplies of carbon, minerals and water are severely limited. Therefore, an altruistic association that maintains an efficient control of these resources may serve both partners better. Rhizodeposition in an arid ecosystem could result in serious loss of carbon. However, carbon losses to the soil would be minimized if carbon recycling is efficiently controlled by the fungus. Bago et al. (2002) reported huge fluxes of photosynthetically fixed carbon from plants to the soil caused by AM fungi. A similar concept was reviewed by Trappe (1981), by which 85% of standing grass plants were estimated to be below ground, where fungi account for 80% of the total soil respiration but constitute only one-eighth of the total biomass.
The morphological variability of hyaline, stained and pigmented hyphae (Fig. 3C) observed at the root surface and the rhizosphere was similar to that found internally. A major exception was the mucilaginous hyphae (Fig. 3A, B, D–F). It is suggested that these hyphae were protected by the mucilage coating external to the root in the rhizosphere. The extension of these hyphae into the soil (Fig. 3B), formation of root hair-like protrusions (Fig. 3D) and connection with sand grains (Fig. 3F) suggest a mycorrhizal function of acquiring nutrients and water.
The term DSE has been applied liberally to any fungus that forms melanized septate hyphae and microsclerotia (Jumpponen 2001). More specifically, dominant grasses and shrubs of arid southwestern USA ecosystems are systemically colonized by structurally variable fungi that exist primarily as protoplasts, as well as hyaline, non-staining and non-pigmented fungal structures in physiologically active roots; stained and melanized hyphae and microsclerotia develop as activity decreases during periods of stress. In an attempt to refine descriptive terms, because of the continuous colonization of root cells as seen in this study and also the continuous colonization of shoot cells (Barrow, unpublished results), it is proposed that these fungi be termed systemic endophytic fungi (SEF). Several endophytes have been isolated and they differ morphologically. Preliminary unpublished identification of two isolates indicates that one belongs to Agaricales and that the other is Aspergillus ustus. Other isolates that are variable in appearance suggest that SEF represent a wide spectrum of unrelated fungal species, consistent with the review of Jumpponen (2001) and Jumpponen and Trappe (1998). Vandenkoornhuyse et al. (2002) also found an unexpected 49 different fungal phylotypes distributed across all fungal phyla associated with roots of a single grass plant. It was not clear whether these fungi were internal colonizers or rhizosphere residents.
In summary, the extensive colonization of native grasses and shrubs by DSE in arid southwestern USA rangelands, as well as their ubiquitous presence in other stressed ecosystems, indicates a significant ecological function (Jumpponen 2001). Our preliminary results suggest that these fungi are prevalent in many grass and shrub species in native ecosystems. Their presence in stressed ecosystems in which AM fungi do not persist suggests that they are better adapted (Kohn and Stasovski 1990). In addition to protecting host organs and cells from stress, systemic colonization suggests that these fungi enhance the performance of plant cells under stress, thus promoting their survival. Their internal morphology differs from traditional mycorrhizal fungi but in some cases they may promote growth through enhanced nutrition (Barrow and Osuna 2002; Haselwandter and Read 1982; Newsham 1999). The methods used in this study only illustrate the extensive colonization and morphological variability of DSE fungi. It is imperative to conduct further studies utilizing innovative microscopy and molecular methods to give a better understanding of the nature and extent of colonization. These results also indicate the need for studies on carbon assimilation, management and bidirectional transport by the fungi.
References
Bago B, Zipfel W, Williams RM, Jun J, Arreola R, Lammers PJ, Pfeffer PE, Shachar-Hill Y (2002) Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis. Plant Physiol 128:108–124
Barrow JR, Aaltonen RE (2001) A method of evaluating internal colonization of Atriplex canescens (Pursh) Nutt. roots by dark septate fungi and how they are influenced by host physiological activity. Mycorrhiza 11:199–205
Barrow JR, Osuna P (2002) Phosphorus solubilization and uptake by dark septate fungi in fourwing saltbush, Atriplex canescens (Pursh) Nutt. J Arid Environ 51:449–459
Barrow JR, Havstad KM, McCaslin BD (1997) Fungal root endophytes in fourwing saltbush, Atriplex canescens, on arid rangelands of southwestern USA. Arid Soil Res Rehabil 11:177–185
Bevege DI (1968) A rapid technique for clearing tannins and staining intact roots for detection of mycorrhizas caused by Endogone spp., and some records of infection in Australian plants. Trans Br Mycol Soc 51:808–810
Brundrett MC, Piche Y, Peterson RL (1983) A new method for observing the morphology of vesicular-arbuscular mycorrhizae. Can J Bot 62:2128–2134
Clay K (1990) Fungal endophytes of grasses. Annu Rev Ecol Syst 21:275–297
Cousin MA (1996) Chitin as a measure of mold contamination of agricultural commodities and foods. J Food Prot 59:73–81
Czarnes S, Hallett PD, Bengough AG, Young IM (2000) Root-and microbial-derived mucilages affect soil structure and water transport. Eur J Soil Sci 51:435 -443
de Cano MS, de Mule MCZ, de Caire O Z, Palma RM, Colombo K (1997) Aggregation of soil particles by Nostoc muscorum Ag. (Cyanobacteria). Exp Bot 60:33–38
Gantar M, Rowell P, Kerby NW, Sutherland IW (1995) Role of extracellular polysaccharide in the colonization of wheat (Triticum vulgare L.). Biol Fertil Soils 19:41–48
Grayston SJ (2000) Rhizodeposition and its impact on microbial community structure and function in trees. Phyton (Horn, Austria) 40:27–36
Haselwandter K, Read DJ (1982) The significance of a root-fungus association in two Carex species of high-alpine plant communities. Oecologia 53:352–354
Henson JM, Butler MJ, Day AW (1999) The dark side of the mycelium: melanins of pathogenic fungi. Annu Rev Phytopathol 37:447–471
Jumpponen A (2001) Dark septate endophytes: are they mycorrhizal? Mycorrhiza 11:207–211
Jumpponen A, Trappe JM (1998) Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytol 140:295–310
Kohn L, Stasovski I (1990) The mycorrhizal status of plants at Alexandra Fiord, Ellensmere Island, Canada, a high Arctic site. Mycologia 82:23–35
Kormanik PP, Bryan WC, Schultz RC (1980) Procedures and equipment for staining large numbers of plant root samples for endomycorrhizal assay. Can J Microbiol 26:536–538
Money NP, Caesar-TonThat TC, Frederick B, Henson JM (1998) Melanin synthesis is associated with changes in hyphopodial turgor, permeability, and wall rigidity in Gaeumannomyces graminis var. graminis. Fungal Genet Biol 24:240–251
Newsham KK (1999) Phialophora graminicola, a dark septate fungus, is a beneficial associate of the grass Vulpia ciliata ssp. ambigua. New Phytol 144:517–524
North GB, Nobel PS (1994) Changes in root hydraulic conductivity for two tropical epiphytic cacti as soil moisture varies. Am J Bot 81:41–63
Parbery DG (1996) Trophism and the ecology of fungi associated with plants. Biol Rev 71:473–527
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Read DB, Gregory P, Bell AE (1999) Physical properties of axenic maize root mucilage. Plant Soil 211:87–91
Roberson EB, Firestone MK (1992) Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl Environ Microbiol 58:1284–1291
Ruotsalainen AL, Vare H, Vestberg M (2002) Seasonality of root fungal colonization in low-alpine herbs. Mycorrhiza 12:29–36
Smith FA, Smith SE (1996) Mutualism and parasitism. Diversity in function and structure in the "arbuscular" (VA) mycorrhizal symbioses. In: Callow JA (ed) Advances in botanical research, vol 22. Academic, London, pp 1–43
Smith SE, Read DJ (eds) (1997) Mycorrhizal symbiosis, 2nd edn. Academic, San Diego, pp 59–60
Sutherland IW (1998) Novel and established applications of microbial polysaccharides. Trends Biotechnol 16:41–46
Trappe JM (1981) Mycorrhizae and productivity of arid and semiarid rangelands. In: Manassah JT, Briskey EJ (eds) Advances in food-producing systems for arid and semi-arid lands, part A. Academic, New York, pp 581–593
Vandenkoornhuyse P, Baldauf SL, Leyval C, Straczek J, Young JPW (2002) Extensive fungal diversity in plant roots. Science 295:2051
Watt M, McCully ME, Canny MJ (1994) Formation and stabilization of rhizosheaths in Zea mays L. Effect of soil water content. Plant Physiol 106:179–186
Young IM (1995) Variation in moisture contents between bulk soil and the rhizosheath of wheat (Triticum aestivum L. cv Wembley). New Phytol 130:135–139
Yu T, Nassuth A, Petersen RL (2001) Characterization of the interaction between the dark septate fungus Phialocephala fortinii and Asparagus officinalis roots. Can J Microbiol 47:741–753
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barrow, J.R. Atypical morphology of dark septate fungal root endophytes of Bouteloua in arid southwestern USA rangelands. Mycorrhiza 13, 239–247 (2003). https://doi.org/10.1007/s00572-003-0222-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-003-0222-0