Abstract
Studies on the prevalence of arbuscular mycorrhizal fungi (AMF) and dark septate endophytic fungi (DSEF) are limited for rhizomatous crops in subtropical ecosystems compared to other terrestrial habitats. Hence, the authors assessed the incidence of AMF and DSEF in roots and diversity of AMF in the rhizosphere of Zingiber montanum and Z. officinale collected from shifting cultivation fields of Manipur, Northeast (NE) India. Both the gingers had dual colonization of AMF and DSEF structures in different cortical cells of the same examined root segments and revealed the Intermediate type of AM morphology. Such endomycorrhizal symbiotic association is being reported for the first time in Z. montanum. Distribution of different AMF and DSEF structures varied significantly across the two ginger species. The total root length colonization with AMF and DSEF was highest in Z. montanum. The maximum spore population of AMF was recorded in Z. officinale soil, whereas the AMF species richness was highest in Z. montanum rhizosphere. Altogether, 13 spore morphotypes of AMF corresponding to eight genera, i.e., Acaulospora, Claroideoglomus, Funneliformis, Glomus, Rhizophagus, Sclerocystis, Scutellospora and Septoglomus were isolated from the field and trap culture soils of both ginger species. Significant positive correlations were recorded between some soil properties and root-colonizing variables of AMF and DSEF. Thus, the occurrence of native AMF and DSEF associations in two important indigenous gingers cultivated in the Jhum fields of hilly terrains reveals the possibility of utilizing them in the future for sustainable agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Members of Zingiberaceae, commonly known as gingers, are among the important horticultural crops mainly cultivated for its aromatic rhizomes which are used since times immemorial as spices, condiments, flavoring agents and for its potential medicinal properties being largely distributed in tropical and subtropical regions of Asia [1]. Manipur being a part of Northeast (NE) India lies within the Indo-Burma mega-biodiversity hot spot region having several indigenous wild and domesticated gingers belonging to 88 species under 19 genera [2], among which Zingiber montanum (J.Koenig) Link ex A.Dietr. (syn. Z. cassumunar Roxb.) and Zingiber officinale Roscoe. are most abundantly found in hilly tracts and valley regions of Manipur and is locally known as Tekhaoyaikhu and Shing, respectively. The fleshy rhizomes of naturally grown mountain ginger (Z. montanum) have a strong camphoraceous odor, bitter taste and are used as folk medicines to combat various ailments and disorders, whereas the common ginger (Z. officinale) is extensively cultivated due to its pungency and high fiber contents [2]. NE India is among the highest productivity area of different Zingiber species in the world, i.e., 5.8 t/ha against the national average of 3.7 t/ha, and considered as one of the organic ginger hubs of India [3].
Arbuscular mycorrhizal (AM) fungi (AMF, phylum Glomeromycota) are the vital components of soil microbial community forming symbiotic associations with the majority of crop plants including some tuberous and rhizomatous ones [4, 5]. AMF have been observed to increase the crop growth and productivity by enhancing the uptake of labile and non-labile mineral elements, especially in weathered field soils of tropical and subtropical areas that are characterized by low percentage of total and available P [6,7,8] and also improve the soil quality, water relations and plant tolerance to abiotic and biotic stresses [9]. However, only approximately 76% of monocot families are reported for their mycorrhizal status till date [6]. Therefore, Wang and Qui [10] proposed the need for examining as many plant species from different land types for their mycorrhizal status to understand the extent of root-colonizing fungi in various habitats. Recently another root colonist, the dark septate endophytic fungi (DSEF) belonging to phylum Ascomycota and characterized by the presence of septate melanized hyphae and microsclerotial structures, has gained increasing interest as they coexist with AMF and protect the plant from different stresses [11, 12]. However, information on DSEF colonization patterns in tropical and subtropical plant roots, particularly to that of NE India, is very limited compared to other climatic zones and temperate ones [13, 14].
Taber and Trappe [15] were the first who investigated the presence of AMF in rhizomes and scale-like leaves of edible ginger (Z. officinale) grown in Hawaii and Fiji, and the benefits of AM symbiosis on growth parameters of ginger species have already been observed in abroad [16,17,18]. Moreover, Muthukumar et al. [19] and Uma et al. [5] also reported the AM status of Z. officinale from South India. Nevertheless, the occurrence of AMF and DSEF associations in mountain ginger (Z. montanum) has not yet been reported till date. Considering the importance of AM fungi for sustainable agriculture, a survey of native AMF species richness in the subtropical habitat of this region would provide insight for their field applications in the future [13, 14]. Hence, the present study aims to determine the AM morphology and root length colonization by different structures of AMF and DSEF as well as the species diversity of AMF in the rhizosphere of two dominant gingers cultivated in the subtropical Jhum fields of Manipur, NE India.
Material and Methods
Study Area and Conditions of Crop Growth
The present work was carried out with two indigenous ginger species, viz., Z. montanum and Z. officinale grown in shifting cultivated fields for the previous five years and situated on the gentle slope of a hillock under the Langol forest extension of Imphal West District, Manipur (location: 24°51′45.0″ N latitude; 93°53′15.7″ E longitude), having elevation of 950 m. a.s.l. Geographically, about 90% of the total area in Manipur is covered by hill ranges being the part of Eastern Himalayas along with 1813 sq km of central valley area [20]. According to the United States Department of Agriculture Classification (USDA), the soil is Ultisol type developed from shale and sandstones and is heterogeneous [20]. The annual mean temperature of the study site varied from 15.3 to 26.8 °C and mean relative humidity between 61 and 88%, while the total rainfall was 1535 mm (ICAR Research Complex for NEH Region, Manipur Centre, Imphal). For each ginger species, three cultivated fields were selected randomly which were situated adjacently on the western side of the hill slope having uniform growth patterns and without any alternate crop grown during the off-seasons.
In NE India, gingers are mainly grown by tribal farmers following traditional practices as a rainfed crop on the raised beds in Jhum or shifting cultivation fields receiving the annual rainfall between 980 and 2050 mm and a temperature range of 12–36 °C. In hilly tracts, planting of the ginger rhizomes is conducted during March–April when the first shower starts and the harvesting is done from November and extends till January [3, 21]. No inorganic fertilizers and biocides were applied during the cultivation of gingers.
Sample Collection
The root and soil samples were collected by digging mineral soil core (0–20 cm depth) around the root zones of five randomly selected plants (6 months old), at a distance of 4–4.5 m apart each, belonging to a ginger species during harvesting period of crop growth, i.e., October–November 2017. Approximately 1 kg of rhizospheric soil was collected from the individual plant of a selected crop species, kept in polythene bag separately, labeled and then brought to the laboratory. After air-drying in shade, one part of the soil corresponding to each plant of a ginger species was utilized for enumeration of AMF spore density and species richness, while the remaining samples were pooled and used for establishing the trap culture and analyzing the physico-chemical characteristics. The roots were gently washed with water to make them free from soil and were fixed in FAA solution till further processing.
Determination of Soil Properties
The texture of the soil samples was assessed by the Bouyoucos Hydrometer method as described by Allen et al. [22], whereas the soil pH and electrical conductivity (EC) were analyzed in the aqueous soil–water (1:1, v/v) solutions using the digital pH and conductivity meter. Organic carbon (OC) of the soil was determined by the rapid titration method [23]. Total nitrogen (N), available phosphorus (P) and exchangeable potassium (K) of the soil samples were evaluated according to Jackson [24]. All the soil properties were assessed in triplicate for each ginger species.
Assessment of Ginger Root Colonization by AMF and DSEF
After washing with distilled water, 15 root segments (1 cm length) of each ginger species were cut, processed for clearing with KOH (2.5%) at 90 °C for 90–120 min in water bath, acidified with HCl (5 N) for 15 min [25], stained in Trypan blue-Lactoglycerol (0.05%) overnight, then mounted in Lactoglycerol on glass slides and examined for the AMF and DSEF structures in stereoscopic compound microscope (Nikon Eclipse Ni-U, Japan) for estimation of their root length colonization (%) which was assessed by magnified intersection method as described by McGonigle et al. [26]. The morphology of AM was categorized as Arum, Paris or Intermediate type depending on the presence of inter- or intracellular nature of AMF structures (arbuscules, hyphae, vesicles, hyphal or arbusculate coils) within the root cortex [27]. The authors could not differentiate the Intermediate-subtypes of AM morphologies as only the squashed root pieces were examined. Moreover, when the parallel running hyphae were intracellular, then the morphology was considered as Intermediate type [28].
Isolation and Identification of AMF Spores
The AMF spores were isolated by wet sieving and decanting technique [29], in which 100 g of soil sample (in triplicate) belonging to each plant of a ginger species was dispensed into 1L of water and the suspension was decanted through 710–37 μm sieve’s series. After washing into beakers, the residues were filtered through girdled filter papers, then spread on glass plates and observed under the stereoscopic microscope (40× magnifications). All the AMF spores were counted, transferred to an individual glass slide by wet needle and then mounted in polyvinyl alcohol-Lacto glycerol (PVLG) either with or without Melzer’s reagent [30]. The non-collapsed AMF spores and sporocarps were identified on the morphological basis, and the sub-cellular features were compared with the culture database established by INVAM (http://invam.cag.wvu.edu/) and Schüssler’s web site (http://www.lrz-muenchen.de/~schuessler/amphylo/amphylo_species.html). The AMF spore population and species richness were indicated as the total number of spores and the number of species in 100 g air-dried soil samples, respectively. The relative abundance (%RA) and isolation frequency (%IF) of each AMF species were calculated according to Dandan and Zhiwei [31].
Establishment of Trap Culture
The composite soil samples along with root fragments corresponding to each ginger species were mixed with autoclaved (120 °C, 15 psi for 30 min. on three consecutive days) coarse sand (1:1 v/v) and filled in triplicate earthen pots (2 kg/pot). Each pot was sown with 10 maize (Zea mays L.) seeds, placed in greenhouse condition (temperature range 20–26 °C, relative humidity between 77 and 90% along with natural daylight and photoperiod) and was watered every alternate day. After 120 days of culture initiation, the developed AMF spores were extracted and identified as stated above.
Statistical Analysis
A paired t test was performed to assess the differences in soil parameters, AMF spore density and root-colonizing fungal structures between two ginger species (SPSS version 20, SPSS Inc., Chicago, Illinois). Pearson’s correlation was applied to determine the relationship between soil characters and endorrhizal colonization levels.
Results and Discussion
To our knowledge, the present finding reveals the first report on AM morphology, AMF and DSEF colonization patterns in the roots and species diversity of AMF in the rhizosphere of Z. motanum under Jhum cultivation system of NE India, whereas Z. officinale have earlier been reported to be associated with AMF [15, 19, 32] and DSEF [5]. The soils of both gingers were sandy loam in texture and slightly acidic (Table 1). Maximum concentrations of EC, total N and available P were found in Z. officinale soil, whereas that of OC and exchangeable K was highest in Z. montanum rhizosphere. No significant difference could be observed between the soil properties (except EC) of the two ginger species (Table 1).
All examined root fragments of both the gingers had dual colonization of AMF and DSEF structures present in different lengths of root cortical cells (Table 2, Fig. 1), and revealed the Intermediate type of AM morphology which is similar to the findings of Uma et al. [5] who observed that 80% of the ginger and spiral ginger species have Intermediate-type AM. When roots are colonized by more than one AMF, as possible in the field condition, the formation of Intermediate-AM morphology, having the features of both Arum and Paris types, might be greater [33].
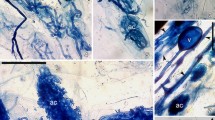
Arbuscular mycorrhizal (a–j) and dark septate endophyte (k–m) fungal colonization in roots of Zingiber officinale (a–e, k) and Zingiber montanum (f–j, l, m). a Appressorium formation in Z. officinale, b, c intercellular hyphae and arbuscules in Z. officinale, d, e intracellular hyphal coils in Z. officinale, f, g intracellular hyphal coil in Z. montanum, h–j vesicles in Z. montanum (ve), k DSE hyphae in Z. officinale, l, m moniliform-DSE hyphae and microsclerotia in Z. montanum. ap, appressorium; ih, intercellular hyphae; ar, arbuscules; at, arbuscular trunk; hc, hyphal coils; ph, intracellular hyphae; ve, vesicle; dsh, dark septate endophyte fungal hyphae (arrow heads); mo-dsh, moniliform-DSE hyphae; ms, microsclerotia. Scale bars = 40 µm
The entry of AMF into roots of Z. officinale was characterized by appressorium (Fig. 1a) and other structures such as arbuscules, intercellular hyphae and intracellular hyphal coils (Fig. 1b–e), whereas in Z. montanum intracellular hyphal coils, inter- and intracellular hyphae and vesicles were observed (Fig. 1f–j). In this study, the root length with total AM colonization (%RLTC) was lower in Z. officinale (56.2%) (Table 2), when compared to those reported for the same plant species from South India by Muthukumar et al. [19], Muthukumar and Tamilselvi [32] and Uma et al. [5] with mean values of 74%, 91% and 63%, respectively. This indicates the variations in mycorrhizal dependency of specific plants in different ecosystems having P-deficient soils [34]. Moreover, at higher altitudes, low temperatures and heavy rainfall followed by soil erosion may also affect the AMF sporulation and subsequently reduce the root colonization levels. Differences in AMF communities and their root-colonizing patterns have been reported among plants, ecosystems, locations and seasons, and therefore, it seems inherent that the coexisting AMF have distinct functional abilities [35].
Soil properties are known to affect AMF functioning and colonization levels [6]. In this study, the soil EC negatively affected the root length colonized by arbuscules (%RLA), although the measured EC concentrations were below to that reported to influence the AMF colonization frequency [36]. Füzy et al. [37] also observed that arbuscule richness in Plantago maritime was negatively correlated with soil EC, which shows that the soil salinity may affect the AMF spore germination, rate of hyphal growth and colonization percentage [38].
The roots of studied ginger species were colonized by different DSEF structures, viz., melanized septate hyphae and moniliform or microsclerotia (Fig. 1k–m), though their hyphae and sclerotia occurred in low percentages (Table 2). The extent of total root length colonization by DSEF, i.e., %RLTDC, was highest in Z. montanum (5.9%) compared to that of Z. officinale (3%) which is similar to the findings of Uma et al. [5] who reported 0–3.9% total root length colonization by DSEF in three different ginger species. In this study, a significant positive correlation was observed between total root length colonized by AMF and DSEF which suggests that these fungal types could coexist together in the same plant roots as also reported by Muthukumar et al. [28]. DSEF are frequently present in the roots of many plant taxa belonging to cold and nutrient-stressed or degraded ecosystems where the colonization rate of AM fungi is lower [11, 39]. Like AMF symbiosis, the host responses to DSEF are variable and usually differ from mutualism to parasitism, depending on the plant and fungal genotype and environmental conditions [40]. Therefore, the mechanism of interactions between mycorrhizal and DSE fungi is inevitable and some strains of DSEF are beneficial to the plant growth and performances [12]. In contrast to a wide knowledge of AMF functioning, the role of DSEF in the ecosystem and the nature of coexistence among two fungal groups are largely unknown. Earlier work on the effect of an ectomycorrhizal and DSE fungal strains on conifer plants has shown a reduction in the DSEF colonization level, and the influence was concluded as the result of competition for nutrients and space and the antagonistic interactions between these two fungal groups [41]. However, such findings are still lacking in the case of AMF and DSEF interactions till date.
The AMF spore density observed in the air-dried rhizosphere soils of Z. montanum (17 spores per 100 g) and Z. officinale (48 spores per 100 g) are comparatively higher to that reported in Z. officinale and other spiral gingers (11–22 spores per 100 g soil) grown in natural and agricultural fields of South India [5, 19]. As reported by Aguilar–Fernández et al. [42], the present findings revealed that crop cultivation by slash-and-burn practices in hilly terrains does not affect the AMF sporulation and community composition adversely for a longer duration. Soil properties are known to affect the AMF population. In the present study, both ginger soils were slightly acidic which might be a factor for low-to-moderate AMF spore population. Rajeshkumar et al. [43] reported that the pH of the soil can influence the status of AMF spores in crop rhizosphere, and slightly acidic soils comparatively harbor a higher number of AM propagules than that of acidic ones. The AMF spore density was significantly and negatively correlated with % OC of studied ginger soils (Table 3). Singh et al. [44] also observed a similar relationship among these variables from the soil of Jhum fallow in NE India. However, the variations in density and diversity of AMF in different crop field soils were found to be influenced by an array of environmental, host and fungal growth factors [6].
A total of 13 and 9 AMF spore morphotypes corresponding to 8 genera, i.e., Acaulospora, Claroideoglomus, Funneliformis, Glomus, Rhizophagus, Sclerocystis, Scutellospora and Septoglomus, were isolated from the field and trap culture soils, respectively, of the selected ginger species (Table 4, Fig. 2). However, Uma et al. [5] isolated a higher number of AMF spore types (18 species) from the root-zone soils of other Zingiberaceae members cultivated in Kerala, India. Such differences in the diversity of AMF species have been previously reported in a variety of habitats and agroecosystems [13, 14, 45]. In this study, the maximum AMF species were recorded in the field cultivated soils of Z. montanum. Three different species of Funneliformis were isolated, whereas the genera such as Acaulospora, Glomus and Sclerocystis were represented with two species each. Spores morphotypes of Claroideoglomus etunicatum, Funneliformis geosporum and Septoglomus constrictum were recovered from the field soils of both ginger species. Funneliformis monosporus, Rhizophagus intraradices and Sclerocystis rubiformis were exclusively recorded in the root-zone soil of Z. montanum, while Glomus macrocarpum was specifically found in Z. officinale soil. Hence, the present findings revealed that the majority of the isolated AMF species belonging to order Glomerales and the genera, i.e., Funneliformis, Glomus, Claroideoglomus, Septoglomus, Rhizophagus and Sclerocystis, contributed higher species richness than the order Diversiporales including Acaulospora and Scutellospora. Uma et al. [5] also recorded 14 AMF species under the order Glomerales and only six from other AM taxonomic groups in gingers and spiral gingers rhizosphere. Glomus has been reported as the most widely distributed genus from different geographical regions around the globe probably due to their high adaptability and smallest sized spores among other AMF taxa which enable them to sporulate in numbers within a limited period and to disperse easily [13, 46].
Arbuscular mycorrhizal fungal spores isolated from field and trap culture soils of studied Zingiber species. a Acaulospora spinosa, b Acaulospora sp., c Claroideoglomus etunicatum, d Funneliformis geosporum, e Funneliformis monosporus, f Funneliformis mosseae, g Glomus macrocarpum, h Glomus multicaule, i Rhizophagus intraradices, j Spores of Sclerocystis rubiformis, k Sporocarp of Sclerocystis taiwanensis, l Septoglomus constrictum, and (m) Scutellospora sp. Scale bars: (a, k, m) = 20 µm and (b, c, d, e, f, g, h, i, j, l) = 40 µm
The spores of Acaulospora sp.1, Funneliformis mosseae and Glomus multicaule were specifically isolated from trap pot soil of Z. montanum, while Acaulospora spinosa, Sclerocystis taiwanensis and Scutellospora sp.1 were exclusively recorded from Z. officinale trap culture soil (Table 4). The occurrence of AMF species in field and trap pot soils of two gingers varied significantly. Bever et al. [46] reported that AMF species isolated from natural field soil may not be always recovered from the trap cultures, because sporulation of a specific AMF may be influenced by the host species through their effects at the time of propagule activation and extent of hyphal development, whereas the trap culture can develop the sporulation of cryptic AMF species that might be unable to regenerate during the sampling period in field. Isolation of AMF spores from the natural field and trap culture soils generally provides a complete understanding of AMF species richness with associated host plant rhizosphere.
Conclusion
The occurrence of both AMF and DSEF in the examined gingers indicates the dynamic nature of diverse root-colonizing fungal communities in shifting cultivation systems. It has been proposed by different workers that the DSEF association generally increases the root functions of native plants growing in multifactorial stressed ecosystems and also emphasizes their potential to function as mutualists along with the mycorrhizal fungi [11, 12, 32]. Further investigations would focus on determining whether compositional variations and root colonization patterns among AMF and DSEF community have a functional role in the growth and productivity of the gingers cultivated in different soil types and climatic conditions.
References
Ravindran PN, Nirmal BK, Shiva KN (2005) Botany and crop improvement of ginger. In: Ravindran PN, Nirmal BK (eds) Ginger: the genus Zingiber. CRC Press, New York
Sharma GJ, Chirangini P, Kishor R (2011) Gingers of Manipur: diversity and potentials as bioresources. Genet Resour Crop Evol 58:753–767
Rahman H, Karuppaiyan R, Kishore K, Denzongpa R (2009) Traditional practices of ginger cultivation in Northeast India. Ind J Trad Knowledge 8(1):23–28
Khade SW, Rodrigues BF (2007) Incidence of arbuscular mycorrhizal (AM) fungi in some angiosperms with underground storage organs from Western Ghats region of Goa. Trop Ecol 48:115–118
Uma E, Muthukumar T, Sathiyadash K, Muniappan V (2010) Mycorrhizal and dark septate fungal associations in gingers and spiral gingers. Botany 88:500–511
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, London
Bagyaraj DJ, Sharma MP, Maiti D (2015) Phosphorus nutrition of crops through arbuscular mycorrhizal fungi. Curr Sci 108(7):1288–1293
Thilagar G, Bagyaraj DJ (2015) Influence of different arbuscular mycorrhizal fungi on growth and yield of Chilly. Proc Natl Acad Sci India Sect B Biol Sci 85:71–75
Chen M, Arato M, Borghi L, Nouri E, Reinhardt D (2018) Beneficial services of arbuscular mycorrhizal fungi—from ecology to application. Front Plant Sci 9:1270. https://doi.org/10.3389/fpls.2018.01270
Wang B, Qui YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
Mandyam K, Jumpponen A (2005) Seeking the elusive function of root-colonizing dark septate endophytic fungi. Stud Mycol 53:173–189
Newsham KK (2011) A meta-analysis of plant responses to dark septate root endophytes. New Phytol 190:783–793
Pandey RR, Chongtham I, Muthukumar T (2016) Influence of season and edaphic factors on endorhizal fungal associations in subtropical plantation forest trees of Northeastern India. Flora 222:1–12
Surendirakumar K, Pandey RR, Muthukumar T (2019) Influence of indigenous arbuscular mycorrhizal fungus and bacterial bioinoculants on growth and yield of Capsicum chinense cultivated in non-sterilized soil. J Agric Sci 157:31–44
Taber RA, Trappe JM (1982) Vesicular-arbuscular mycorrhiza in rhizomes, scale-like leaves, roots, and xylem of ginger. Mycologia 74:156–161
da Silva MF, Pescador R, Rebelo RA, Stürmer SL (2008) The effect of arbuscular mycorrhizal fungal isolates on the development and oleoresin production of micropropagated Zingiber officinale. Braz J Plant Physiol 20:119–130
dos Santos R, Girardi CG, Pescador R, Stürmer SL (2010) Effects of arbuscular mycorrhizal fungi and phosphorus fertilization on post vitro growth of micropropagated Zingiber officinale Roscoe. R Bras Ci Solo 34:765–771
Kawamoto I, Habte M (2011) Enhancement of arbuscular mycorrhizal fungal status of an established ginger crop through a mycorrhizal onion companion crop. J Soil Sci Plant Nutr 57:659–662
Muthukumar T, Senthikumar M, Rajangam M, Udaiyan K (2006) Arbuscular mycorrhizal morphology and dark septate fungal associations in medicinal and aromatic plants of Western Ghats, Southern India. Mycorrhiza 17:11–24
Sehgal JL, Sen TK, Chamuah GS, Singh RS, Nayak DC, Saxena RK, Baruah U, Maji UK (1993) Soils of Manipur for land use planning. National bureau of soil survey and land use planning, Nagpur
Yadav RK, Yadav DS, Rai N, Sanwal SK, Sarma P (2004) Commercial prospects of ginger cultivation in North-Eastern region. ENVIS Bulletin: Himal Ecol 12(2):1–5
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C (1974) Chemical analysis of ecological materials. Blackwell Scientific Publications, Oxford
Walkley A, Black IA (1934) An examination of the Det Jareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Jackson ML (1971) Soil chemical analysis. Prentice Hall, New Delhi
Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA-mycorrhizas. Mycol Res 92:486–488
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JL (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Dickson S (2004) The Arum–Paris continuum of mycorrhizal symbioses. New Phytol 163:187–200
Muthukumar T, Sathiyadash K, Valarmathi V (2018) Arbuscular mycorrhizal and dark septate endophyte fungal associations in plants of different vegetation types in Velliangiri hills of Western Ghats, Southern India. Acta Bot Hung 60:185–222
Gerdeman JW, Nicolson TJ (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235–244
Schenck NC, Perez Y (1990) Manual for identification of VA mycorrhizal fungi, 2nd edn. INVAM University of Florida, Gainesville
Dandan Z, Zhiwei Z (2007) Biodiversity of arbuscular mycorrhizal fungi in the hot-dry valley of the Jinsha river, Southwest China. Appl Soil Ecol 37:118–128
Muthukumar T, Tamilselvi V (2010) Occurrence and morphology of endorrhizal fungi in crop species. J Agron Trop Subtrop Agroecosyst 12:593–604
Dickson S, Smith FA, Smith SE (2007) Structural differences in arbuscular mycorrhizal symbioses: more than 100 years after Gallaud, where next? Mycorrhiza 17:375–393
Smith SE, Jakobsen I, Grønlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156:1050–1057
Öpik M, Moora M, Liira J, Zobel M (2006) Composition of root colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J Ecol 94:778–790
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280
Füzy A, Biró B, Tóth T (2010) Effect of saline soil parameters on endomycorrhizal colonisation of dominant halophytes in four Hungarian sites. Span J Agric Res 8:144–148
Latef AAHA, Miransari M (2014) The role of arbuscular mycorrhizal fungi in alleviation of salt stress. In: Miransari M (ed) Use of microbes for the alleviation of soil stresses. Springer, New York
Yamato M, Iwasaki M (2002) Morphological types of arbuscular mycorrhizal fungi in roots of understory plants in Japanese deciduous broad leaved forests. Mycorrhiza 12:291–296
Lingfei L, Anna Y, Zhiwei Z (2005) Seasonality of arbuscular mycorrhizal symbiosis and dark septate endophytes in a grassland site in southwest China. FEMS Microbiol Ecol 54:367–373
Reininger V, Sieber TN (2012) Mycorrhiza reduces adverse effects of dark septate endophytes (DSE) on growth of conifers. PLoS ONE 7(8):e42865. https://doi.org/10.1371/journal.pone.0042865
Aguilar-Fernández M, Víctor JJ, Varela-Fregoso L, Gavito ME (2009) Short-term consequences of slash-and-burn practices on the arbuscular mycorrhizal fungi of a tropical dry forest. Mycorrhiza 19:179–186
Rajeshkumar PP, Hosagoudar VB, Gopakumar B (2013) Mycorrhizal association of Ochlandra travancorica in Kerala, India. J Threat Taxa 5(2):3673–3677
Singh SS, Tiwari SC, Dkhar MS (2003) Species diversity of vesicular–arbuscular mycorrhizal (VAM) fungi in jhum fallow and natural forest soils of Arunachal Pradesh, North Eastern India. Trop Ecol 44:207–215
Wang FY, Liu RJ, Lin XG, Zhou JM (2003) Comparison of diversity of arbuscular mycorrhizal fungi in different ecological environments. Acta Ecol Sin 23:2666–2671
Bever JD, Schultz PA, Pringle A, Morton JB (2001) Arbuscular mycorrhizal fungi: more diverse than meets the eye, and the ecological tale of why. Bio Sci 51:923–931
Acknowledgments
The authors gratefully acknowledge the financial assistance from the Net-work Project on Application of Microorganisms in Agriculture and Allied Sector (AMAAS), sponsored by the Indian Council of Agricultural Research, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement This study reveals the first report on AM fungal diversity in the rhizosphere soil, and AM morphology, AM and DSE fungal colonization patterns in the roots of Z. montanum under shifting cultivation system of North Eastern India.
Rights and permissions
About this article
Cite this article
Pandey, R.R., Loushambam, S. & Srivastava, A.K. Arbuscular Mycorrhizal and Dark Septate Endophyte Fungal Associations in Two Dominant Ginger Species of Northeast India. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 90, 885–894 (2020). https://doi.org/10.1007/s40011-019-01159-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-019-01159-w