Abstract
Background
In Japan, palisade vessels (PV) are used to distinguish the esophagogastric junction (EGJ). Elsewhere, the EGJ is defined by the upper end of the gastric folds (GF) and PV are considered difficult to detect. This study evaluated the detection rate of PV in Western patients with Barrett’s esophagus (BE) using white light imaging (WLI) and narrow band imaging (NBI), and quantified any discordance between Western and Japanese criteria for the EGJ.
Methods
In 25 BE patients, the presence and location of PV and GF were determined and biopsies were obtained. High-quality images of the EGJ were collected under different conditions (insufflations–desufflation, WLI–NBI, forward-retroflex approach), resulting in eight different images per patient. The presence of PV on each still image was assessed by a panel of six Western and Japanese endoscopists with expertise in BE.
Results
PV were observed in ≥ 1 images by a majority of the panel (≥ 4 raters) in 100 % of patients during insufflation versus 60 % during desufflation (p < 0.001). WLI and NBI detected PV in 100 and 92 %, respectively (p = 0.50). Interobserver agreement of the panel was ‘moderate’ (κ = 0.51). During endoscopy PV were located a median of 1 cm distal of the GF in 15 patients (63 %), with intestinal metaplasia (IM) in this discordant zone, in 27 % of patients.
Conclusions
PV are visible in most Western BE patients and are best inspected during insufflation. The location of the GF and PV differed in a substantial group of patients, partially with IM in this discordant zone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Barrett’s esophagus (BE) is a premalignant condition for the development of esophageal adenocarcinoma (EAC) [1, 2]. The prevalence of BE has been estimated at 1.6 % in Europe and 1.7–5.6 % in the USA [3–6]. In contrast, for Japan, a prevalence up to 19.9 % has been reported in studies where the endoscopic diagnosis was histologically confirmed [7, 8], and even up to 37.4 % in studies solely based on endoscopic diagnosis [8, 9]. Among BE patients, the proportion of longer BE segments is generally higher in Western countries, whereas in Japan, short BE segments are more frequently found [3, 8, 10]. Progression rates to esophageal neoplasia are higher in longer BE segments [11, 12]. The combination of BE segment length and progression rates also seems to be reflected in the difference in estimated incidence of EAC between Western and Japanese BE patients (2.58 vs. 0.3 per 100,000, respectively) [13, 14].
A factor that may contribute to this difference in BE detection is the definition of the esophagogastric junction (EGJ) as the distal margin of the BE segment. In Western guidelines, the upper end of the gastric mucosal folds is considered the landmark for the EGJ [2]. This landmark is easily detectable in Western patients. However, its position may be inconsistent by factors such as air insufflation or deep inspiration. Therefore, the position of the upper end of the gastric folds (GF) is assessed in deflated condition [15]. However, this inconsistency influences the detection of short BE and studies have shown that the interobserver agreement for BE segments shorter than 1 cm is poor [15].
The Japanese guidelines consider the most distal extent of the palisade vessels (PV) to be the endoscopic definition of the EGJ [16]. PV are thin, longitudinal vessels present in the mucosal layer within the lower esophageal sphincter, descending into the submucosa once entering the cardia. PV are most easily assessed in a distended esophagus. Although always in a consistent location, Western endoscopists generally do not use the PV as a landmark since they are faint and cannot always be clearly visualized in Western BE patients [15].
Over the past decade, endoscopic imaging has improved markedly with new techniques such as high resolution, magnification, and image-enhanced endoscopy. Narrow band imaging (NBI) is an established optical chromoendoscopy technique that highlights mucosal surface patterns and microvascular details by utilizing short wavelength light, and may thus improve visualization of PV [17]. In the latest version, the NBI mode has increased brightness combined with dual-focus high-resolution endoscopy. This may facilitate the detection of PV in the Western BE population.
The aims of this study were to prospectively evaluate the detection rate of PV in Western BE patients, to quantify any discordance between the Western and Japanese criteria for the distal margin of the Barrett segment, and to evaluate the clinical relevance of this discordance.
Methods
Patient selection
For this prospective observational study, consecutive patients were enrolled between March 2012 and May 2013 at the St. Antonius Hospital (Nieuwegein, The Netherlands), a tertiary center for the treatment of BE and early esophageal neoplasia. This study was approved by the local institutional review board. Written informed consent was obtained from all study patients. Patients were considered eligible if they met the following criteria: (1) age between 18 and 80 years; (2) BE with minimum length of ≥ 2 cm as pointed out by prior endoscopic examination; (3) scheduled for surveillance endoscopy; (4) written informed consent.
Patients were excluded if: (1) previous biopsies of the most distal 2 cm BE showed high-grade dysplasia or adenocarcinoma; (2) any prior endoscopic treatment for Barrett’s dysplasia or cancer; (3) any prior surgical intervention at the distal esophagus or proximal stomach; (4) any visible abnormalities in the distal esophagus at study endoscopy; (5) presence of severe stricture in the distal esophagus impairing passage of the endoscope at study endoscopy; (6) presence of esophageal varices at study endoscopy.
Endoscopic procedure
Endoscopy was performed by a Western endoscopist with extensive experience in the diagnosis and treatment of BE and early esophageal neoplasia (B.W.) assisted by a Japanese endoscopist (O.G.), under conscious sedation (midazolam or propofol). Initial standard surveillance imaging of the entire Barrett’s segment was performed according to international guidelines using a HDTV endoscopy system (GIF-HQ190 endoscope and EXERA III endoscopy system, Olympus Medical Systems, Hamburg, Germany) [18].
In all patients considered eligible for inclusion, endoscopic landmarks (such as the diaphragmatic pinch, and the maximum and circumferential extent of the Barrett’s segment) were recorded on a case record form. The distance of the GF to the incisors was determined in inflated and deflated condition. Then, in case of visible PV, their location was assessed in inflated and deflated state. Any difference in distance between the top of the GF and the distal end of the PV, both assessed in their optimal condition according to current guidelines (deflated and inflated, respectively), was considered a ‘zone of discordance’.
Subsequently, high-quality still images (no magnification) of the EGJ in overview were obtained, further subdivided into eight categories: forward approach–white light imaging (WLI)–insufflation (A); forward–WLI–desufflation (B); forward–NBI–insufflation (C); forward–NBI–desufflation (D); retroflex–WLI–insufflation (E); retroflex–WLI–desufflation (F); retroflex–NBI–insufflation (G); retroflex–NBI–desufflation (H). Thus, per patient, eight high-quality images were collected, anonymized, and stored.
Finally, two biopsies were obtained from the cardia (< 1 cm below the distal end of the PV, or < 1 cm below the GF in case of absence of PV), two biopsies from the ‘zone of discordance’ when present, and four-quadrant biopsies 1 cm proximal of the GF (according to the Seattle protocol for random biopsy during Barrett surveillance [19]). Biopsies were obtained with a standard biopsy forceps (SwingJaw 2.45 mm, Olympus Medical Systems, Hamburg, Germany). Endoscopic images of every biopsy procedure from the zone of discordance were collected to document the accuracy of targeting these biopsies. Follow-up or additional treatment endoscopy was scheduled according to international guidelines [18].
Histology
The biopsies were fixated in buffered 10 % formalin for at least 24 h, embedded in paraffin, cut into 4-μm slides and stained with hematoxylin and eosin (H&E), alcian blue, and P53 immunostaining according to the routine biopsy processing protocol. All biopsies were evaluated by an expert pathologist in BE (C.S.). The presence of intestinal metaplasia (IM) was scored and the revised Vienna classification was used for grading the dysplasia [20].
Image assessment
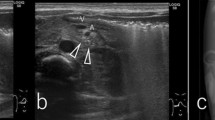
All endoscopic images were incorporated in a slideshow (Adobe Acrobat 10, Adobe Systems Incorporated, USA) with the same size and resolution. The order of images was randomized using the Statistical Software Package (SPSS version 20.0.0.1 for Windows, Chicago, IL, USA). A panel of three Western endoscopists (E.S., B.S., and R.B.), with expertise in the endoscopic detection and treatment of early neoplasia in BE, and three Japanese endoscopists (T.U., J.H., and Y.O.), with experience in the detection of PV, scored all images for the presence of PV (Fig. 1).
Different endoscopic images for the assessment of palisade vessels. The presence of palisade vessels was assessed in endoscopic images in eight conditions by a panel of six endoscopists: forward approach–white light imaging (WLI)–insufflation (a); forward–WLI–desufflation (b); forward–narrow band imaging (NBI)–insufflation (c); forward–NBI–desufflation (d); retroflex–WLI–insufflation (e); retroflex–WLI–desufflation (f); retroflex–NBI–insufflation (g); retroflex–NBI–desufflation (h)
Beforehand, all observers were informed about PV by a 5-min automated presentation. In this presentation, the background, recognition, and schematic images of PV were discussed followed by a subset of 16 endoscopic images obtained under different conditions (comparable to the images used in the experiment) in which the PV were pointed out.
The presence of PV was scored in all images on a three-point scale (present–in doubt–absent). The ‘in doubt’ category was added to prevent random guessing in case of uncertainty by an endoscopist from the panel. The panel was blinded for the results of the real-time endoscopy and for the histological outcome.
Study outcomes
The primary outcome of this study was the proportion of Western BE patients with visible PV on endoscopy using WLI and NBI. The secondary outcomes of this study were: (1) the proportion of patients with visible PV with WLI or NBI, specified by the approach (forward or retroflex); (2) the interobserver agreement for the visualization of the PV in each combination of conditions (eight categories as pointed out earlier); (3) proportion of patients with a ‘zone of discordance’; (4) proportion of biopsies containing IM in the cardia, in the zone of discordance and just proximal to the top of the GF.
Image assessment and statistical analysis
Data for analysis were available from the real-time (location of endoscopic landmarks and histologic presence of IM) as well as from the image assessment. For the image assessment, four images per condition (for example NBI) were available for analysis. Images in which PV were seen with certainty by the panel were desired for analysis. Therefore, all images scored as “in doubt” were considered “absent”, resulting in a binary “present” or “absent” analysis. Individual images were used for a per-image analysis. Per-image PV were defined as ‘visible’ when considered present by a majority (≥ 4) of the panel. For a per-patient analysis, PV were considered present in a patient in a certain condition when at least one of the four images contained ‘visible’ PV as scored in the per-image analysis.
The analyses of the proportion of patients with visible PV using WLI versus NBI (and specified per approach) and the interobserver agreement were based on the image assessment, for which the effect of insufflation was first confirmed by analysis (since insufflation is known to be a factor of influence), followed by the effects of type of imaging and then approach. The proportion of patients with visible PV using WLI versus NBI was analyzed in a per-image and a per-patient analysis. The interobserver agreement was analyzed based on a per-image analysis.
The analyses of the proportion of patients with a zone of discordance and the proportion of biopsies containing IM were based on the real-time data with a per-patient analysis. In addition, a per-biopsy analysis was performed for the presence of IM in biopsies.
Statistical analysis was performed with SPSS version 20.0.0.1. For descriptive statistics, mean with standard deviation was used for normal distributed data and median with interquartile range (IQR) for variables with a skewed distribution. Since multiple images were collected per patient, paired analysis was performed: continuous data were compared with either a paired t test (normally distributed data) or a Wilcoxon signed-rank test (not normally distributed data); proportions were compared with McNemar’s test. Interobserver agreement was calculated with the Fleiss kappa for multiple observers. The outcome was interpreted according to the classification by Landis and Koch [21] (0 ‘poor’ agreement; 0.00–0.20 ‘slight’ agreement; 0.21–0.40 ‘fair’ agreement; 0.41–0.60 ‘moderate’ agreement; 0.61–0.80 ‘substantial’ agreement; 0.81–1.00 ‘almost perfect’ agreement).
Sample size estimation
Although assessed in patients with a Japanese background, the rate of visualization of PV found in an earlier study was 17 % with WLI, and 58 % with NBI [17]. Based on these percentages, we anticipated that by using a two-sided equivalence test (nQuery Adviser, version 7.0) with a 5 % significance level, a power of 80 and a 15 % drop-out, 25 patients needed to be enrolled in this study to assess the detection rate of PV with WLI versus NBI in Western patients.
Results
Patients and images
The baseline patient characteristics of the 25 patients are listed in Table 1. One image of each combination of conditions per patient resulted in a total of 200 images for image assessment.
Optimal visibility of palisade vessels
Per-image analysis showed 115 images [58 % (95 % CI 51–64)] in which PV were considered visible. Of these, 81 images [70 % (95 % CI 62–78)] were in insufflation and 34 images [17 % (95 % CI 26–43)] in desufflation (p < 0.001). In the per-patient analysis, this resulted in visible PV in 25 patients [100 % (95 % CI 87–100)] during insufflation [in median 4 (IQR 2–4) of the four available insufflation images per patient], whereas PV were recognized in 15 patients [60 % (95 % CI 41–77)] in deflated condition [in median 1 (IQR 0–3) of the four desufflation images per patient]; p < 0.001.
Since the effect of insufflation was confirmed, further analysis was performed with the 100 images in inflated condition to value the clinically relevant effects of type of imaging (WLI vs. NBI) and the approach (forward vs. retroflex). Per-image analysis showed that PV were visible in 42/50 WLI images [84 % (95 % CI 71–92)] and in 39/50 NBI images [78 % [95 % CI 65–87)]; p = 0.44. In the per-patient analysis this resulted in 25 patients [100 % (95 % CI 86–100)] with visible PV during WLI [in median 2 (IQR 1–2) of the 2 WLI images per patient] and in 23 patients [92 % (95 % CI 74–99)] during NBI [in median 2 (IQR 1–2) of the 2 NBI images per patient]. As shown in Table 2, further specification per approach (forward or retroflex, or a combination thereof) did not show any differences.
Interobserver agreement
Per-image analysis showed that the overall interobserver agreement for the presence of PV in images was ‘moderate’ [κ 0.51 (95 % CI 0.47–0.54)]. Table 3 displays the presence of PV with the corresponding interobserver agreement in the eight different combinations of conditions. The overall interobserver agreement was ‘moderate’ within both subgroups of the panel: κ 0.53 (95 % CI 0.45–0.61) amongst the Japanese endoscopists and κ 0.42 (95 % CI 0.34–0.50) amongst the Western endoscopists. In every condition, the agreement amongst Western endoscopists was slightly lower compared to the agreement of the Japanese endoscopists, but in optimal condition (inflated) remained still ‘fair’ to ‘moderate’.
Defining the esophagogastric junction
Figure 2 shows the presence and location of the GF and PV during real-time endoscopy. PV were observed in 24/25 [96 % (95 % CI 80–100)] patients, in line with the photo assessment of the panel. With both landmarks measured in optimal condition, 15 of these 24 patients [63 % [95 % CI 41–81)] had a ‘zone of discordance’ with the top of the GF located median 1 cm (range, 1–2 cm) proximal to the PV.
Findings during real-time endoscopy. The presence and location of the gastric folds and palisade vessels was assessed during insufflation and desufflation (a, b). Fifteen of 24 subjects with visible palisade vessels had a ‘zone of discordance’ when both landmarks were assessed in their optimal condition (i.e., gastric folds with desufflation, palisade vessels with insufflation) (c). The length of this zone of discordance was median 1 cm (range, 1–2 cm). PV palisade vessels, GF gastric folds, ZoD zone of discordance
Intestinal metaplasia in different zones
Figure 3 shows the per-biopsy and a per-patient analysis of the presence of IM in biopsies obtained from the different zones in the 15 patients with a zone of discordance. In four [27 % (95 % CI 8–55) of these patients, IM was found in one or more biopsies in this zone of discordance [16 % [95 % CI 6–35] of all 25 patients].
Presence of intestinal metaplasia. Histology results from the 15 patients with a “zone of discordance”. A per-biopsy analysis (left graph) of the presence of IM in the biopsies obtained per zone showed a significant difference between the three zones. A per-patient analysis (right graph), in which a zone was considered ‘positive for IM’ when one or more biopsies in that zone contained metaplasia, showed that 4/15 patients (27 %) had at least one biopsy with IM in that zone. All four patients also had IM in biopsies obtained above the gastric folds. In patients with no zone of discordance (n = 10), IM was found in 0/20 cardiac biopsies and in 25/40 biopsies obtained proximal of the top of the gastric folds (in per patient analysis: 0 and 100 % of patients, respectively). ZoD zone of discordance, GF gastric folds, IM intestinal metaplasia
Low-grade dysplasia was observed in three biopsies in three different patients, all obtained proximal to the upper end of the GF (i.e., Barrett’s epithelium). No dysplasia was found in biopsies obtained in the cardia or from any ‘zone of discordance’.
Discussion
In 2006, the Prague classification was introduced to describe the endoscopic extent of BE. This classification used the top of the GF as landmark for the distal BE segment [15]. The working group responsible for this classification considered the use of the PV for this purpose but argued that in most BE patients the PV are not visible with standard endoscopic imaging and may be less clear in case of reflux esophagitis. Other investigators, however, have claimed that the PV are the appropriate landmark for this distal end of the BE segment. Yet, the vast majority of publications related the Japanese population only [8, 17, 22–25]. Western guidelines use the upper end of GF as the distal landmark for BE [15]. In contrast, Japanese guidelines have continued to use the location of the PV for this purpose. In this study, we found that current endoscopic imaging techniques allow detection of PV in the majority of our Dutch patients with BE. In addition, we found that in 63 % of these patients there is a discrepancy in the location of the GF and the PV.
Current Japanese guidelines state that PV are best assessed in a distended esophagus [16]. Data in our study confirm this: PV were detected in a lower number of images in deflated condition. Further assessment of the influence of type of imaging and approach was therefore performed in the images in inflated condition. Remarkably, by the majority of our panel, PV were observed in 100 % of patients with conventional WLI, whereas this was in 92 % of patients using NBI. In the study by Hamamoto et al. [17]., the detection rate of PV with WLI was much lower compared to NBI. The use of high-definition techniques in our study may explain the loss of benefit for NBI over WLI in the detection of PV. Equal to the type of imaging, approach (forward or inversion) did not seem to significantly improve the detection of PV.
In our per-image analysis, the overall interobserver agreement of our panel was ‘moderate’ (κ 0.51). The ‘moderate’ interobserver agreement was mainly for images in inflated condition, whereas the interobserver agreement was only ‘fair’ for images in deflated condition. The study by Kusano et al. [26] found an ‘excellent’ overall interobserver agreement (κ 0.88) for the detection of PV. This was, however, performed during real-time non-BE-related endoscopy with a single presence/absence-score for visible PV in a per-patient analysis, and not specified to certain conditions. Moreover, the agreement was based on the scores of two endoscopists versus six in our study.
For our study, we used two ‘groups of observers’: three Western endoscopists, with extensive experience in the diagnosis and treatment of BE but unfamiliar using PV for describing BE, and three Japanese endoscopists, familiar with using PV as landmark for the EGJ. The interobserver agreement amongst Japanese endoscopists ranged from κ 0.47 to 0.62 for ‘insufflation’ images. The three Western endoscopists had a slightly lower, but not significant, interobserver agreement (κ 0.34–0.49) which may reflect their relative inexperience in using PV as a BE landmark.
During the real-time endoscopy, the location of the GF was scored first by our two endoscopists, followed by the assessment of PV. In 63 % of patients with visible PV, the upper ends of the GF were located more proximal than the distal end of the PV. The median length of this zone of discordance of Western and Japanese guidelines was 1 cm. In a study by Ogiya et al., a zone of discordance was found in 17 % of patients, although it remains unclear whether the GF were located proximal to the PV in these cases. Moreover, this was retrospectively assessed on endoscopy images [23]. No other endoscopic studies have directly evaluated this zone of discordance.
The primary aim of this study was to contribute to the knowledge of the EGJ and the value of GF and PV. Yet, does this zone of discordance have any clinical consequences? In other words, would the use of PV as a BE landmark change clinical care in Western patients (Table 4)? For patients with long BE segments (as in this study), the use of the PV would result in an increased measured length of the BE segment in patients with a zone of discordance; but surveillance and treatment strategies would not change. However, in a patient with a small segment of columnar-lined epithelium around the EGJ, the use of different landmarks may result in different conclusions and following clinical steps. One might hypothesize that if the PV are used, more patients may be considered to have a short segment BE on endoscopy, which will be biopsied according to the Seattle protocol to detect IM in a subset of patients who will then be surveilled or treated according to protocol. If the upper end of the GF is used as a landmark, patients with a short segment of columnar epithelium in this zone of discordance are not considered to have BE. Even when found to have IM in this area, they are considered to have IM of the cardia, which is not considered an indication for surveillance. In addition, certain recent guidelines are slowly changing their perspective on efficacy of surveillance for ultrashort (< 1 cm) non-dysplastic BE segments and on the length of surveillance intervals for non-dysplastic 1–3 cm BE segments [27, 28]. Factors that may play a role are the high interobserver agreement for < 1 cm BE segments [15], a low yield of IM [29] in these shorter segments, a low risk for malignancy [11, 30, 31], and the probable poor cost-effectiveness of surveillance for these short non-dysplastic BE segments.
In our study, 27 % of patients with a zone of discordance had IM detected in this area, whereas in none of the patients IM was detected below the PV. Although no statistical significance was reached since the study was not powered for this endpoint, this finding is remarkable. Earlier studies have shown that in 25 % of the normal population IM can be detected in biopsies obtained from the cardia [32, 33]. Although in this study a limited number of biopsies were collected from the cardia and zone of discordance, one might question the incidences of cardiac IM found in earlier studies. Hypothetically, biopsies in these studies may have been collected just distal to the upper end of the GF, possibly from zones of discordance. The IM found in those specific biopsies may then be considered esophageal instead of cardiac. A study powered on this issue may give more answers.
Strengths of this study were the involvement of both a Western endoscopist, with extensive experience in BE, and a Japanese endoscopist, familiar with the use of PV, during the real-time endoscopy. Moreover, all biopsies were evaluated by a pathologist with extensive expertise in BE. However, this study has its limitations. First, only endoscopic still images and no videos were used for scoring the presence of PV. Moreover, images were scored by two specific groups of endoscopists: Japanese endoscopists familiar with PV, and Western endoscopists from tertiary care centers for the treatment of BE. Thirdly, the study was performed in a small population of patients with relatively long Barrett’s segments containing no dysplasia or low-grade dysplasia. Therefore, the role of dysplasia and length of the BE segment on the visibility of PV could not be assessed. Additionally, due to prescripted proton pump inhibitors for their BE, only a few of the patients had reflux esophagitis, a factor that may disturb the detection of PV. Fourth, this study lacks the data concerning the presence of H. pylori and atrophic gastritis, since this is not routinely scored in our population due to the low prevalence, which may hinder interpretation of the data. Next, the study was not powered on the presence of IM or the detection of a zone of discordance, but solely on the detection of PV with WLI and NBI. Although a trend was seen in the presence of IM between the different areas, the relatively low number of patients and consequent low amount of biopsies collected limits sufficient interpretation of the histological results of this study. Finally, the zones of discordance were relatively small (median 1 cm): although biopsies were obtained by an expert endoscopist in BE with additional photo documentation, sampling error cannot be ruled out.
In conclusion, with the current improved imaging systems, PV can be detected in most Western patients with BE. In a substantial group of patients, there is a discrepancy between the location of the upper end of the GF and the distal end of the PV. In this area, IM can be found in a substantial group of patients. Further studies on the clinical relevance of the difference in location of the EGJ and on the incidence of zones of discordance in non-BE patients (according to Western criteria) are needed to provide answers to the questions raised above. Until then, we do not advocate taking biopsies distal of the upper end of the GF as the clinical implications appears to be limited.
Abbreviations
- BE:
-
Barrett’s epithelium
- EAC:
-
Esophageal adenocarcinoma
- EGJ:
-
Esophagogastric junction
- NBI:
-
Narrow band imaging
- PV:
-
Palisade vessels
- WLI:
-
White light imaging
References
Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet. 2009;373(9666):850–61.
American Gastroenterological A, Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140(3):1084–91.
Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129(6):1825–31.
Fan X, Snyder N. Prevalence of Barrett’s esophagus in patients with or without GERD symptoms: role of race, age, and gender. Dig Dis Sci. 2009;54(3):572–7.
Hayeck TJ, Kong CY, Spechler SJ, et al. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010;23(6):451–7.
van Soest EM, Dieleman JP, Siersema PD, et al. Increasing incidence of Barrett’s oesophagus in the general population. Gut. 2005;54(8):1062–6.
Fujiwara Y, Higuchi K, Shiba M, et al. Association between gastroesophageal flap valve, reflux esophagitis, Barrett’s epithelium, and atrophic gastritis assessed by endoscopy in Japanese patients. J Gastroenterol. 2003;38(6):533–9.
Amano Y, Kushiyama Y, Yuki T, et al. Prevalence of and risk factors for Barrett’s esophagus with intestinal predominant mucin phenotype. Scand J Gastroenterol. 2006;41(8):873–9.
Okita K, Amano Y, Takahashi Y, et al. Barrett’s esophagus in Japanese patients: its prevalence, form, and elongation. J Gastroenterol. 2008;43(12):928–34.
Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano–Monghidoro study. Gut. 2008;57(10):1354–9.
Pohl H, Arash H, Ell C, et al. Length of Barrett’s esophagus and cancer risk—implications from a population based study. Gastroenterology. 2014;146(5):S-122.
Anaparthy R, Gaddam S, Kanakadandi V, et al. Association between length of Barrett’s esophagus and risk of high-grade dysplasia or adenocarcinoma in patients without dysplasia. Clin Gastroenterol Hepatol. 2013;11(11):1430–6.
Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119(6):1149–58.
Shibata A, Matsuda T, Ajiki W, et al. Trend in incidence of adenocarcinoma of the esophagus in Japan, 1993–2001. Jpn J Clin Oncol. 2008;38(7):464–8.
Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131(5):1392–9.
Aoki T. Report of research committee on definition of Barrett’s esophagus. Chiba: Japanese Society of Esophageal Diseases; 2000. p. 20–23 (in Japanese).
Hamamoto Y, Endo T, Nosho K, et al. Usefulness of narrow-band imaging endoscopy for diagnosis of Barrett’s esophagus. J Gastroenterol. 2004;39(1):14–20.
Committee ASoP, Evans JA, Early DS, et al. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc.;76(6):1087–94.
Levine DS, Haggitt RC, Blount PL, et al. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus. Gastroenterology. 1993;105(1):40–50.
Schlemper RJ, Kato Y, Stolte M. Review of histological classifications of gastrointestinal epithelial neoplasia: differences in diagnosis of early carcinomas between Japanese and Western pathologists. J Gastroenterol. 2001;36(7):445–56.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Hoshihara Y, Kogure T. What are longitudinal vessels? Endoscopic observation and clinical significance of longitudinal vessels in the lower esophagus. Esophagus. 2006;3(4):145–50.
Ogiya K, Kawano T, Ito E, et al. Lower esophageal palisade vessels and the definition of Barrett’s esophagus. Dis Esophagus. 2008;21(7):645–9.
Kumagai Y, Yagi M, Aida J, et al. Detailed features of palisade vessels as a marker of the esophageal mucosa revealed by magnifying endoscopy with narrow band imaging. Dis Esophagus. 2012;25(6):484–90.
Kinjo T, Kusano C, Oda I, et al. Prague C&M and Japanese criteria: shades of Barrett’s esophagus endoscopic diagnosis. J Gastroenterol. 2010;45(10):1039–44.
Kusano C, Kaltenbach T, Shimazu T, et al. Can Western endoscopists identify the end of the lower esophageal palisade vessels as a landmark of esophagogastric junction? J Gastroenterol. 2009;44(8):842–6.
Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63(1):7–42.
Whiteman DC, Appleyard M, Bahin FF, et al. Australian clinical practice guidelines for the diagnosis and management of Barrett’s Esophagus and Early Esophageal Adenocarcinoma. J Gastroenterol Hepatol. 2015;30(5):804–20.
Melson J, Desai V, Greenspan MM, et al. Negative surveillance endoscopy occurs frequently in patients with short-segment non-dysplastic Barrett’s esophagus. Dis Esophagus. 2015;28(7):660–5.
Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut. 2012;61(7):970–6.
Thomas T, Abrams KR, De Caestecker JS, et al. Meta analysis: cancer risk in Barrett’s oesophagus. Aliment Pharmacol Ther. 2007;26(11–12):1465–77.
Goldblum JR, Vicari JJ, Falk GW, et al. Inflammation and intestinal metaplasia of the gastric cardia: the role of gastroesophageal reflux and H. pylori infection. Gastroenterology. 1998;114(4):633–9.
Morales TG, Sampliner RE, Bhattacharyya A. Intestinal metaplasia of the gastric cardia. Am J Gastroenterol. 1997;92(3):414–8.
Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990;43(6):543–9.
Conflict of interest
Prof. J.J.G.H.M. Bergman received research support for IRB-approved studies (Olympus Endoscopy, Cook Medical, Boston Scientific Corporation, GI Solutions Covidien, ERBE and Ninepoint Medical), financial support for training programs (GI Solutions Covidien) and honorarium-consultancy-speakers fees (Cook Medical, Boston Scientific Corporation and GI Solutions Covidien). Prof. Dr. B.L.A.M. Weusten received research support for IRB-approved studies (GI Solutions Covidien, ERBE and C2 Therapeutics), and consultancy fees (Boston Scientific Corporation and C2 Therapeutics). D.W. Schölvinck, O. Goto, C.A. Seldenrijk, R. Bisschops, J. Horii, Y. Ochiai, E.J. Schoon, B.E. Schenk, T. Uraoka, M.G.H. van Oijen, and N. Yahagi have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schölvinck, D.W., Goto, O., Seldenrijk, C.A. et al. Detection of palisade vessels as a landmark for Barrett’s esophagus in a Western population. J Gastroenterol 51, 682–690 (2016). https://doi.org/10.1007/s00535-015-1136-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1136-2