Abstract
Background
The larynx and hypopharynx are common sites for head and neck cancer, which shares many risk factors with upper digestive tract disease. Patient survival with malignancies depends on stage at the time of diagnosis. Endoscopic screening of the hypopharynx is neither routinely performed in clinical practice nor has it been evaluated in a formal study.
Methods
This is a prospective pilot study of patients undergoing routine EGD. Demographic data were collected from patients prior to the procedure. All patients in the study underwent an EGD and prior to performing the standard portion of the EGD procedure, the endoscopist evaluated the larynx and hypopharynx with both white light endoscopy (WLE) and narrow band imaging (NBI). Details of the procedure, including ability to see all anatomic structures, time spent, complications, and findings, were recorded.
Results
A total of 111 patients were included in the study. The exam of the laryngopharynx was completed in 87 % of patients (97/111). Reasons for incomplete exam included intubated patients (2/14), inadequate sedation (9/14), and inability to see the entire hypopharynx (3/14). The mean time of the WLE was 20.2 s, while the NBI evaluation took 15.6 s for a mean and 35.8 s for the entire exam of the larynx and hypopharynx. Minor procedural complications occurred in 3/11 (2.7 %) of the patients and included hypotension, tachycardia, and hypoxia. There were 6 patients who had hypopharyngeal abnormalities seen on both WLE and NBI (5.4 %) and were subsequently referred to otolaryngology. Of the six referrals, one patient had a vocal cord biopsy showing leukoplakia, while the others were deemed normal anatomic variants.
Conclusions
Evaluation of the hypopharynx can be accomplished by gastrointestinal endoscopists at the time of EGD in the vast majority of patients in a safe manner while adding only about 35 s to the overall exam time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The larynx and hypopharynx are common sites for head and neck cancer, which shares many risk factors with upper digestive tract disease. Patient survival with malignancies depends on stage at the time of diagnosis. Endoscopic evaluation of the hypopharynx is neither routinely performed in clinical practice nor has it been evaluated in a formal study.
During the past two decades, the survival rate for patients with laryngeal cancer has decreased throughout the United States, and it is currently the only U.S. cancer to have worse outcomes over time [1]. According to the National Cancer Institute (NCI), the 5-year survival rate is 76 % for localized laryngeal/hypopharyngeal tumors, whereas regional and distant tumors have a 5-year survival rate of 41 and 34 %, respectively (SEER Cancer Statistics Review, 2002–2008). Laryngeal cancer is difficult to diagnose because symptoms such as coughing, hoarseness, and sore throat are often mistaken for common problems, and more severe symptoms, such as swallowing dysfunction, typically do not present until later stages, leading to late diagnosis, more involved treatments, increased treatment cost, increased mortality, and increased risk for comorbidity and subsequent tumors [2]. Furthermore, current diagnostic techniques such as white light endoscopy do not always adequately identify head and neck cancer [3]. Narrow band imaging (NBI) is a newer imaging technique that has been shown to be a useful tool for cancer detection and is widely available. According to the NCI, upper aerodigestive tract carcinomas (lung, esophagus, and head and neck) have a high coincidence as they share common risk factors, namely tobacco and alcohol, which causes up to 75 % of head and neck cancers with the most common subsites being the oral cavity, oropharynx, larynx, and hypopharynx [4]. Barrett’s Esophagus and gastrointestinal reflux disease (GERD) are also commonly associated with squamous cell carcinoma of the laryngopharynx. This preliminary report will present a study that supports the attempted visualization of laryngeal and hypopharyngeal abnormalities during esophagogastroduodenoscopy (EGD) exams.
Although the review of literature above supports the argument for early detection of laryngeal and hypopharyngeal abnormalities, there is no current standard organized screening exam available for laryngeal cancer. This lack of an exam for early detection is likely due to potentially limited cost/benefit ratio; there are 43.8 million smokers in the US yet there are only 12,000 new cases of laryngeal cancer per year [1]. However, millions of EGD’s are performed annually throughout the U.S. [5]. Despite the visual availability of the larynx and hypopharynx in this at-risk population, visualization of the larynx and hypopharynx is not commonly performed at the time of EGD. In fact, a recent review article in Gastrointestinal Endoscopy highlighted the lack of evidence regarding both safety and time requirements of pharyngolaryngeal exam during EGD and called for more research to be done [6]. The increase in successive tumors due to general late stage diagnosis amplifies the need for better pathways for early tumor identification. This study sought to evaluate the safety and feasibility of laryngeal and hypopharyngeal imaging by WLE and NBI during diagnostic EGD. The Division of Otolaryngology–Head and Neck Surgery and the Division of Gastroenterology at the University of Wisconsin School of Medicine and Public Health collaborated to collect and analyze data on visualizing the laryngeal and hypopharyngeal area during diagnostic EGD.
Materials and methods
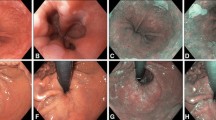
This is an Institutional Review Board (IRB) approved, collaborative, and prospective pilot study between the Division of Otolaryngology–Head and Neck Surgery and the Division of Gastroenterology at the William S. Middleton Veteran’s Administration Hospital in Madison, WI. The study took place over a 6 month period and examined patients undergoing diagnostic EGD. The participating gastroenterologists attended an hour-long educational presentation given by a faculty otolaryngologist (SHD) including basic laryngophayngeal anatomy, epidemiology, pathology, and characteristics of pathology on WLE and NBI. A follow-up session with the same otolaryngologist to review anatomy and pathology was then done immediately prior to the start of the study. Exclusion criteria included suspected risk of upper gastrointestinal hemorrhage, patients less than age 18, or patients unable to give consent. Demographics including age, gender, alcohol and tobacco history, and history of upper aerodigestive cancer were recorded prior to the procedure. Tobacco use was considered positive if there was greater than 10 pack-years of use (information about chewing tobacco was not specifically acquired). Alcohol use was considered to be moderate if the patient averaged two or more alcoholic drinks per day, which was based off of the Centers for Disease Control’s (CDC) definition of alcohol consumption that defines one drink per day for women and two per day for men as moderate. The patients’ blood pressure, electrocardiogram, and oxygen saturation were measured prior to sedation and throughout the procedure per protocol. Sedation medications included midazolam, fentanyl, and benadryl. The patient was positioned in the left lateral decubitus position and a bite-block was used. All EGDs were performed using a standard upper endoscope (Model number GIfh 180, Olympus Optical Co, LTD, Tokyo, Japan). During the procedure, the endoscopist first evaluated the laryngopharyngeal area with WLE, and then with NBI before performing the standard EGD procedure (See Fig. 1A, B). The details and durations of the evaluations during WLE and NBI were recorded and specifically included intubation, difficulty with sedation, frequency and site of lesions or anomalies, and how often the larynx, hypopharynx, and all of the subsites could be seen and examined. Specific subsites examined were the vocal cords/glottis, supraglottis, right and left pharyngeal walls, and right and left piriform sinuses. If the patient was found to have an anomaly, then they were referred to the Division of Otolaryngology–Head and Neck Surgery for further evaluation to determine the final pathology, which was also recorded. All adverse events were recorded, as well as reasons for incomplete examinations. The following events were recorded and considered adverse events: a decline in oxygen saturation less than 90 %, systolic blood pressure less than 90, and heart rate less than 50 or greater than 120. Similar sedation adverse event criteria have been used in previous endoscopy studies [7].
Results
A total of 111 patients were included in the study, 101 of whom were over the age of 50 (See Table 1). The population consisted of 105 males. Fifty-four percent (60/111) had a tobacco smoking history of 10 pack-years or more, with the average smoking history being 40.55 pack-years. Forty-three percent (48/111) of the study group had a history of moderate to heavy alcohol use. Three patients had a history of aerodigestive cancer, including two with a history of laryngeal cancer and one with a history of oral cancer. Examination of the laryngopharynx was completed in 87 % of patients (97/111) (See Fig. 2). Fourteen exams were incomplete, meaning not all subsites could be seen by both WLE and NBI. Reasons for this were endotracheal tube intubation which prohibited visualization of the entire larynx and hypopharynx (2/14), inadequate sedation (9/14), and inability to see the entire hypopharynx (3/14) (See Fig. 3). Of the exams that were not limited by sedation or an endotracheal tube, the examiner was unable to fully visualize the right piriform sinus on one exam and the right laryngopharyngeal wall on two exams. For 5 of the 14 incomplete exams, the patient had no laryngeal/pharyngeal exam at all, while 9 of the 14 had partial laryngeal/pharyngeal exams. Of these nine partial exams, one included a WLE exam but no NBI due to coughing, and two were partial due to the fact that the patient was intubated and the endotracheal tube made visualization of the entire hypopharynx and larynx difficult. In two more partial exams, the right laryngeal wall or right piriform sinus was not able to be adequately viewed. In the remaining four partial exams, patients started coughing; so a part of the exam was then omitted. In all 14 of the incomplete exams, the incomplete or partial exam took place during visualization of the larynx. Evaluation of the hypopharynx and larynx had no impact on the EGD exam, and all patients then had a complete and successful examination of the esophagus/stomach/duodenum.
WLE was performed first with an average exam time being 20.2 s, followed by NBI evaluation averaging 15.6 s, and adding a mean of 35.8 s to the standard EGD exam time.
Three patients (2.7 %) had minor procedural adverse events during the laryngeal and hypopharyngeal exam, one with hypoxia, one with hypotension, and one with tachycardia. There were no major adverse events (bleeding or perforation) due to the exam. All adverse events were self-limited.
Six patients (5.4 %) had hypopharyngeal abnormalities seen on both WLE and NBI and were referred to the Division of Otolaryngology–Head and Neck Surgery. No lesion was seen with NBI that was not seen with WLE. The abnormalities seen by the gastrointestinal endoscopist were: one patient showed a plaque-like lesion on the vocal cord, one showed an aphthous ulcer on the laryngeal wall, two showed a mucosal irregularity, and three showed a small nodule (two on the glottis and one in the piriform sinus). Four of these abnormalities were deemed normal anatomic variants on follow-up exam with transnasal flexible fiberoptic laryngoscopy, while the patient with the plaque-like lesion had vocal fold leukoplakia (See Fig. 4), followed by a biopsy that showed hyperkeratosis. The patient showing leukoplakia had a smoking history of 20 pack-years with no alcohol or aerodigestive cancer history. One patient did not follow-up for the appointment with Otolaryngology–Head and Neck Surgery.
Overall, the larynx and hypopharynx were visible in 87 % of the exams (97/111), 14 of the exams being incomplete due to anatomy, intubation, or coughing/intolerance. Of the completed exams, the larynx, hypopharynx, and subsites were visible 100 % of the time. The most difficult subsites to view were the right laryngeal wall and the right piriform sinus. With regard to final pathology, four follow-up exams resulted in a normal laryngeal exam, making the false positive rate 4/5 (80.0 %). One patient had vocal fold leukoplakia, making the true positive rate 1/5 (20.0 %).
Discussion
The NCI defines screening as “checking for disease when there are no symptoms, which may find a disease at an early stage, leading to better chance of curing the disease” (NCI Dictionary of Cancer Terms). Screening people at risk for cancer has been proven effectively for multiple cancers, for example colorectal [8], breast and cervical [9], lung [10], oral [11], and skin cancer [12]. Laryngeal cancer is the only U.S. cancer to have worse survival rates over time; however, there is currently no screening test for it and hypopharyngeal cancer is one of the most deadly with approximately 88 % of patients presenting with stage III or IV disease [13]. This pilot study was designed and performed to assess the safety and feasibility of visualizing the larynx and hypopharynx using WLE and NBI imaging in those undergoing diagnostic EGD in an at-risk population. Gastroenterology patients were chosen because the larynx and hypopharynx are potentially visible prior to the endoscope being passed into the esophagus. There is also a common coincidence of disease between the gastrointestinal system and the larynx and hypopharynx, because of their shared risk factors, namely tobacco and alcohol.
Early laryngeal cancer is typically asymptomatic, leading to late diagnosis. Hypopharyngeal cancer, in particular, is difficult to diagnose early because symptoms such as coughing, hoarseness, and sore throat are often mistaken for common problems, and more severe symptoms, such as swallowing dysfunction, typically do not present until later stages, leading to late diagnosis, more involved treatments, increased mortality, and increased risk for comorbidity and subsequent tumors [14]. Once a tumor has grown from localized to regional or distant, the 5-year survival rate for laryngeal cancer drops 30–40 %, from 76.4 to 41.8 % for regional and 34.8 % for distant (SEER Cancer Statistics Review, 2002–2008), so finding early cancer is necessary. The cost for treating advanced (T3/T4) laryngeal cancer is on average 2.5 times more expensive than the treatment for early (T1/T2), further supporting the argument for early detection. Three of the most common types of pre-malignant lesions and changes in the epithelium that lead to cancer are leukoplakia, erythroplakia, and pachydermia. These epithelial changes are often asymptomatic [15], a reason for the high rate of late diagnosis. The NCI states that the stage of the detected cancer determines prognosis, supporting the fact that early detection is the best method for survival.
We hypothesized that this method of evaluation would be safe due to the relatively low number of adverse events during diagnostic EGD. Our results supported this hypothesis, as only 2.7 % (3/111) patients had minor, self-limiting adverse events, a typical number for routine EGD. There were no major adverse events. The frequency of serious morbidity associated with upper endoscopy is about 0.5 % and the procedure-related mortality is estimated at between 0.03 and 0.05 %. Cardiopulmonary adverse events are responsible for most of the morbidity and are primarily related to the administration of sedative and analgesic drugs [16]. Since the three adverse events in our study were minor and none of these patients suffered injury or damage, the results suggest that evaluation of the larynx and hypopharynx is safe.
Based off of feedback from gastroenterologists, we also hypothesized that laryngeal and hypopharyngeal evaluation would be feasible during EGD. Two important factors that need to be considered when discussing feasibility are the ability to fully examine the larynx/hypopharynx and the time needed to complete the exam. In 87 % (97/111) of the examinations, the larynx, hypopharynx, and all of the subsites were visible, with the right laryngeal wall and right piriform sinus being the most difficult subsites to view. It is unclear why the right laryngeal wall and right piriform sinus were more difficult to evaluate, but anatomy or patient positioning in the left lateral decubitus position could be playing a role. As with most procedural training, as gastroenterologists perform the exam more frequently, they will likely improve in their ability to evaluate all of the subsites. The most common reasons for an incomplete examination were coughing/gagging due to inadequate sedation or obstructed view from an endotracheal tube. Most patients undergoing diagnostic EGD are not intubated; however, if a patient does have an endotracheal tube, the exam will likely be inadequate for early detection of laryngeal/hypopharyngeal lesions. Importantly, the average exam time of the hypopharynx and larynx added only 35 s to the total time of the EGD, meaning this exam does not unreasonably burden a busy endoscopist. Moreover, all EGD exams were then subsequently completed even in patients in whom examination of the larynx and hypopharynx could not be performed. This suggests that examining the upper airway during EGD does not affect the ability to complete a total EGD exam.
In this study, there were 6 patients who had laryngeal/hypopharyngeal lesions seen on EGD. Of these, five had follow-up examinations done by otolaryngology. Four of the five (80 %) were deemed to have normal anatomical variants, while one patient (20 %) had leukoplakia with a vocal cord biopsy showing hyperkeratosis, one of the most common pre-malignant lesions leading to cancer. This would not have been detected early without evaluation of the larynx during EGD. Our study was designed to assess for safety and feasibility and a much larger study would be necessary to evaluate for specificity and sensitivity of finding laryngeal/hypopharyngeal pathology.
The addition of NBI to the imaging armamentarium may enhance detection rates. The common initial diagnostic technique used in otolaryngology is white light endoscopy which is cost effective, but typically only has a success rate of 67 % for sensitivity and 70 % for specificity [17]. NBI is an imaging method that relies on the penetration of narrow band blue light (415 nm) and green light (540 nm) in order to enhance aspects of the mucosal surface [18]. The sensitivity and specificity of NBI to superficial esophageal squamous cell carcinomas or head and neck carcinomas was 98 and 87 %, respectively, in a study of patients with a known history of esophageal squamous cell carcinoma [2]. While it is not currently used for evaluating head and neck cancers, it is one of the most effective techniques for the early diagnosis of head and neck cancer, supported by a study done at the National Cancer Center Hospital in Tokyo [2]. NBI is presently being used in gastroenterology as an effective imaging tool for the esophagus, stomach, and colon [3], making its application to the larynx an easy transition. NBI has the potential to become an effective, standard method for evaluating the larynx and hypopharyx after more research and education are provided.
There may also be a value in collaborating with those who perform bronchoscopy since the larynx can also be viewed with bronchoscopy but generally also is not focused upon since laryngeal examination at the time of bronchoscopy is not standard of care. While not as common as in esophageal cancer, laryngeal cancer is also linked with lung cancer [19]. Twenty-five percent of patients with non-small cell lung carcinoma were found to have a second primary tumor, the majority of which were head and neck cancers [20]. Bronchoscopists could also participate in early detection for head and neck abnormalities while performing bronchoscopy.
While we believe this study was of high quality, it does have some limitations. One weakness would be that our patient population consisted of veterans so was predominantly male, and had a high reported history of tobacco and alcohol use; therefore, raising the question of whether the results are generalizable to the population at large. A larger study of the general population undergoing routing EGD will provide more information about generalizability. A larger study would also be necessary to evaluate for sensitivity and specificity of laryngeal and hypopharyngeal cancer detection rates as this study was not designed to determine the accuracy of laryngeal screening, only the feasibility. Another limitation was that only tobacco smoking history in pack-years was recorded in our data, so we do not have information on other forms of tobacco use, such as chewing tobacco, a known risk factor for oral-pharyngeal cancer. Also, the number of false negatives is unknown since patients without noted abnormalities during EGD did not have routine follow up with Otolaryngology–Head and Neck Surgery; however, this limitation speaks only to the inability to determine accuracy of the exam, not the safety or feasibility of the procedure which were the primary endpoints. Future studies will be able to better evaluate this detection method and avoid the limitations that we encountered.
In conclusion, this study found evaluation of the larynx and hypopharynx during diagnostic EGD examination can be accomplished in the majority of patients in a safe manner while only adding 35 s to overall exam time. In other words, visualization of the larynx and hypopharynx during an EGD exam is safe and feasible. This was a preliminary study and the participating endoscopists had little experience with examination of the hypopharynx/larynx, but as they gain experience and education, the ability to complete a full exam and the detection rate for laryngopharyngeal abnormalities may increase. We did not find a difference in detection of lesions between WLE and NBI, which was most likely due to the very small sample size of pathology. As more research and education is provided on the value of NBI for the evaluation of head and neck cancer, it could also become a valuable tool in the early detection of laryngeal and hypopharyngeal abnormalities. While our study indicated that early detection of hypopharyngeal and laryngeal abnormalities is safe and feasible, we cannot say if it is effective until further research is done.
Ultimately, gastroenterology and otolaryngology could be nationally joined to work toward a common goal. Screening for laryngeal and hypopharyngeal cancer could be implemented as routine procedure across the nation and may decrease morbidity and disease-specific mortality in the United States.
References
Hoffman HT, Porter K, Karnell LH (2006) Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope 116:1–13
Muto M, Minashi K, Yano T (2010) Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol 28:1566–1572
East JE, Tan EK, Bergman JJ (2008) Meta-analysis: narrow band imaging for lesion characterization in the colon, oesophagus, duodenal ampulla and lung. Aliment Pharmacol Ther 28:854–867
Nonaka S, Saito Y, Oda I (2010) Narrow-band imaging endoscopy with magnification is useful for detecting metachronous superficial pharyngeal cancer in patients with esophageal squamous cell carcinoma. J Gastroenterol Hepatol 25:264–269
Biacabe B, Gleich LL, Laccourreye O, Hartl DM, Bouchoucha M, Brasnu D (1998). Silent gastroesophageal reflux disease in patients with pharyngolaryngeal cancer: further results. Head Neck 20(6):510–514
Emura F, Baron TH, Gralnek IM (2013) The pharynx: examination of an area too often ignored during upper endoscopy. Gastrointest Endosc 78:143–149
Sipe BW, Rex DK, Latinovich D, Overley C, Kinser K, Bratcher L, Kareken D (2002) Propofol versus midazolam/meperidine for outpatient colonoscopy: administration by nurses supervised by endoscopists. Gastrointest Endosc 56(2):324
Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD (2012) Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 366(8):687–696
Magnus MC, Ping M, Shen MM (2011) Effectiveness of mammography screening in reducing breast cancer mortality in women aged 39–49 years: a meta-analysis. J Womens Health 20:845–852
National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. NEJM 365:395–409
Kujan O, Glenny A, Duxbury J, Thakker N, Sloan P (2005) Evaluation of screening strategies for improving oral cancer mortality: a cochrane systematic review. J Dent Educ 69:255–265
Aitken JF, Youl PH, Janda M, Lowe JB, Ring IT, Elwood M (2006) Increase in skin cancer screening during a community-based randomized intervention trial. Int J Cancer 118:1010–1016
al. HEe (2012) SEER Cancer Statistics Review, 1975–2009
Spector JGea (2001) Delayed regional metastases, distant metastases, and second primary malignancies in squamous cell carcinomas of the larynx and hypopharynx. Laryngoscope 111:1079–1087
Probst R, Grevers G (2006) Basic otorhinolaryngology: a step-by-step learning guide. Thieme, New York
Sieg A, Hachmoeller-Eisenbach U, Eisenbach T (2001) Prospective evaluation of complications in outpatient GI endoscopy: a survey among German gastroenterologists. Gastrointest Endosc 53(6):620–627
Kulapaditharom B, Boonkitticharoen V (2001) Performance characteristics of fluorescence endoscope in detection of head and neck cancers. Ann Otol Rhinol Laryngol 110:45–52
Piazza C, Peretti G (2008) Narrow-band imaging: a new tool for evaluation of head and neck squamous cell carcinomas. Review of the literature. Acta Otorhinolaryngol Ital 28:49–54
Piccirillo JF, Lacy PD, Basu A (2002) Development of a new head and neck cancer-specific comorbidity index. Arch Otolaryngol Head Neck Surg 128:1172–1179
Duchateau CS, Stokkel MP (2005) Second primary tumors involving non-small cell lung cancer: prevalence and its influence on survival. Chest 127:1152–1158
Acknowledgments
None.
Disclosures
Shanlee Stevens, Dr. Johnson, Dr. Pfau, and Dr. Dailey have no conflict of interest or financial ties to disclose.
Funding
No specific funding was used for this study or paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stevens, S.M., Johnson, E.A., Pfau, P.R. et al. Visual evaluation of the larynx and hypopharynx during esophagogastroduodenoscopy: a safety and feasibility study. Surg Endosc 29, 1209–1215 (2015). https://doi.org/10.1007/s00464-014-3796-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3796-z