Abstract
Purpose
This report describes the results of an observational, retrospective cohort study, evaluating the use of iron sucrose (IS) and red blood cell (RBC) transfusions in patients with cancer in routine clinical practice in France. A parallel investigated cohort treated with ferric carboxymaltose (FCM) has been reported earlier.
Methods
Data of patients with a solid tumour or haematological malignancy who have received IS or an RBC transfusion during 2010 from 3 months prior (M−3) to 3 months post first treatment (M+3) were analysed.
Results
Data from 46 patients who had received IS (400 mg median total iron dose) and 357 patients who had received RBC transfusions as first treatment (baseline) were included. Median haemoglobin levels improved from 9.9 g/dL (interquartile range 9.2; 11.0 g/dL) at baseline to 12.4 g/dL (11.4; 13.1 g/dL) at M+3 in IS-treated patients and from 8.2 g/dL (7.8; 8.8 g/dL) at baseline to 10.1 g/dL (8.8; 11.1 g/dL) in transfused patients. An erythropoiesis-stimulating agent was given to 54.3 and 28.9% of patients in the IS and the RBC transfusion groups, respectively, resulting in slightly better mean haemoglobin increase in both groups (2.4 vs 1.5 g/dL and 2.0 vs 1.6 g/dL, respectively). No severe nor serious adverse reaction and no hypersensitivity reactions were reported.
Conclusion
Both IS and RBC transfusions effectively increased Hb levels in patients with cancer. IS was safe and well tolerated in this population. Considering prior reported results with FCM, using FCM may reduce ESA dose requirements and the required number of infusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with cancer frequently suffer from anaemia and/or iron deficiency (ID) [1, 2]. In a recent study, anaemia (Hb <12 g/dL) was found in 33.0% of patients with solid tumours and 33.9% of patients with haematological malignancies [2]. ID (transferrin saturation [TSAT] <20%) was found in 45.9 and 35.4%. ID can develop in patients with underlying inflammation, limiting the release of iron form intracellular stocks and availability for erythropoiesis [3, 4] (functional ID), or due to blood loss during surgery or insufficient iron uptake (absolute ID).
Anaemia is associated with impaired quality of life (QoL) and may be related to impaired response to cancer treatment and overall survival [5–8]. ID, even without anaemia, can be associated with impaired physical function and fatigue symptoms [2, 9–11]. When left untreated, ID can develop into anaemia [3]. Established treatment options for anaemia in cancer patients include the administration of erythropoiesis-stimulating agents (ESAs), red blood cell (RBC) transfusions and intravenous (i.v.) or oral iron [3, 12–14]. Guidelines and experts strongly recommend the reduction or prevention of RBC transfusions and restricted usage of ESAs at the lowest required dose. Controlled clinical studies in anaemic cancer patients have shown that addition of i.v. iron to ESA treatment improves Hb levels compared to no or oral iron supplementation [3, 15, 16]. Additional controlled and observational studies have suggested that also patients without concomitant ESA treatment can benefit from i.v. iron [17–21].
The most widely used i.v. iron preparation is iron sucrose, an iron(III)-hydroxide sucrose complex [22]. Depending on the country, iron sucrose is approved for administration at maximum single doses of 200–300 mg, until the calculated total iron deficit is reached. Iron preparations that can be given at higher single doses and are used commonly in cancer patients include ferric carboxymaltose [20, 21] and low molecular weight iron dextran [23].
The study reported here evaluated data on the management of anaemia in cancer patients in France to assess the feasibility and conditions of use of iron sucrose and RBC transfusions in routine clinical practice beyond the limiting criteria of clinical trials.
Methods
Study design and population
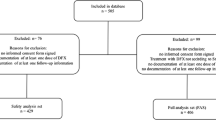
OncoFer was designed as an observational, retrospective and prospective study in 45 active centres in France. Eligible cancer patients had to be at least 18 years of age, diagnosed with a solid tumour or a haematological malignancy, provide informed consent and receive either i.v. iron sucrose (Venofer®, Vifor Pharma, Switzerland) or RBC transfusion in 2010. The study design, methods and data from prospectively enrolled, ferric carboxymaltose-treated patients were previously published [21]. Retrospective enrolment was performed in reversed consecutive order of the patients’ last i.v. iron or RBC transfusion treatment. Patients receiving an investigational anaemia therapy or being enrolled in or having not completed another investigational anaemia study by ≥30 days before baseline were excluded. The study was conducted in compliance with the International Conference on Harmonisation Good Clinical Practice, the Declaration of Helsinki and local guidelines and regulations. Ethics approval was granted by the Ethics committee CPP Ile-de-France VI Hôpital Pitié Salpétrière based on the submitted study protocol.
The primary objective was to describe the administration of iron sucrose and RBC transfusions, including dose, frequency, route and duration of administration and treatment location.
Patient management and data collection
Decisions on diagnostic tests and treatments were left to the investigator’s discretion according to their routine practice. Data of a 6-month period comprising a period from 3 months prior (M−3) to 3 months after (M+3) the first treatment (baseline) were included. Anonymised data on laboratory tests, concomitant medications, adverse and serious adverse drug reactions (related or possibly related to the study drug) were collected from patient records and described in more detail previously [21].
Data analysis
Data are shown for the overall cohort and stratified by treatment with iron sucrose or RBC transfusions without iron sucrose. Categorical data are summarised by number and percentage; continuous data are presented with mean and median values and interquartile range (Q1; Q3) or full range (minimum-maximum). The statistical analysis was performed by ICTA PM (Fontaine-les-Dijon, France).
In order to assess the actual treatment effect of iron sucrose or RBC transfusion, data were censored once a therapy other than that defined at baseline was received. All Hb and iron status data of patients who received an RBC transfusion after the first iron sucrose dose or more than 1 month before the first iron sucrose dose were censored from analysis during 1 month after the RBC transfusion. Patients who received an RBC transfusion within 1 month before the first iron sucrose dose were excluded.
Quality control of 30 randomly selected patient records confirmed that the accepted 1‰ rate of inconsistencies was respected.
According to recommendations for sample size calculations for descriptive prevalence studies [24], a sample size of 405 patients was estimated based on the desired observed frequencies (i.e. half of the total width of the expected CI) and the required precision of ≥5% (i.e. 95% CI). Furthermore, a 5% rate of lost to follow-up or non-evaluable patients over the 3-month follow-up period was anticipated based on an approximately 10% data loss reported in the ECAS study after 6-month follow-up [1].
Results
Patient characteristics
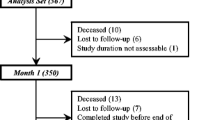
The study set comprised data from 417 cancer patient; 403 patients were included in the analysis set, 46 in the iron sucrose group and 357 in the RBC transfusion group (Fig. 1). Median age of patients was 63 years (25–79 years) in the iron sucrose and 64 years (21–92 years) in the RBC transfusion group; 47.8 and 51.8% were male, respectively (Table 1).
The majority of patients (82%) presented solid tumours (Table 1). Haematologic malignancies were only reported in the RBC transfusion group. Both groups had similar rates of metastatic disease (63.0 and 57.7%, respectively) and ongoing cytotoxic chemotherapy at baseline.
Baseline C-reactive protein (CRP) was assessed in 19.6% of patients in the iron sucrose group and 36.7% in the RBC group. The percentage of tested patients with elevated baseline CRP values was 77.8 and 86.3%, respectively. Median baseline creatinine levels were 59.6 and 76.9 μmol/L and remained stable over time. Abnormal creatinine levels (>80 μmol/L) were observed in 30.0 and 15.8% of patients in the iron sucrose group at baseline and M+3, respectively, and in 43.2 and 32.3% of patients in the RBC transfusion group at baseline and M+3, respectively.
Patient follow-up
Among patients in the analysis set, 63.0% (n = 29) and 62.7% (n = 224) have completed the study at the theoretical month 3 post-baseline visit (M+3) in the iron sucrose and RBC group, respectively. No patient withdrew consent (Fig. 1).
During the observation period, 8 patients (17.4% of deaths) in the iron sucrose group and 95 patients (26.5%) in the RBC transfusion group died. The most frequent causes of death in the iron sucrose and RBC transfusion groups were disease progression (75.0% [n = 6] and 83.2% [n = 79], respectively) and general physical health deterioration (12.5% [n = 1] and 10.5% [n = 10], respectively). Other reasons included cirrhosis, dyspnoea, haemoptysis, haemorrhage, hypoxic pneumopathy and pulmonary oedema (1.1% [n = 1] each in the RBC transfusion group) and one case of unknown reason in the iron sucrose group 12.5% [n = 1]). All deaths were considered unrelated to the study drug.
Circumstances of iron administration and RBC transfusions
The median total iron dose in the iron sucrose group was 400 mg (100–1600 mg), administered in a median of 3 doses (1–7 doses). One dose of iron sucrose was sufficient in 28.3% of the patients, but the majority required multiple iron doses (Fig. 2a). Iron sucrose treatment was still ongoing in the majority of patients at M+1 (60.9%), but only small fractions of patients required iron sucrose at M+2 and M+3 (9.1 and 11.1%, respectively) (Fig. 2b). In all patients with documented mode of administration (n = 40), iron sucrose was administered by undiluted push injection, and in the majority of patients given via a central vein (88.4 vs 11.6% via peripheral vein). The reason for treatment initiation was anaemia in 60.9% of patients (n = 28), ID with anaemia in 26.2% of patients (n = 12) and ID without anaemia in 10.9% of patients (n = 3), based on physician assessment. In one patient, treatment with iron sucrose was initiated because of oral iron intolerance (2.2%). Iron status was only assessed in 37.0% of patients (n = 17) in the iron sucrose group and based on TSAT (76.5%, n = 13), serum ferritin levels (35.3%, n = 6) and other markers (41.2%, n = 7).
Iron sucrose was administered by nurses in the vast majority of cases, and only occasionally by physicians (92.7 vs 7.3%, respectively). A total of 65.1% of the patients received at least one administration of iron sucrose in the hospital, and 37.2% received at least one administration at home.
In the RBC transfusion group, a median of two transfusions (range 1–28) was administered. The reason for treatment initiation was anaemia in 95.8% of patients (n = 342) and ID with anaemia in 1.1% of patients (n = 4). Additional reasons for RBC transfusions included bleeding during surgeries in 2.3% of patients (n = 8), pancytopenia and thrombocytopenia (n = 1, 0.3%), and ‘guidelines without further reason’ (n = 1). Analysis of uncensored data showed that from M−3 to M+3, RBC transfusions were also given to 30.4% of the iron sucrose group overall (Table 2), and post-baseline to 26.1% of patients. The mean number of RBC transfusions given in the iron sucrose group was 1 (1–5).
Additional treatment with an ESA was given to 54.3% of patients in the iron sucrose group and to 28.9% in the RBC transfusion group (Table 2). The median total ESA dose was 454,286 IU (285,714; 752,381 IU) in the iron sucrose group and 357,143 IU (183,810; 610,714 IU) in the RBC transfusion group. The monthly cumulative median ESA dose increased from 94,286 IU (77,143; 147,619 IU) at baseline to 132,857 IU (132,857; 132,857 IU) at M+3 in the iron sucrose group and from 97,143 IU (71,429; 147,619 IU) to 132,857 IU (110,714; 177,143 IU) in the RBC transfusion group.
Effectiveness of iron sucrose and RBC transfusions—Hb evolution over time
In iron sucrose-treated patients, median Hb increased steadily from 9.9 g/dL at baseline to 12.4 g/dL at M+3 (Fig. 3a). Notably, median Hb levels at M+3 slightly exceeded those at M−3 (12.0 g/dL [9.1; 12.7 g/dL]) and did not appear to reach a plateau. In the subgroup of patients who only received one administration of iron sucrose, Hb levels only increased 0.5 g/dL between baseline and M+3 (from 10.5 to 10.9 g/dL, n = 13), while in patients receiving two or more iron sucrose administrations, a more substantial Hb increase ranging from 2.8 to 3.2 g/dL was achieved.
Evolution of Hb from 3 months before (M−3) to 3 months after (M+3) the initial i.v. iron treatment or RBC transfusion, stratified by different patient characteristics. a Median Hb over time by type of therapy (iron sucrose group: 9.9 g/dL [9.2; 11.0 g/dL] at baseline, 10.4 g/dL [9.5; 11.3 g/dL] at M+1, 11.6 g/dL [10.4; 12.5 g/dL] at M+2 and 12.4 g/dL [11.4; 13.1 g/dL] at M+3; RBC group: 8.2 g/dL [7.8; 8.8 g/dL] at baseline, 9.9 g/dL [8.6; 11.2 g/dL] at M+1, 9.9 g/dL [8.6; 11.0 g/dL] at M+2 and 10.1 g/dL [8.8; 11.0 g/dL] at M+3). b Mean Hb in the iron sucrose group stratified by ESA use. c Mean Hb in the RBC transfusion group stratified by ESA use. Graphs are based on censored patient data
In the RBC transfusion group, median baseline Hb (8.2 g/dL) was substantially lower than in the iron sucrose group. Hb levels steeply increased to 9.9 g/dL (8.6; 11.2 g/dL) at M+1, where they plateaued at approximately 10.0 g/dL, and remained lower than at M−3 (11.0 g/dL [9.7; 12.0 g/dL]).
In patients of the iron sucrose group with or without additional ESA treatment, mean Hb levels increased by 2.4 and 1.5 g/dL from baseline to M+3, respectively (Fig. 3b). In the RBC transfusion group, the increase in Hb levels between baseline and M+3 was 2.0 vs 1.6 g/dL in patients with and without additional treatment with ESA, respectively (Fig. 3c). In the iron sucrose group, Hb levels continued to increase between M+1 and M+3 regardless of concomitant ESA therapy. In the RBC transfusion group, a slow but continuous increase of Hb levels was observed only in patients who also received ESA.
Censoring data did not significantly influence the calculated median Hb levels at the end of the observational period. At M+3, data from 2 patients were censored in the iron sucrose group, resulting in an uncensored HbM+3 of 12.3 g/dL [11.3, 13.0 g/dL] compared with censored HbM+3 of 12.4 g/dL [11.4, 13.1 g/dL]. In the RBC transfusion group, data from 4 patients were censored, resulting in an uncensored HbM+3 of 10.0 g/dL [8.8, 11.1 g/dL] compared with censored HbM+3 10.1 g/dL [8.8, 11.1 g/ dL].
Iron status parameters serum ferritin and TSAT
Among iron sucrose-treated patients, median serum ferritin levels increased from 64 ng/mL [17; 260 ng/mL] at baseline to 446 ng/mL [237; 1349 ng/mL] at M+1, 394 ng/mL [224; 632 ng/mL] at M+2 and 561 ng/mL [194; 1302 ng/mL] at M+3. Ferritin levels of RBC-treated patients increased from 456 ng/mL [161; 912 ng/mL] at baseline to 959 ng/mL [337; 4097 ng/mL] at M+1, 839 ng/mL [567; 1688 ng/mL] at M+2 and 2224 ng/mL [905; 3499 ng/mL] at M+3.
TSAT of iron sucrose-treated patients varied between 12.5% [9.0; 14.0%] at baseline to 14.0% [9.2; 40.1%] at M+1, 14.5% [11.5; 21.0%] at M+2 and 13.5% [12.0; 23.0%] at M+3. Among transfused patients, TSAT changed from 9.5% [9.0; 15.1%] at baseline to 26.0% [12.0; 16.0%] at M+1, 25.0% [17.8; 39.7%] at M+2 and 40.0% [39.0; 84.0%] at M+3.
Tolerability, adverse reactions
Neither severe nor serious adverse drug reactions, including adverse reactions leading to death, were reported in the iron sucrose and RBC transfusion groups (Table 3).
Only two patients (3.7%) in the iron sucrose group experienced adverse drug reactions. For one patient each, hypophosphataemia and ineffective drug were reported (1.9% each). In another patient, the second dose of iron sucrose was not administered as planned, because of general status alteration, which was not reported as an adverse drug reaction since the administration was not initiated. Due to local regulations at the time of treatment, the adverse reactions ‘off-label use’ and ‘drug administration error’ were assigned to all patients treated with iron sucrose.
In the RBC transfusion group, 11 patients (3.0%) had also received iron sucrose which was reported as adverse drug reaction ‘off-label use’.
Discussion
This retrospective observational study in cancer patients with anaemia documented routine practice conditions for the use of iron sucrose or RBC transfusions in France during the year 2010. The study also included a cohort of ferric carboxymaltose-treated patients that was prospectively enrolled in 2011 and analysed and published separately [21].
Total iron sucrose doses were divided into three or more separate administrations in more than half of patients although the median total dose per patient was only 400 mg iron. In the formerly reported ferric carboxymaltose cohort, most patients received their iron treatment as a single dose at baseline despite a substantially higher median total iron dose (1000 mg) [21]. Approximately two thirds of iron sucrose administrations have been performed in the hospital, mainly given by nurses. Nowadays, all oncology patients receive i.v. iron treatment in the outpatient setting according to the European Medicines Agency’s published measures to maximise the safe use of i.v. iron, requiring availability of staff and equipment to recognise and manage hypersensitivity reactions [25].
One quarter of iron sucrose-treated patients received additional RBC transfusions and one half additional ESA treatment. In the recently published evaluation of treatment practice with ferric carboxymaltose, only 15.3% of iron-treated patients have received additional RBC transfusions and 35.7% an ESA treatment [21]. Whether this reflects a general trend in treatment practice complying with guidelines and recommendations to minimise or prevent RBC transfusions and ESA doses [3, 12–14] cannot be concluded from the available data due to differences in demographics and disease characteristics of the study populations [21]. Overall, the rather small number of patients who have received iron sucrose administration compared to those who have received RBC transfusions as first treatment (46 vs 357; 11.4%) is in line with studies that investigated the practice in diagnosis and treatment of anaemic cancer patients in France and across nine European countries [26, 27].
In order to assess the actual effect of the treatments on Hb levels, data were censored once a patient received another therapy than defined at baseline (iron sucrose or RBC transfusions); however, overall results were similar in the censored and non-censored populations. Both treatments were associated with restoration of median Hb to levels that patients had 1 month prior to treatment. However, only the iron sucrose group showed a continuous improvement in median Hb during the entire post-treatment observation period of 3 months, even slightly exceeding the Hb level from 3 months prior to the first treatment. The time course and extent of Hb response were similar to those in the formerly reported ferric carboxymaltose cohort, provided that a smaller fraction of ferric carboxymaltose-treated patients has received an additional ESA [21].
The Hb response in the RBC group at 1 month post-treatment was not followed by further improvement, and the final Hb remained slightly below the already subnormal Hb level from 3 months prior the first treatment. These results are consistent with the fact that in cases where anaemia is caused by ID, RBC transfusions only correct the symptoms, but not the underlying cause of anaemia when given without additional iron treatment. Notably, patients in the RBC group had lower median Hb levels throughout the entire 3-month pre-treatment period and experienced a steep decrease in Hb during the month prior to the RBC transfusion. Earlier initiation of an effective anaemia treatment such as iron sucrose or ferric carboxymaltose might have avoided the experience of severe anaemia and the need for the still complex and risky rescue treatment with RBC transfusions.
In line with studies that showed the efficacy of i.v. iron when given without an additional ESA [17–21], also patients who received iron sucrose alone (i.e. without additional ESA) responded well to the iron treatment and achieved mean Hb levels around 12 g/dL. There were no substantial differences in Hb response between patients in the RBC group that have received additional ESA or not. Notably, the respective subgroups with or without ESA treatment had similar Hb levels at baseline and 3 months prior to the first treatment, suggesting that they were at least comparable within the treatment group (iron sucrose or RBC, respectively).
Patients treated with iron sucrose generally achieved repletion of iron stores (serum ferritin); however, the improvements in TSAT were rather modest and low utilisation of iron status assessment at baseline (37.0% overall, 28.3% for TSAT) limits the generalisation of this observation. Formerly reported results in patients treated with ferric carboxymaltose showed a substantial improvement in TSAT with most patients achieving normal TSAT of >20% [21].
RBC transfused patients had already high ferritin levels at baseline that increased to levels of almost 1000 ng/mL and above, and also TSAT rised to 40% and more. These non-physiological high levels of iron status parameters may be caused by release of labile iron from transfused red blood cells before they were ingested by the macrophages of the reticuloendothelial system. Premature release of iron in the circulation can lead to unspecific binding of iron to proteins and oxidative stress [28, 29].
The study confirmed that iron sucrose, given at a median total dosage of 400 mg iron, is well tolerated. Neither a hypersensitivity reaction nor drug-related serious adverse events were reported. Although the number of patients was limited in this study, these observations are in line with previous reports showing the good tolerability of iron sucrose, particularly in comparison to iron dextran and/or ferric gluconate [30–34]. Furthermore, the absence of related serious adverse event in this iron sucrose-treated cohort and the formerly reported ferric carboxymaltose-treated cohort [21] suggests comparable safety profiles of these two compounds. The overall mortality and completion rate are comparable with other populations of cancer patients with metastatic disease (63.0%). Also, RBC transfusions were well tolerated without serious adverse reactions during the 3-month post-treatment observation period. However, large population-based studies and a meta-analysis in the oncology setting suggest an increased long-term risk of mortality, morbidity and cancer recurrence in transfused patients [35–38].
The rate of patients with abnormal creatinine levels suggests a considerable prevalence of renal conditions, which may contribute to anaemia in this patient population.
Notably, the study population included both patients with solid tumours and patients with haematological malignancies. However, the latter comprised only 13.9% of the study population, and prior studies in patients with solid tumours or with lymphoproliferative disorders [15, 39] did not suggest that iron sucrose may act differently in patients with solid tumours or haematological malignancies, respectively. Therefore, no separate analysis of these sub-populations has been performed. In general, the observational nature of the study is associated with some limitations compared with a controlled study, including a less well-defined patient population (e.g. site of primary disease) and a low proportion of patients having all relevant laboratory values assessed. Conversely, an observational study like this allows for evaluation of a treatment’s feasibility in a broader patient population in routine clinical practice.
Iron sucrose was considered as not indicated in oncology and haematology according to the Marketing Authorisation at the time of treatment, and therefore the adverse drug reaction ‘off-label use’ was recorded for each patient receiving iron sucrose. However, the indication of iron sucrose ‘where there is a clinical need for a rapid iron supplyʼ can also include cancer patients, for example during or after treatment with ESAs that rapidly increases iron demand [40].
According to the SmPC, iron sucrose should only be given when ‘the diagnosis of iron deficiency is based on appropriate laboratory tests (Hb, serum ferritin, TSAT, serum iron etc.)ʼ [41]. However, in practice, ID was only tested in less than half of the patients (40%) prior to iron sucrose treatment. The high prevalence of elevated CRP levels confirms that cancer is often associated with chronic inflammation. Therefore, ID in cancer patients should be assessed by markers such as TSAT, which can detect both functional and absolute iron deficiency. Indeed, TSAT was used more commonly than serum ferritin or other markers among patients in this study. The estimated high prevalence of functional iron deficiency in this population further supports the use of i.v. iron instead of a first treatment with oral iron, because oral iron is not expected to be effective when absorption and utilisation of iron are blocked due the presence of inflammation.
Overall, this small study confirms that iron sucrose can be safely and effectively used in patients with cancer. Despite a comparative analysis of results in this cohort and formerly reported use of ferric carboxymaltose in this setting is not possible, the use of ferric carboxymaltose may provide some benefits in terms of lower ESA dose requirements, better response of iron parameters and lower number of infusions. RBC transfusions confirmed their feasibility as potential rescue treatment; however, recovery of Hb levels is not as effective as with i.v. iron, and unphysiologically high levels of iron status parameters warrant reevaluation of this treatment option.
References
Ludwig H, Van BS, Barrett-Lee P, Birgegard G, Bokemeyer C, Gascon P et al (2004) The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 40:2293–2306
Ludwig H, Muldur E, Endler G, Hubl W (2013) Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol 24:1886–1892
Aapro M, Osterborg A, Gascon P, Ludwig H, Beguin Y (2012) Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of intravenous iron. Ann Oncol 23:1954–1962
Weiss G, Goodnough LT (2005) Anemia of chronic disease. N Engl J Med 352:1011–1023
Cella D, Kallich J, McDermott A, Xu X (2004) The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: results from five randomized clinical trials. Ann Oncol 15:979–986
Hudis CA, Van BS, Chang J, Muenstedt K (2004) rHuEPO and treatment outcomes: the clinical experience. Oncologist 9(Suppl 5):55–69
Crawford J, Cella D, Cleeland CS, Cremieux PY, Demetri GD, Sarokhan BJ et al (2002) Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 95:888–895
Crommelin DJ, de Vlieger JS, Weinstein V, Muhlebach S, Shah VP, Schellekens H (2014) Different pharmaceutical products need similar terminology. AAPS J 16:11–14
Brownlie T, Utermohlen V, Hinton PS, Haas JD (2004) Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr 79:437–443
Favrat B, Balck K, Breymann C, Hedenus M, Keller T, Mezzacasa A et al (2014) Evaluation of a single dose of ferric carboxymaltose in fatigued, iron-deficient women—PREFER a randomized, placebo-controlled study. PLoS One 9:e94217
Krayenbuehl PA, Battegay E, Breymann C, Furrer J, Schulthess G (2011) Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 118:3222–3227
Aapro MS, Link H (2008) September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. Oncologist 13(Suppl 3):33–36
National Comprehensive Cancer Network Inc (2014). NCCN Practice Guidelines in Oncology; Cancer and Chemotherapy-Induced Anemia - v.2.2014. Accessed 5 March 2014. http://www.nccn.org/professionals/physician_gls/PDF/anemia.pdf
Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL et al (2010) American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 116:4045–4059
Bastit L, Vandebroek A, Altintas S, Gaede B, Pinter T, Suto TS et al (2008) Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J Clin Oncol 26:1611–1618
Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR (2007) Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 12:231–242
Athibovonsuk P, Manchana T, Sirisabya N (2013) Prevention of blood transfusion with intravenous iron in gynecologic cancer patients receiving platinum-based chemotherapy. Gynecol Oncol 131:679–682
Dangsuwan P, Manchana T (2010) Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. Gynecol Oncol 116:522–525
Kim YT, Kim SW, Yoon BS, Cho HJ, Nahm EJ, Kim SH et al (2007) Effect of intravenously administered iron sucrose on the prevention of anemia in the cervical cancer patients treated with concurrent chemoradiotherapy. Gynecol Oncol 105:199–204
Steinmetz T, Tschechne B, Harlin O, Klement B, Franzem M, Wamhoff J et al (2013) Clinical experience with ferric carboxymaltose in the treatment of cancer- and chemotherapy-associated anaemia. Ann Oncol 24:475–482
Toledano A, Luporsi E, Morere JF, Scotte F, Laribi K, Barriere J et al (2016) Clinical use of ferric carboxymaltose in patients with solid tumours or haematological malignancies in France. Support Care Cancer 24:67–75
Beguin Y, Jaspers A (2014) Iron sucrose—characteristics, efficacy and regulatory aspects of an established treatment of iron deficiency and iron-deficiency anemia in a broad range of therapeutic areas. Expert Opin Pharmacother 15:2087–2103
Auerbach M, Silberstein PT, Webb RT, Averyanova S, Ciuleanu TE, Shao J et al (2010) Darbepoetin alfa 300 or 500 μg once every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. Am J Hematol 85:655–663
Naing L, Winn T, Rusli BN (2006) Practical issues in calculating the sample size for prevalence studies. Arch Orofac Sci 1:9–14
European Medicines Agency (2013). Assessment report for: Iron containing intravenous (IV) medicinal products. Accessed 22 June 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/IV_iron_31/WC500150771.pdf
Ludwig H, Aapro M, Bokemeyer C, Glaspy J, Hedenus M, Littlewood TJ et al (2014) A European patient record study on diagnosis and treatment of chemotherapy-induced anaemia. Support Care Cancer 22:2197–2206
Spielmann M, Luporsi E, Ray-Coquard I, BS d, Azria D, Lasocki S et al (2012) Diagnosis and management of anaemia and iron deficiency in patients with haematological malignancies or solid tumours in France in 2009-2010: the AnemOnHe study. Eur J Cancer 48:101–107
Geisser P, Burckhardt S (2011) The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics 3:12–33
Van Wyck DB (2004) Labile iron: manifestations and clinical implications. J Am Soc Nephrol 15(Suppl 2):S107–S111
Aronoff GR, Bennett WM, Blumenthal S, Charytan C, Pennell JP, Reed J et al (2004) Iron sucrose in hemodialysis patients: safety of replacement and maintenance regimens. Kidney Int 66:1193–1198
Charytan C, Schwenk MH, Al-Saloum MM, Spinowitz BS (2004) Safety of iron sucrose in hemodialysis patients intolerant to other parenteral iron products. Nephron Clin Pract 96:c63–c66
Haddad A, Abbadi R, Marji A (2009) Use of iron sucrose in dialysis patients sensitive to iron dextran. Saudi J Kidney Dis Transpl 20:208–211
Van Wyck DB, Cavallo G, Spinowitz BS, Adhikarla R, Gagnon S, Charytan C et al (2000) Safety and efficacy of iron sucrose in patients sensitive to iron dextran: north American clinical trial. Am J Kidney Dis 36:88–97
Bailie GR (2012) Adverse events associated with intravenous iron preparations: a comparison of reported rates. Clin Adv Hematol Oncol 10:600–602
Al-Refaie WB, Parsons HM, Markin A, Abrams J, Habermann EB (2012) Blood transfusion and cancer surgery outcomes: a continued reason for concern. Surgery 152:344–354
Amato AC, Pescatori M. Effect of perioperative blood transfusions on recurrence of colorectal cancer: meta-analysis stratified on risk factors. Dis Colon rectum. 1998;41: 570–585.
Bakkum-Gamez J, Dowdy S (2014) Retooling the pap smear for ovarian and endometrial cancer detection. Clin Chem 60:22–24
Halabi WJ, Jafari MD, Nguyen VQ, Carmichael JC, Mills S, Pigazzi A et al (2013) Blood transfusions in colorectal cancer surgery: incidence, outcomes, and predictive factors: an American College of Surgeons National Surgical Quality Improvement Program analysis. Am J Surg 206:1024–1032
Hedenus M, Birgegard G, Nasman P, Ahlberg L, Karlsson T, Lauri B et al (2007) Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: a randomized multicenter study. Leukemia 21:627–632
Brugnara C, Chambers LA, Malynn E, Goldberg MA, Kruskall MS (1993) Red blood cell regeneration induced by subcutaneous recombinant erythropoietin: iron-deficient erythropoiesis in iron-replete subjects. Blood 81:956–964
Vifor Pharma UK Limited (2013). Summary of Product Characteristics (UK), Venofer®. Accessed 16 August 2012. http://www.medicines.org.uk/emc/medicine/24168/SPC/Venofer+(iron+sucrose)/
Acknowledgements
Preparation and conduct of the study have been sponsored by Vifor France SA. Data Management and biostatistical analyses were performed by ICTA PM, France, and funded by Vifor France SA. Medical writing support in the preparation of the manuscript was provided by Daniela Kenzelmann-Brož, SFL Regulatory Affairs & Scientific Communication, Switzerland, and funded by Vifor Pharma Ltd., Switzerland.
The authors would like to thank the local investigators who actively participated in this study: Grégory Dendleux, Centre Hospitalier Pierre Oudot; Gérard Dine, Saïd Brahimi, Audrey Echegasd, Nadia Ali Amar, Centre Hospitalier Troyes; Jean-Loup Mouysset, Clinique Rambot-Provencale; Abderraouf Radji, Centre Frederic Joliot; Laurent Zelek, Kader Chouahnia, Gaetan Des Guetz, Marie-Christine Pailler, Thierry Bouillet, Hopital Avicenne; Pierre Bory, Centre Hospitalier Bastia Cedex; Mihaela Achille, Emmanuel Achille, France Campos, Institut De Cancerologie Prive De L’orangerie; Hamah Lamallem, Hopital Americain De Paris; Faress Husseini, Hopital Louis Pasteur; Claude Boiron, Anne Donnadieu, Centre Rene Huguenin; Bertrand Mennecier, Nouvel Hopital Civil; Jean-Philippe Wagner, Centre De Radiothérapie De Dunkerque; Jérôme Dauba, Hopital Layne; Isabelle Gabelle, Chu De Grenoble; Joel Castelli, Pascale Revole, Pascal Bourlet, Centre Hospitalier De Castelluccio; Younes El Masmoudi, Clinique Des Murlins; Delphine Borchiellini, Axel Leysalle, Centre Antoine Lacassagne; Dominique Jaubert, Nathalie Bonichon-Lamichhane, Hortense Laharie, Christophe Debelleix, Clinique Tivoli; Alain Zannetti, Elouen Boughalem , Centre Hospitalier Cholet Cedex; Louis-Marie Dourthe, Clinique Sainte Anne; Anne Floquet, Dominique Bechade, Marie Sire, Institut Bergonie; Isabelle Ray-Coquard, Centre Leon Berard; Jean-Pierre Crumbach, Centre Hospitalier Freyming Merlebach Cedex; Philippe Agape, Tawfiq Henni, Tahar Touahri, Jean-Francois Paitel, Centre Hospitalier Felix Guyon - Allée Des Topazes; Valérie Moulin, Aurélie Ferru, Patrick Bouchaert, Chu La Miletrie; Dominique Spaeth, Fabien Brocard, Célia Becuwe, Centre D’oncologie De Gentilly; Fabienne Watelle, Centre Hospitalier Dr. Schaffner; Nadine Paillot, Caroline Cuvier, Marjorie Lallom, Sylvie Giacchotti, Hopital De Mercy; Sandrine Richard, Julie Gachet, Hopital Europeen Georges Pompidou; Marielle Guillet, Jean-Christophe Souquet, Lionel Wander, Hopital Croix Rousse; Marc Espie, Florence Coussy, Sylvie Giacchetti, Caroline Cuvier, Marjorie Lallom, Hopital Saint Louis; Karima Yakendji Bekredda, Polyclinique Sainte Marguerite; Laurent Bastit, Clinique Pasteur; Alain Saad, Selva David, Tony Nakry, Centre Hospitalier Beziers Cedex; Elisabeth Perez, Patricia Zunic, Groupe Hospitalier Sud Reunion; Jean-Philippe Metges, Institut De Cancérologie Et D’hématologie - Hôpital Morvan, Hugues Bourgeois, Clinique Victor Hugo; Philippe Laplaige, Polyclinique De Blois; Nathalie Denizon, Centre Hospitalier Le Mans Cedex 9; Michaël Finaud, Djamal Hadjadj Aoul, Velardocchio, Clinique Vert Coteau; Alain Ducolone, Elisabeth Quoix, Nouvel Hopital Civil; Jean-Pierre Lotz, Hopital Tenon; Anne Mercier-Blas, Xavier Artignan, Centre Hospitalier Prive Saint Gregoire; Jean-Louis Lacaze, Audrey Eche, Institut Claudius Regaud; Olivier Romano, Hopital Prive La Louviere.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author’s disclosures of potential conflicts of interest
Elisabeth Luporsi, Alain Toledano, Dominique Spaeth, Marc Espié and Stéphanie Perot reported no conflict of interest. Florian Scotté and Roland Bugat reported consultancy to Vifor Pharma.
Ladan Duvillié and Isabelle Pithois Merli are employees of Vifor Pharma France. The authors are fully responsible for content and editorial decisions for this paper. All authors had access to the primary study data. All authors reviewed the paper and approved the final version.
Rights and permissions
About this article
Cite this article
Luporsi, E., Toledano, A., Spaeth, D. et al. Use of iron sucrose and red blood cell transfusions in anaemic cancer patients in France (OncoFer study). Support Care Cancer 25, 973–982 (2017). https://doi.org/10.1007/s00520-016-3489-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3489-3