Abstract
Morphological changes in the nuclear degeneration of the synergid (mainly the synergid that receives the pollen tube) and antipodal cells in Triticum aestivum were studied. Although located in the same embryo sac, and derived from the same megaspore, nuclear degeneration of the synergid and antipodal cells differs greatly. Nuclear degeneration in the synergid is characterized by pycnosis, i.e., total chromatin condensation, nuclear deformation and distinct shrinkage in volume, followed by the formation of an irregular and densely stained mass—the degenerated nucleus—while the nucleolus disappears prior to the degradation of chromatin. In contrast, in the nuclear degeneration of antipodal cells, chromatin is only partly condensed and the nuclear volume changes only slightly after the distinct chromatin condensation. Chromatolysis then occurs, i.e., stainable contents disappear while the nuclear envelope is retained. The nucleoli persist after the disappearance of the chromatin. The possible functions of nuclear degeneration of synergid and antipodal cells are discussed, especially with respect to the guidance of pollen tube growth and the proliferation of free-nuclear endosperm. The degeneration of synergids and antipodal cells in T. aestivum are distinct forms of programmed cell death, regarded as cytoplasmic cell death and nuclear degradation in advance of cell death, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “programmed cell death” was coined in 1965 by Lockshin and Williams (Lockshin and Williams 1965). Programmed cell death (PCD) is an active process of cell death, regulated by a number of genes, commonly occurring during development of multicellular organisms (Greenberg 1996; Martin et al. 1994). Cell death occurs in a predictable “programmed” fashion, and is necessary and beneficial for the normal development of multicellular organisms (Vaux and Korsmeyer 1999).
From embryogenesis to fertilization, PCD occurs at specific sites and times throughout the life of flowering plants, including somatic embryogenesis, differentiation of the vascular system, sloughing of root cap cells, leaf senescence, sex determination, tapetal cell deterioration, the abortion of nonfunctional megaspores, death of nucellar cells and stylar transmitting tissue, degeneration of synergids and antipodal cells, suspensor growth and endosperm development (Greenberg 1996; Wang et al. 1996; Wei et al. 2002; Wu and Cheung 2000).
Degeneration of the synergid and antipodal cells via PCD plays an important role during fertilization and subsequent embryogenesis in flowering plants. Generally, the necessity for synergid degeneration during the fertilization process has been assumed to be closely related to the guidance of the pollen tube into the embryo sac, facilitating the subsequent release of sperm from the pollen tube, and transport of sperm to the respective female nuclei (Russell 1992; Vijayaraghavan and Bhat 1983; Yang 1994). A considerable body of literature concerning the ultrastructural changes of synergid degeneration has accumulated in the last few decades; most researchers have examined cytoplasmic changes (Jensen and Fisher 1968; Mogensen 1972; You and Jensen 1985; reviewed by Vijayaraghavan and Bhat 1983), whereas little attention has been paid to nuclear changes. Although there have been several reports with micrographs showing the degenerating synergid nuclei singly at various stages (Fisher and Jensen 1969; Guo and Hu 1995, 1997; Janson and Willemse 1995; Coimbra and Salema 1999; Yu et al. 1994; Huang and Russell 1994; Sornsathapornkul and Owens 1999), none have dealt with the sequence or pattern of nuclear degeneration. As antipodal cells are thought to have a function relevant to the absorption and transport of nutrients to the embryo sac, most investigations have focused on the ultrastructures of cytoplasm and cell wall adapted to high metabolic activity and transportation (Bohdanowicz and Turala-Szybowska 1985, 1987; Dong and Yang 1989; Engell 1994; You and Jensen 1985), while reports concerning antipodal cell degeneration are scarce (Zhang et al. 1988).
These gaps in the study of nuclear degeneration of the synergid and antipodal cells may be related to the limitations of transmission electron microscopy. Primarily, compared to optical microscopy, the restricted scope of observation and sample capacity of electron microscopy inevitably increases the difficulty in observing the dynamic changes in the nuclear degenerative process. Use of this technique to observe the degenerating synergid nucleus presents further challenges. Specifically, it is difficult to distinguish the degenerating nucleus against the darkly stained cytoplasmic background, especially after entrance and discharge of the pollen tube. These technical difficulties may have limited the number of reports on degenerating synergid nuclei.
Difficulties in standard transmission electron microscopy may be resolved by using Steedman’s wax sectioning and fluorochrome 4′, 6-diamidino-2-phenylindole (DAPI) staining. Steedman’s wax is a low-melting-point wax, which has cutting properties similar to those of paraffin, i.e., material embedded in Steedman’s wax can be cut into ribbon-sections (Steedman 1957). At present, this technique is used extensively for immunofluorescence labeling of the cytoskeleton in plant cells (Brown et al 1996; Baluška et al 1992; He et al. 2002). DAPI is a DNA fluorochrome with high sensitivity, specificity and stability (Zhu and Lin 1986). Applying Steedman’s wax for specimen embedding and fluorochrome DAPI for tissue staining in the investigation of nuclear degeneration of the synergid (mainly the synergid that receives the pollen tube) and antipodal cells in Triticum aestivum, we have not only overcome the above-mentioned limitations, but have also acquired an accurate description of nuclear morphological changes by successively observing the focal planes in ribbon-sections. Furthermore, the images obtained from sections are sharper in contrast to the background than those from direct application of DAPI to the isolated embryo sac. In the present study, we also compare differences in synergid and antipodal nuclear degeneration and discuss the possible functions of this nuclear degeneration.
Materials and methods
Four to five central spikelets of T. aestivum at various stages of development were collected from the field at the Chinese Academy of Agricultural Sciences, Beijing, P.R. China, in May 2002. Ovaries were removed from two florets at the base of each spikelet and divided into 12 stages: before anthesis (stages 1–10), at anthesis (stage 11, when most palea and lemma were closed, and only a few yellow-colored anthers were exposed), and after anthesis (stage 12, spikes appeared bulky, most anthers were exposed and mainly white-colored). For collection of specimens in the first 10 stages before anthesis, spikelets at different stages were picked according to their length from the top of the awns of the spike to the base of the flag leaf blade. Spikelets were collected beginning at about 9–10 cm in length, then continuously at every 1 cm increase in length until anthesis.
Ovaries were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.0) overnight at room temperature. Following a brief rinse in PBS, selected specimens were dissected under a stereomicroscope. Embryo sacs were directly isolated and stained with DAPI. Other specimens were dehydrated in a graded ethanol series, then gradually infiltrated at 37°C with mixtures of ethanol and Steedman’s wax (a 9:1, w/w mixture of polyethylene glycol 400 distearate and 1-hexadecanol; Aldrich) in proportions of 2:1, 1:1 and 1:2 (v/v), soaking for 2 h at each step. After three soakings in pure Steedman’s wax, specimens were placed in a mold allowing the wax to polymerize at room temperature (He et al. 2002).
Longitudinal sections of ovaries were cut at 8 μm in thickness with a rotary microtome. Ribbons were mounted on coverslips coated with poly-l-lysine (2 mg/ml, MW: 1.5–3×105). The sections were first dewaxed in ethanol, and then saturated with distilled water. Coverslips with sections were mounted on a microscope slide containing a drop of DAPI (1 μg/ml) in TAN buffer [17% (w/v) sucrose, 20 mM Tris-HCl (pH 7.6), 0.5 mM EDTA, 1.2 mM spermidine, 7 mM 2-mercaptoethanol and 0.4 mM PMSF; Nemoto et al. 1988], and the surplus dye was blotted. The margins of coverslips were then sealed with nail polish.
Specimens were examined with an epifluorescence microscope and a Leica TCS-NT (or Leica TCS-SP2) confocal laser scanning microscope (CLSM).
Results
States and sizes of nuclei in the mature embryo sac
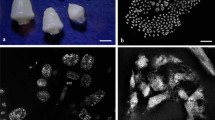
The embryo sac of T. aestivum is of Polygonum type. The mature embryo sac contains an egg apparatus composed of an egg and two synergids at the micropylar end, a central cell with two polar nuclei in the middle, and a mass of 20–30 antipodals at the chalazal end (Fig. 1). The polar nuclei are usually adjacent to the egg apparatus. The egg nucleus is centrally located, while the synergid nuclei are situated in the middle of the cell, near the micropylar end and appressed to the lateral wall (Figs. 2, 3). The egg and synergid nuclei are similar in size, but the former is closer to the chalazal end. The polar nuclei are comparatively large, while the antipodal nuclei are the largest in the mature embryo sac of T. aestivum, varying between one and three times the size of the synergid nuclei (Fig. 1).
An isolated mature embryo sac of Triticum aestivum, showing two synergid nuclei, an egg nucleus, two polar nuclei and many antipodal nuclei, examined in an epifluorescence microscope after 4′, 6-diamidino-2-phenylindole (DAPI) staining. SN Synergid nucleus, EN egg nucleus, PN polar nuclei, AN antipodal nuclei. Bar 50 μm
Normal synergid nucleus
Normal synergid nucleus, identical to that in Fig. 2 and nearly spherical. Note the evenly dispersed chromatin in whole nucleus
The nucleus of one synergid begins to degenerate. a The degenerating synergid nucleus showing nuclear deformation and chromatin condensation. In one portion of the nucleus, the chromatin begins to condense into a compact mass emitting bright blue fluorescence (arrow). b The normal synergid nucleus in the same embryo sac as in a
a–d Successive focal planes of the same embryo sac, showing further nuclear degeneration. The degenerating synergid nucleus shrinks substantially in volume. The whole nucleus becomes pycnotic and gives a very bright, compact and slender appearance. Note that the nucleolus (arrow) is visible. e A normal nucleus (left) and a degenerating nucleus (right) in the two synergids, respectively. Note that both synergids are similar in shape
The pollen tube penetrates into the synergid containing a pycnotic nucleus. a The degenerating synergid nucleus, in which the nucleolus has disappeared, converts into an irregular and compact mass of chromatin. The synergid changes its shape after receiving the pollen tube. b The normal synergid nucleus in the same embryo sac as in a
Difference in DAPI fluorescence intensity among nuclei of component cells inside and around the embryo sac
The nucellar nuclei around the embryo sac of T. aestivum are relatively small and emit intensely bright blue fluorescence, while the nuclei of synergids in the mature embryo sac are large with less vivid fluorescence. The synergid cytoplasm and nucleolar zones do not take up DAPI staining (Figs. 2, 3). The level of nuclear fluorescence of the egg and central cell resembles that of the synergids. However, the fluorescence of polar nuclei is fainter (Fig. 1). In contrast with all other nuclei in the embryo sac, antipodal nuclei emit an intense fluorescence (Fig. 1).
Nuclear degeneration in the synergid
At stages 1–3, the nuclei of both synergids are nearly spherical and emit bright blue fluorescence. The chromatin disperses evenly over the whole nucleus, and some subtle structures, such as granules or flocculi, are visible (Figs. 2, 3). At stage 9 in one of the two synergids, the nucleus becomes tadpole-shaped and begins to shrink in volume. The chromatin, instead of being evenly dispersed, becomes partially condensed into a compact mass with fluorescence increasing in intensity (Fig. 4a). As the area of the condensed chromatin expands, the whole nucleus becomes pycnotic and assumes a very bright, compact and slender appearance, but the nucleolus still persists (Fig. 5a–e). At stage 11, the pollen tube penetrates a synergid containing a pycnotic nucleus and discharges its contents. Simultaneously, that synergid changes in shape. The nucleolus disappears from the nucleus, which had converted into an irregular and compact mass of chromatin (Fig. 6a). However, few changes are found in the other synergid nucleus; it remains the same as at stages 1–3 (Figs. 4b, 5e, 6b).
Nuclear degeneration in the antipodal cells
At stages 1–3, the antipodal nuclei are nearly spherical and relatively small, emitting bright, even and diffuse blue fluorescence. Few subtle structures such as granules and flocculi are visible (Fig. 7). Later, in certain antipodal cells, a part of nuclear periphery appears undulated, the chromatin is only slightly condensed, the nuclear DNA is concentrated, and the fluorescence from the nuclei remains even, but is brighter and more condensed than previously (Fig. 8). At stage 12, the majority of the embryo sacs are in karyogamy. In certain embryo sacs, the division of free-nuclear endosperm has already initiated. In most antipodal cells, the chromatin is strongly condensed and aggregated into compact clusters (Figs. 9, 10), while in some other antipodal cells, the condensed chromatin begins to gradually disappear, with little remaining at the nuclear periphery. The nucleoli of these antipodal cells persist (Figs. 11, 12). The antipodal nuclei seem to enlarge gradually before the extensive condensation of their chromatin.
Normal antipodal nuclei emit bright, even and diffuse blue fluorescence
An antipodal nucleus (arrow) begins to degenerate, emitting even, but brighter and more condensed blue fluorescence. Part of the nuclear periphery takes on an undulated appearance (arrowheads)
The chromatin is strongly condensed and aggregated into compact clusters (arrows)
The chromatin is strongly condensed and aggregated into compact clusters (arrows)
The condensed chromatin gradually disappears, with little remaining at the periphery of the nucleus (arrows). Nucleoli (Nu) persist
The condensed chromatin gradually disappears, with little remaining at the periphery of the nucleus (arrows). Nucleoli (Nu) persist
Discussion
Morphological changes, especially in nuclei, are of great importance in identifying PCD. In apoptosis of animal cells, changes in nuclear morphology follow a standard sequence, characterized by condensation of chromatin, distribution of condensed chromatin into crescents along the periphery of the nuclear envelope, blebbing of the nucleus and, finally, separation of the nucleus into discrete masses of condensed chromatin (Kerr et al. 1972; Martin et al. 1994). However, it is clear that PCD is not synonymous with apoptosis. In general, PCD is a functional term, used to describe cell death that is a normal part of the life of a multicellular organism. Apoptosis, on the other hand, is a descriptive term, introduced by Kerr et al. (1972) to describe a type of cell death exhibiting a distinct set of morphological features. Not all PCD processes are apoptotic, and often have other distinct characteristics (Martin et al. 1994). Hence, during the process of PCD in plant development, although chromatin condensation is always present, the pattern of nuclear degeneration varies, especially the events of nuclear degeneration at the late stages. From our observations of nuclear degeneration of the synergid and antipodal cells in T. aestivum, we propose two different patterns of nuclear degeneration: nuclear pycnosis in the synergid, in which chromatin is condensed into one densely stained mass; and chromatolysis in the antipodal cells, i.e., chromatin disappears via degradation or leakage following chromatin condensation. This information, coupled with that from review of the relevant literature, has led us to conclude that nuclear degeneration during PCD in plant development involves at least four types:
- Apoptotic:
-
Nuclear degeneration is characterized by condensation of chromatin toward the nuclear periphery followed by formation of lobes and fragmentation into highly electron-dense pieces (Eleftheriou 1986; Wang et al. 1996).
- Disintegrating:
-
Nucleus undergoes deformation with mild chromatin condensation, and partial destruction of nuclear envelope leads to leakage of nuclear matrix and eventually nuclear disruption (Wei et al. 2002).
- Chromatolytic:
-
Disappearance of stainable contents and retention of the nuclear envelope at late stages of nuclear degeneration (Evert 1977; Li et al. 2003).
- Pycnotic:
-
Whole nucleus shrinks in volume, condensed into a compact mass of chromatin (Cao et al. 2003; Coimbra and Salema 1999; Guo and Hu 1995, 1997; Yu et al. 1994).
According to You and Jensen (1985), the cytoplasm in both synergids in T. aestivum shows similar signs of degeneration before pollination. However, in the present study, only one synergid nucleus underwent substantial degeneration, while the other remained nearly unchanged even after the pollen tube penetrated the embryo sac. It is interesting that the pollen tube penetrates only the synergid that contains the degenerating nucleus. Therefore, we speculate that further degeneration of the nucleus might be a signal guiding the penetration of the pollen tube into that synergid, in a scenario in which the two synergids are at similar states of cytoplasmic degeneration.
The chalaza is the most important site for transporting nutrients to the embryo sac. The antipodal cells are located only at the chalazal pole of the embryo sac, near the end of the funicular vascular strands, and seem to be critical for absorption and transport of nutrients to the central cell and the egg apparatus, and later to the endosperm and developing embryo (Bohdanowicz and Turala-Szybowska 1985, 1987; Engell 1994). Zhang et al. (1988) studied the structural changes during degeneration of antipodal complex and its contribution to endosperm formation in wheat caryopsis. They proposed that the degenerating antipodal complex exported its cell contents continually to support the proliferation and enlargement of the adjacent free-nuclear endosperm, thereby simultaneously supporting both material transport and nutrient supply. They also observed uneven distribution and gradual decrease of the nuclear heterochromatin, as well as extrusion and migration of nuclear material to the free-nuclear endosperm during the degeneration of the antipodal complex. In the present investigation, we observed similar nuclear changes, i.e., condensation and disappearance of nuclear chromatin after fertilization in antipodal cells of T. aestivum. It is possible that nuclear materials are degraded and transported to the free-nuclear endosperm for nuclear division. Moreover, the nuclear degeneration of these antipodal cells is asynchronous, which might be beneficial to the transport and reuse of the degraded products by spreading nutrient provision over a longer time-frame.
In the antipodal cells of barley, when chromatin condenses into compact clusters, organelles in the cytoplasm remain in a normal state (Engell 1994). Likewise, during the degeneration process of the antipodal cells in wheat, the structure of organelles in the cytoplasm remains intact, while cytoplasmic degeneration is characterized by a decrease in cell content without the accumulation of vesicles, multivesicular bodies and disintegrated nuclear fragments—a common phenomenon in the process of nucellus degeneration (Zhang et al. 1988). We therefore speculate that the nucleus is the primary degenerating structure in the process of the antipodal cell death, while the cytoplasm retains normal function in nutrient transport and supply. Similarly, the nucleus degenerates first during PCD of the starchy endosperm cells in rice (Wei et al. 2002). Here, in contrast with the rapid nuclear degradation, cytoplasmic organelles maintain their metabolic functions for a longer time, and seed reserves are continually synthesized and accumulate despite nuclear degeneration during the PCD process. Two possible mechanisms could facilitate this continuation of cytoplasmic activity after nuclear degeneration (Wei et al. 2002): (1) nuclei maintain their essential relevant functions during the process of degeneration, and (2) sufficient mRNA was transcribed before nuclear disruption. These long-lived mRNAs may remain active, allowing cytoplasmic organelles to maintain regular function despite the lack of a nucleus. These mechanisms may also occur in antipodal cell degeneration.
In barley, when the endosperm becomes cellular, the antipodal cells quickly degenerate (Engell 1994). Wall ingrowths along the embryo sac commonly exist in the central cells of most angiosperms before and after fertilization (Coimbra and Salema 1999; Dong and Yang 1989; Guo and Hu 1997; Sornsathapornkul and Owens 1999; Wallwork and Sedgley 2000; review by Huang and Russell 1992). Formation of these ingrowths may play an important role in transporting nutrients to the embryo sac, especially in those embryo sacs lacking antipodal cells, or during the embryogenesis process after degeneration of the antipodal cells. The preferential degeneration of the nucleus is likely advantageous to the rapid collapse of antipodal cells at the time of cellular endosperm formation. Furthermore, degraded polysaccharide products from the degenerating antipodal cells might participate in the growth process of the cell wall in the endosperm (Engell 1994).
On the whole, the degeneration of synergids and antipodal cells in T. aestivum illustrate two distinct forms of PCD. The synergid degeneration is cytoplasmic cell death (CCD) (Gunawardena et al. 2001), in which cytoplasmic degeneration precedes nuclear changes such as condensation of chromatin; while the degeneration process of antipodal cells is initiated by nuclear degradation, similar to apoptosis in animal cells.
References
Baluška F, Parker JS, Barlow PW (1992) Specific patterns of cortical and endoplasmic microtubules associated with cell growth and tissue differentiation in roots of maize (Zea mays L.). J Cell Sci 103:191–200
Bohdanowicz J, Turala-Szybowska K (1985) Ultrastructure of endopolyploid antipodals in Aconitum vulparia Rchb. I. Antipodals in the mature embryo sac. Protoplasma 127:163–170
Bohdanowicz J, Turala-Szybowska K (1987) Ultrastructure of endopolyploid antipodals in Aconitum vulparia Rchb. II. Antipodals in the period of free nuclear endosperm. Protoplasma 140:13–21
Brown RC, Lemmon BE, Olsen OA (1996) Polarization predicts the pattern of cellularization in cereal endosperm. Protoplasma 192:168–177
Cao J, Jiang F, Sodmergen, Cui KM (2003) Time-course of programmed cell death during leaf senescence in Eucommia ulmoides. J Plant Res 116:7–12
Coimbra S, Salema R (1999) Ultrastructure of the developing and fertilized embryo sac of Amaranthus hypochondriacus L.. Ann Bot 84:781–789
Dong J, Yang HY (1989) An ultrastructural study of embryo sac in Oryza sativa L.. Acta Bot Sin 31:81–88
Eleftheriou EP (1986) Ultrastructural studies on protophloem sieve elements in Triticum aestivum L. nuclear degeneration. J Ultrastruct Mol Struct Res 95:47–60
Engell K (1994) Embryology of barley. IV. Ultrastructure of the antipodal cells of Hordeum vulgare L. cv. Bomi before and after fertilization of the egg cell. Sex Plant Reprod 7:333–346
Evert RF (1977) Phloem structure and histochemistry. Annu Rev Plant Physiol 28:199–222
Fisher DB, Jensen WA (1969) Cotton embryogenesis: the identification, as nuclei, of the x-bodies in the degenerated synergid. Planta 84:122–133
Greenberg JT (1996) Programmed cell death: a way of life for plants. Proc Natl Acad Sci USA 93:12094–12097
Gunawardena AHLAN, Pearce DM, Jackson MB, Hawes CR, Evans DE (2001) Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 212:205–214
Guo FL, Hu SY (1995) Cytological evidence of biparental inheritance of plastids and mitochondria in Pelargonium. Protoplasma 186:201–207
Guo FL, Hu SY (1997) Ultrastructural aspects of the female germ unit in Pelargonium hortorum. Acta Bot Sin 39:193–199
He Q, You RL, Sodmergen, Bao WM (2002) Preprophase band loses its function as a cytokinetic apparatus in mitosis of neck canal mother cell. Protoplasma 220:105–109
Huang BQ, Russell SD (1992) Female germ unit: organization, isolation, and function. Int Rev Cyt 140:233–293
Huang BQ, Russell SD (1994) Fertilization in Nicotiana tabacum: cytoskeletal modifications in the embryo sac during synergid degeneration. A hypothesis for short-distance transport of sperm cells prior to gamete fusion. Planta 194:200–214
Janson J, Willemse MTM (1995) Pollen tube penetration and fertilization in Lilium longiflorum (Liliaceae). Am J Bot 82:186–196
Jensen WA, Fisher DB (1968) Cotton embryogenesis: the entrance and discharge of the pollen tube in the embryo sac. Planta 78:158–183
Kerr JFR, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Li DH, Yang X, Cui KM, Li ZL (2003) Morphological changes in nucellar cells undergoing programmed cell death (PCD) during pollen chamber formation in Ginkgo biloba. Acta Bot Sin 45:53–63
Lockshin R, Williams C (1965) Programmed cell death. II. Endocrine potentiation of the breakdown of the intersegmental muscles of silkworms. J Insect Physiol 11:803–809
Martin SJ, Green DR, Cotter TG (1994) Dicing with death: dissecting the components of the apoptosis machinery. Trends Biochem Sci 19:26–30
Mogensen HL (1972) Fine structure and composition of the egg apparatus before and after fertilization in Quercus gambelii: the functional ovule. Am J Bot 59:931–941
Nemoto Y, Kawano S, Nakamura S, Mita T, Nagata T, Kuroiwa T (1988) Studies on plastid-nuclei (nucleoids) in Nicotiana tabacum L. I. Isolation of proplastid-nuclei from cultured cells and identification of proplastid-nuclear proteins. Plant Cell Physiol 29:167–177
Russell SD (1992) Double fertilization. Int Rev Cyt 140:357–388
Sornsathapornkul P, Owens JN (1999) Ultrastructure and histochemistry of the ovule, fertilization, and formation of the zygote in a tropical Acacia hybrid (Acacia mangium Willd. × Acacia auriculiformis A. Cunn. ex Benth.). Int J Plant Sci 160:229–240
Steedman HF (1957) A new ribboning embedding medium for histology. Nature 179:1345
Vaux DL, Korsmeyer SJ (1999) Cell death in development. Cell 96:245–254
Vijayaraghavan MR, Bhat U (1983) Synergids before and after fertilization. Phytomorphology 33:74–84
Wallwork MAB, Sedgley M (2000) Early events in the penetration of the embryo sac in Torenia fournieri (Lind.). Ann Bot 85:447–454
Wang H, Li J, Bostock RM, Gilchrist DG (1996) Apoptosis: a functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8:375–391
Wei CX, Lan SY, Xu ZX (2002) Ultrastructural features of nucleus degradation during programmed cell death of starchy endosperm cells in rice. Acta Bot Sin 44:1396–1402
Wu HM, Cheung AY (2000) Programmed cell death in plant reproduction. Plant Mol Biol 44:267–281
Yang HY (1994) Recent advances in research on the mechanism of synergid degeneration during fertilization process. Chinese Bull Bot 11:1–5
You R, Jensen WA (1985) Ultrastructural observations of the mature megagametophyte and the fertilization in wheat (Triticum eastivum). Can J Bot 63:163–178
Yu HS, Huang BQ, Russell SD (1994) Transmission of male cytoplasm during fertilization in Nicotiana tabacum. Sex Plant Reprod 7:313–323
Zhang WC, Yan WM, Lou CH (1988) The structural changes during the degeneration process of antipodal complex and its function to endosperm formation in wheat caryopsis. Acta Bot Sin 30:457–462
Zhu C, Lin CT (1986) Optical properties and applications of a new DNA fluorochrome 4′, 6-diamidino-2-phenylindole. J Wuhan Bot Res 4:91–102
Acknowledgements
The Peking University Health Science Center (Beijing, P.R. China) provided excellent CLSM facilities and technical support. We thank Dr. Qun He for instruction in the use of Steedman’s wax, Prof. Shu-Jin Shen for correction in English writing and Dr. Cheng-Jun Ji for careful review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
An, LH., You, RL. Studies on nuclear degeneration during programmed cell death of synergid and antipodal cells in Triticum aestivum. Sex Plant Reprod 17, 195–201 (2004). https://doi.org/10.1007/s00497-004-0220-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-004-0220-1