Abstract
A detailed study into the structural features of the multilevel antipodal complex of wheat Triticum aestivum L. embryo sac was performed at different stages of the complex’s differentiation after double fertilization. The heterogeneity of nuclei ploidy in individual antipodal complexes caused by the asynchrony of the endoreduplication rounds of the nuclear DNA was revealed. The nuclei ploidy of basal, middle, and apical layers of the complexes was measured at the early, middle, and late stages of differentiation. At the early stage of differentiation, the nuclei ploidy of the antipodal complex’s basal layer adjacent to the chalasal region of the nucellus of the embryo sac reaches 13 C, the nuclei of the apical layer cells that contact the endosperm syncytium reaches 63 C, and the nuclei of the middle layer located between the basal and apical layers reach 30 C. At the middle stage of differentiation, the nuclei ploidy in the basal layer increases to 17 C. The nuclei ploidy of the apical layer cells increases to 95 C, and nuclei ploidy of the middle layer increases to 45 C. At the stage of late differentiation, the nuclei ploidy in the basal layer increases to 24 C; the apical layer ploidy increases to 215 C; the middle layer ploidy increases to 63 C. Changes in the shape and structure of the nuclei during differentiation were revealed. They manifest themselves in heterogeneity in shape, size and structure of chromatin; the formation of individual polytene chromosomes; nuclear membrane invaginations; and the variation in the number of nucleoli. Data on the distribution and structure of cytoplasmic organelles of the antipodal cells, endoplasmic reticulum, dictyosomes, mitochondria, and microtubules at different stages of differentiation of the antipodal complexes are fundamentally new. The increased number of cytoplasmic organelles was revealed. During the differentiation, prolong cisterns of the granular reticulum are replaced by concentric rings, mitochondria and plastids of extended and cupped shape appear, and the microtubule network is rebuilt. The features of the antipodal complex’s cell structure may reflect changes in the functions of the antipodal complex during the differentiation. At the early stage, all cells of the complex perform an osmoregulatory function, and cells of different layers of the complex specialize at the middle stage of differentiation. The ploidy level of cell nuclei with polytene chromosomes reflects their functional significance in the formation of endosperm at the nuclear stage of development, and, subsequently, of normal full-fledged grain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Polyploid cells are formed in many tissues of animals and plants, and polytenic chromosomes are formed in the nucleus only in rare cases to ensure the high synthetic activity of cells that perform specialized functions. The structure of polytene chromosomes has been studied in detail for Diptera. In such polytene chromosomes, somatic conjugation between homologous chromosomes and homologous chromatid sites in the same functional state has been detected (Zhimulev, 1994, 1996).
Polyploid nuclei with giant polytene chromosomes of another type were observed in the decidual and trophoblast cells of mammals located between the fetal placenta and the maternal organism (Zybina, 1986) and in the antipodal cells of the female gametophyte of cereals located in close proximity to the ovuleus conducting system and occupying the border position between the endosperm formed as a result of double fertilization and the area of the placento-chalase (Poddubnaya-Arnoldi, 1976; Batygina, 1994). The similarity in the structure of plant and animal cells' giant chromosomes suggests the similarity of their functions. These cells belong to the tissues that perform trophic and barrier functions between the maternal organism and the tissues formed after fertilization. A secretory function is also common to cells with such polytene chromosomes (Zybina, 1986; Chaban et al., 2011).
The antipodal complex’s cells of the embryo sac in cereals are characterized by a long period of functioning (relative to the period of seed development) as compared with other plant species (Poddubnaya-Arnoldi, 1976). In addition, it is precisely in cereals that giant polytene chromosomes form in antipodal cells’ nuclei. Therefore, the antipodal cells of the embryo sac in cereals are a unique object for solving the fundamental problems of cell biology and a convenient model for studying the structure of the nuclei and the stages of reforming polytene chromosomes during ontogenesis.
Earlier, Chaban et al. described in detail the formation stages of an antipodal complex before fertilization. It was shown for the first time that the three initial cells of the complex undergo an unequal number of asynchronous divisions during proliferation. In the early stages of the antipodal complex’s proliferation, mitosis occurs in the basal and apical cells, whereas the cells of the apical zone only divide in the later stages. The formed complex consists of three tiers of cells: the basal tier adjacent to the chalasal zone of the nucellus, the apical tier bordering the central cell and, subsequently, the endosperm, and the middle tier located between the basal and apical tiers. During this period of development, antipodal nuclei do not differ in size and DNA content from the nuclei adjacent to the nucellus cells. After the completion of proliferation before double fertilization, the process of endopolyploidization of antipodal cells’ genomes is initiated, which is accompanied by a total rearrangement of chromatin, and, as a result, giant chromosomes form in the nuclei (Chaban et al., 2011).
The most important stage in the female gametophyte’s development in cultivated cereals is the formation of endosperm tissue in it, the normal development of which causes the full formation of these plants’ grain. The key role in this process is played by cells of the antipodal complex of the embryo sac. It was shown that the grain is not formed or unperformed grain is formed when the development of antipodal cells’ complexes of barley-rye and wheat-rye amphidiploids is disturbed (Brink and Cooper, 1944; Rigin and Orlova, 1974). The earlier-described secretion of the nucleus components and the nucleolus into the cytoplasm of antipodal cells and, later, into the endosperm, suggests that the synthesis of substances necessary for the forming endosperm at the nuclear stage is one of the main functions of antipodal cells (Chaban et al., 2011). The unique structural features of antipodal cells are explained by their functions at various stages of the complex’s differentiation, when all the key processes in their nuclei and cytoplasm occur.
Studying the fine structure of nuclei’s and organelles’ chromatin from the cytoplasm in antipodal cells during the complex’s differentiation is of fundamental importance for understanding the processes of somatic polyploidy in plant cells.
Current research on antipodal cells from the embryo sac of cereals (Kuwada, 1910; Terada, 1928; Morrison, 1954; Hoshikawa, 1960; Diboll and Larson, 1966; Diboll, 1968; Kaltsikes, 1973; Kaltsikes et al., 1975; Petrova et al., 1985; You and Jensen, 1985; Engell, 1994; Maeda and Miyake, 1996; 1997; Lazarev and Chentsov, 2004; An and You, 2004; Chaban, 2008; Chaban et al., 2011; Chetoor and Evans, 2015) are fragmentary and do not give a clear idea about the structure of these cells at different stages of their differentiation.
The goal of this work is to study the structural and functional features of the antipodal complex’s cells at the stage of differentiation after double fertilization of the wheat embryo sac.
MATERIALS AND METHODS
The antipodal complex’s cells (n = 21) from the embryo sac of hexaploid wheat (Triticum aestivum L.) Moscovskaya 39 variety (2n = 42) were the object of the study. The ovules at different stages of development were extracted from wheat ears.
For immunocytochemical staining, ovules were fixed for 2 h in 4% paraformaldehyde (Sigma, United States) on PBS (pH 7.2). To identify DNA and argentophilic proteins, the nucleolus of the ovule was fixed in a mixture of ethanol and glacial acetic acid in a 3 : 1 ratio. To study cell ultrastructure, the ovule was fixed in 2.5% glutaraldehyde solution on 0.1 M Zorensen’s phosphate buffer (pH 7.3) with sucrose (0.015 g/mL).
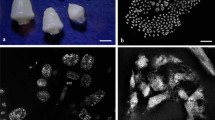
After fixation, embryo sacs containing the endosperm, the embryo, and antipodal cells were isolated from ovule tissue using dissecting needles under a binocular loupe according to Petrova (1970). A general view of ovules and isolated embryo sacks are presented in Figs. 1a and 1b.
General view of ovules, embryo sac, and complexes of wheat Triticum aestivum L. antipodal cells at the stage of differentiation. (a) Individual ovules isolated from the spike, scale: 3 mm. (b) Embryo sac with antipodal complex at the stage of early differentiation, scale: 250 μm; ac—antipodal complex. (c, d) Complexes of antipodal cells at the (c) early and (d) late stages of differentiation. * Cytoplasmic vacuoles of antipodal cells, scale: 70 μm; ac—antipodal complex.
The morphology of antipodal cells’ nuclei was studied after staining with hematoxylin Carrachi (Merck, Germany). DNA staining was performed according to the Völgen method with Schiff’s reagent (Sigma, United States) and DAPI fluorochrome (4,6-diamidino-2-phenylindole) (Sigma, United States). In order to identify RNP-products in the antipode nuclei, regressive RNA contrasting was performed according to (Monneron and Bernhard, 1969). Nucleol argentophilic proteins were detected after Ag-Nor staining (Lazareva and Chentsov, 2004).
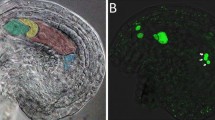
To study the morphology and assess the DNA content of the nucleus, cells were stained with DAPI fluorochrome (4,6-diamidino-2-phenylindole) contained in the final medium Vectashield with DAPI (Vector Laboratories, United States). The amount of DNA of the antipodal cells’ nuclei was estimated in the images of the complexes’ nuclei (PlanApo×20 lens) (Figs. 2a, 2b, 2c) using the ImageJ software. After subtracting the background (“Tresholding”), the program identified the individual nuclei of the antipodal complex (“Analyze particles”) and determined the luminous intensity indices per square of each nucleus (“Integrated Optical Density”). As a reference point, the luminescence intensity for the nuclei of the endosperm triploid cells was measured on the same images.
For indirect immunocytochemical detection of the nucleolus’ fibrillarin, mouse monoclonal antibodies (1 : 50) against fibrillarin clone 38F3 (Abcam, United States) and sheep antibodies against mouse Ig conjugated with fluorochrome Alexa 488 (Sigma, United States) were used at a 1 : 400 dilution.
For indirect immunocytochemical detection of microtubules, mouse monoclonal antibodies against α-tubulin clone DM1α (Sigma, United States) (1 : 100 dilution) and goat antibodies against mouse IgG conjugated with Alexa 488 fluorochrome (Sigma, United States) (1 : 1000 dilution) were used.
For indirect immunocytochemical detection of EPR, the Golgi apparatus and mitochondria, mouse monoclonal antibodies (2 : 100 dilution) against KDEL (Abcam, United Kingdom), mouse monoclonal antibodies (2 : 100 dilution) against a 58K protein of the Golgi apparatus (Sigma Aldrich, United States), and rabbit polyclonal antibodies (1 : 100 dilution) against cytochrome c (Agrisera, Sweden) were used. As second antibodies, donkey antibodies against mouse Ig conjugated with Alexa 488 fluorochrome (Sigma, United States) at a 1 : 1000 dilution and donkey antibodies against rabbit Ig conjugated with Alexa 488 fluorochrome (Abcam, United Kingdom) were used.
Stained preparations were put in Mowiol (Hoechst, Germany). The preparations were studied using a Leica light microscope (with N Plan ×40 and ×100/1.25 lenses, oil) equipped with a DFC 295 digital camera and Leica Аpplication Suite Version 3.4.0 software; Axiovert 200M inverted fluorescence microscope (Carl Zeiss Inc.) (with PlanApo ×20 and × 63/1.4 lenses, oil) equipped with a black and white Carl Zeiss AxioCam digital camera and AxioVision 3.1 software.
A three-dimensional reconstruction of the granular reticulum’s localization and the dictomy of the antipodal cells’ Golgi apparatus was carried out using images obtained with a Nicon Yokogawa confocal microscope with a standard set of filters and PlanApo objectives ×20, ×60 and ×100 (scanning step of 0.5 µm, 25 images in the Z-stack).
To study the ultrastructure of antipodal cells, fixed embryo sacs were put into Epon 812 (Sigma, United States) according to the standard procedure. Ultrathin sections were obtained using an LKB III microtome (Sweden) and stained according to the Reynolds method (Reynolds, 1963). Ultrathin sections were studied using a JEM-1011 transmission electron microscope (JEOL, Japan) with a GATAN ES500W digital camera.
Image processing was performed in ImageJ and Photoshop CS6 software.
RESULTS
Investigation into the differentiation process of the antipodal embryo sac in T. aestivum cells after double fertilization has shown that the cells are unevenly increasing in size at this stage of development due to numerous rounds of endoreduplication. An analysis of the structure of antipodal complexes of 50 germinal bags (1000 cells) showed that the basal layer of the antipodal complex was formed by 5–6 cells, the apical layer had 8–10, and the middle one had 10–12 cells at the differentiation stage.
At the early stage of differentiation, all cells of the antipodal complex have an oval shape, rounded nuclei of similar size, and cytoplasm with one or two large vacuoles (Fig. 1c). However, they later begin to differ in morphology, which is manifested in a significant increase in the size of the nuclei of individual cells and the appearance of separate polytene chromosomes in them (Fig. 1d).
Antipodal Cells’ Nuclei
The amount of DNA of the antipode nuclei (1000 cells) of complexes at different stages of differentiation was determined on total preparations of 50 embryo sacs stained with DAPI in the ImageJ software. The DNA content in the cell nuclei of the antipodal complex at different stages of differentiation is given in Table 1.
The obtained data showed that, within the same antipodal complex, the cell nuclei differ in DNA content at all stages of differentiation. At the same time, cells from different tiers of each complex differ mar-kedly in size and nuclei ploidy. The nuclei ploidy of cells adjacent to the endosperm is significantly higher than in the nuclei of other tiers’ cells. At the same time, cells of the lowest ploidy are located in the basal tier adjacent to chalase.
All the nuclei of antipodal cells at the early stage of differentiation of the complex have a rounded shape, and no individual polytene chromosomes were found (Fig. 2a). Antipodal cells of the basal layer with small rounded nuclei with fibrillar chromatin retain their morphology until the end of the differentiation’s middle stage. The cell nuclei of the middle and apical tiers increase markedly in size, and giant polytene chromosomes are found in them (Fig. 2b). At the late stage of differentiation, polytene chromosomes are observed in the cell nuclei of all tiers, and the nuclei acquire an elongated-oval shape (Fig. 2c).
By staining the components of the nucleus with hematoxylin Carrachi (Fig. 3b), Ag-Nor staining of argentophilic nucleolus proteins (Fig. 3c), and immunocytochemical detection of the major protein of the dense fibrillar component of the nucleolus – fibrillarin (Fig. 3d), it was shown that, the nuclei contain from one to four giant nucleoli and several zones of localization of mini-nucleoli at all stages of differentiation.
Main structural domains of the nuclei of antipodal cells at the stage of differentiation of the complex. (a) Chromosomes (Schiff’s reagent, Fellgen method); (b) chromosomes and nucleoli of the nucleus (Hematoxylin Carrachi); (c) nucleoli and mininucleoli (Ag-NOR staining); (d) nucleoli and mininucleoli (antibodies against fibrillarin, 38F3 clone), chromosomes (DAPI); (e, f) nuclei of antipodal complexes at the early and middle stages of differentiation. ChN—chalasal nucellus; ns—nucleolus; ch—chromosomes; * condensed regions of polytene chromosomes; arrows—mininucleoli. Scale: 30 μm.
A schematic representation of the of the antipodal cells’ nuclei structure at different stages of their dif-ferentiation is presented in Fig. 4.
Nuclei of antipodal cells at different stages of differentiation of the complex. Hematoxylin Carrachi. (a) Fibrillar endopolyploid nuclei of the tiers of the complex at an early stage of differentiation. (b, c) Isolated individual polytene chromosomes of the nuclei of the middle and apical tiers at the middle and late stages of differentiation. Scale: 30 μm.
The study of the antipodal cells’ ultrastructure showed that the nuclear membrane is permeated with pore complexes and forms numerous invaginations at the middle and late stages of differentiation (Figs. 5a, 5b).
Ultrastructure of nuclei of wheat embryo sac antipodal cells at the stage of differentiation. (a) Polytene chromosomes (ch), giant nucleolus (ns) with numerous heterogeneous fibrillary centers (fc) and vacuoles (v), mininucleolus (mns) in the nucleus of the antipodal cell at an early stage of differentiation. Scale: 10 μm. (b) Invaginations of the nuclear membrane of the nucleus at the early and middle stages of differentiation. Scale: 5 μm.
At an early stage of differentiation, polytene chromosomes are formed by a smaller number of chromatids in contrast to chromosomes in the nuclei at the middle and late stages of differentiation. In the nuclei, giant nucleoli with numerous heterogeneous fibrillary centers immersed in an extensive dense fibrillar component surrounded by numerous granules are detected (Fig. 5a).
In the area of polytene chromosomes, polymorphic structures (Figs. 6a–6c) containing RNA and RNP and associated with the surface of polytene chromosomes (Fig. 6d) are identified.
Polymorphism of structures containing RNA and RNP in the nuclei of cells of antipodal complexes at the stage of differentiation. (a–c) Structures associated with the surface of chromosomes (arrows) in the nuclei of antipodal cells. (d) RNA and RNP of the nucleus of antipodal cells (Bernard method). ch—Chromosomes; rnp—ribonucleoproteids. Scale: (a) 3 μm; (b–d) 1 μm.
Antipodal Cells’ Cytoplasm
The use of immunocytochemical staining in the cytoplasm of antipodal cells that are at the stage of differentiation revealed the localization features of its granular reticulum, dictyosomes, mitochondria, and microtubules.
To localize the main organelles involved in the secreting function, a three-dimensional reconstruction of the antipodal cells’ structure was carried out after immunocytochemical detection of the granular reticulum (Fig. 7b) and dictyosomes (Fig. 7d). At an early stage of differentiation, the granular reticulum and dictyosomes are located throughout the entire cytoplasm of the antipodal cells. In the cells of the basal layer, such a pattern is observed during the entire stage of differentiation. In the cells of the middle and apical tiers of the complex, dictyosomes are located on the periphery of the cell and near the invaginations of the nuclear membrane at the middle and late stages of differentiation (Fig. 7). Numerous mitochondria are located throughout the entire cytoplasm of the cells of the antipodal complex (Figs. 8a, 8b).
Localization of the endoplasmic reticulum and dictyosomes in the cytoplasm of the cells of antipodal complexes of wheat embryo sacs. Immunocytochemical staining of granular reticulum (KDEL) and dictyosomes (58K). (a) Granular reticulum on the optical section; (b) distribution of granular reticulum in the cell volume (Z-projection 25 sections); (c) dictyosomes on the optical section; (d) distribution of dictyosomes in the cell volume (Z-projection 25 sections). Scale: 30 μm.
Localization of mitochondria and microtubules at the stage of differentiation of cells of the antipodal complex. (a, b) Mitochondria (antibodies to cytochrome c) at the early and late stages of differentiation. (d, f) Bundles of microtubules (antibodies to tubulin, DM1α clone) in the cytoplasm of antipodal cells at the early and middle stages of differentiation. (c, e) Nuclei of antipodal cells. Scale: (a, b) 30 μm; (c–f) 20 μm.
At the early stage of differentiation in the cytoplasm of antipodal cells, a thin network of numerous bundles of short microtubules is detected (Fig. 8d). At the late stage of differentiation, dense extended bundles of microtubules are detected (Fig. 8f).
When studying the ultrastructure of the cytoplasm of antipodal cells at the stage of differentiation, numerous organelles are clearly visualized.
At an early stage, the granular reticulum has the shape of extended cisterns. At the late stage of diffe-rentiation, the granular reticulum is represented by concentric closed cisterns, inside of which there can be mitochondria and plastids (Fig. 9a). The number of such concentric cisterns located one inside the other can reach ten at the late stage of differentiation (Fig. 9b). Stacks of dictyosomes of the Golgi apparatus consisting of 4–10 cisterns are identified (Fig. 9c).
Ultrastructure of the endoplasmic reticulum and dictyosomes at the stage of differentiation of cells of the antipodal complex. (a, b) Multilayer concentric cisterns of granular reticulum (epr) at the early and middle stages of differentiation. Individual dictyosomes of the Golgi Apparatus (ga). Plastids (p) and mitochondria (m). Chromosome (ch), nuclear envelope (ne). Scale: 3 μm.
Mitochondria are numerous, they are difficult to distinguish from plastids (Figs. 9a, 10b). However, the mitochondrial matrix is lighter than that of the plastids. Many mitochondria at the late stage of differentiation are in the shape of a bowl (Fig. 10a). The form of plastids is diverse and varies from oval to cupped (Fig. 10b). In the cytoplasm of antipodal cells at all stages of differentiation, numerous ribosomes are detected. At the late stage of differentiation there are numerous large lipid droplets in the cytoplasm (Fig. 10b).
Ultrastructure of mitochondria and plastid of the cells of the antipodal complex at the stage of differentiation. (a) Cupped mitochondria (m), cisterns of granular reticulum (epr). (b) Dictyosomes of the Golgi apparatus (ga), plastids (p), lipid droplets (l), pore complexes (pc) of the antipodal cell. Nuclear envelope (ne) and the telomere region of the polythene chromosome (ch) of the antipodal cell nucleus. Scale: (a) 6 μm; (b) 3 μm.
DISCUSSION
In a number of early cytological works, a brief description of the ultrastructure of the antipodal cells of various cereals—Zea mays L. (Diboll and Larson, 1966; Diboll, 1968), Oryza sativa L. (Maeda et al., 1996, 1997), Hordeum vulgare L. (Engell, 1994), and Triticumaestivum L. (Morrison, 1954; Hoshikawa, 1960; You and Jensen, 1985)—was given. It was shown that the nuclei of the antipodal cells in cereals increase in size during ontogenesis. The nuclei contain one or several large nucleoli with vacuoles. The nuclear envelope forms numerous invaginations (Morrison, 1954; Hoshikawa, 1960; Diboll and Larson, 1966; Diboll, 1968; You and Jensen, 1985; Engell, 1994; Maeda et al., 1996, 1997).
Chaban et al. showed that the sizes of the nuclei of antipodal wheat cells at the stage of differentiation after fertilization are different: the nucleus of the apical layer cells from the antipodal complexes reach the largest size. In the composition of the giant chromosomes, individual fibrillar structures, chromonemes, are revealed, the nucleolus size increases, and the cells lengthen (Chaban et al., 2011).
In our study, the cells of antipodal complexes at different stages of differentiation after fertilization were studied and we showed that separate giant polytene chromosomes are detected at the middle and late stages of differentiation in the nuclei of antipodal wheat cells of Triticum aestivum L. (Figs. 2b, 2c, 3a, 3b, 3f). The central regions of polytene chromosomes are condensed. In the telomere region, the chromatids of the chromosomes are not conjugated; they are sepa-rated and are in contact with the nuclear envelope (Figs. 3a, 3b, 3f). In each nucleus, at the stage of differentiation, one or three large nucleoli are detected (Figs. 3b, 3c, 3d). The nuclear envelope with numerous pores forms invaginations (Figs. 5, 10b).
Petrova et al. investigated the structure of antipodal nuclei in the embryo sac of Hordeum vulgare L. and observed similar pictures (Petrova et al., 1985).
Antipodal cell complexes of Triticum aestivum L. with nuclei of different morphologies were described earlier by Lazareva and Chentsov. At the early stage of ontogenesis in the nuclei of the antipodal cells, in addition to large nucleoli, they detected mininucleoli using Ag-Nor staining and immunocytochemical detection of fibrillarin (Lazareva and Chentsov, 2004). Our data confirm the facts they obtained (Fig. 3c).
Bennett et al. measured the amount of DNA in the antipodal nuclei of different embryo sacks of Triticum aestivum L. fixed at certain time intervals after flowe-ring and revealed nuclei with low and high DNA content among the antipodal nuclei of each complex (Bennett et al., 1973). In our work, we first linked the DNA content of the antipodal cells’ nuclei from Triticum aestivum L. to their morphology and topology, showing that they differ in ploidy, nuclear structure, and location in the antipodal complex. In each complex, cells with nuclei containing from 5 to 24 C were located in the basal layer, and they hardly changed the morphology of the nuclei. Antipodal cells with medium (from 30 to 63 C) and high (from 63 to 215 C) DNA content were located in the middle and apical tiers of the complex, and the structure of their nuclei significantly changed during differentiation: cell nuclei increased in size and they had individual polytene chromosomes. The cells of the apical tier of the complex have always been tightly associated with endosperm cells, and their nuclei have undergone the greatest number of genomic endoreduplication rounds (up to 215 С).
We found structures associated with different parts of chromosomes in all the nuclei of antipodal cells at the stage of differentiation (Figs. 6a–6c). Using regressive RNA contrasting, we also detected RNA and RNP on the surface of chromosomes.
Using three-dimensional reconstruction of cell images after immunocytochemical staining, we showed that the localization of the granular reticulum and dictyosomes involved in cells’ secretory activity changes at different stages of differentiation of the antipodal complex. In the early stages, they are evenly distributed throughout the cytoplasm; later, most of the organelles are detected near the plasma membrane and in the nuclear membrane’s invaginations.
A similar ultrastructure of cytoplasmic organelles of antipodal cereal cells was observed in Zea mays L. (Diboll and Larson, 1966; Diboll, 1968), Oryza sativa L. (Maeda and Miyake, 1996, 1997), and Hordeum vulgare L. (Engell, 1994). We found that the dictyosomes of the antipodal cells of Triticum aestivum L. consist of 4–10 cisterns. In barley, rice, and corn, the dictyomes have a similar structure (3–6 cisterns). Granular reticulum in the form of extended cisterns and concentric rings were detected in antipodal cells of Triticumaestivum L. (You and Jensen, 1985), Hordeum vulgare L. (Engell, 1994), Oryza sativa L. (Maeda and Miyake, 1996, 1997), and Zea mays L. (Diboll and Larson, 1966). In the antipodal cells of Hordeum vulgare L., the smooth reticulum had the shape of concentric rings, and the granular reticulum formed extended cisterns (Engell, 1994). We did not find this pattern in antipodal cells of Triticum aestivum L. Granular and smooth reticulum had the shape of both concentric rings and extended cisterns. We have shown that the antipodal cells’ granular reticulum cisterns from Triticum aestivum L. more often have the shape of concentric rings, and that the number of concentric cisterns inserted into each other increases at the later stages of differentiation. Such a structure of the reticulum is observed in the embryo sacs of Triticum aestivum L., in the syncytial endosperm of which a wave of mitosis is detected. This pattern was not found in the antipodal cells’ cytoplasm of other cereals.
In the antipodal cells of wheat, numerous polymorphic plastids similar in morphology to mitochondria were detected. A similar plastid structure was observed in the antipodal cells of Triticum aestivum L. (You and Jensen, 1984) and Hordeum vulgare L. (Engell, 1994). The diversity of mitochondrial structures of antipodal cells was noted in Triticum aestivum L. (You and Jensen, 1985), Hordeum vulgare L. (Engell, 1994), Oryza sativa L. (Maeda and Miyake, 1996, 1997), and Zea mays L. (Diboll and Larson, 1966). In the studied antipodal cells of wheat, polymorphic mitochondria were also found. We found that cupped mitochondria and numerous lipid droplets appear in the antipodal cells’ cytoplasm in the late stages of differentiation of the complex. The presence of lipid droplets has been shown earlier in the cytoplasm of the antipodal cells of Zea mays L. (Diboll and Larson, 1966). Plastids synthesize amino acids, fatty acids, nucleotides, and mitochondria. They are involved in the synthesis of amino acids and nucleotide derivatives. Increased size and changed structure of these organelles may indicate an increase in their functional load in the process of differentiation of the complex.
It should be noted that all cells of antipodal complexes have large vacuoles, often facing the endosperm.
The data obtained suggest that the cells of the basal and apical tiers are not only different in structure but also perform different functions at the stages of diffe-rentiation. At the stage of early differentiation, all antipodal cells perform an osmoregulatory function, as evidenced by the presence of large vacuoles in the cells on both sides of the nucleus. At the middle and late stages of development, isolated giant polytene chromosomes are detected in the cells, which indicates a high metabolic activity of these cells. It is likely that the cells of the middle and apical tiers with the highest degree of polytenisation translate a variety of protein-coding RNAs that ensure the development and protection of the emerging endosperm syncytium.
REFERENCES
An, L.-H. and You, R., Studies on nuclear degeneration during programmed cell death of synergid and antipodal cells in Triticum aestivum, Sex Plant Reprod., 2004, vol. 17, pp. 195–201.
Batygina, T.B., Khlebnoe zerno (The Bread Grain), Leningrad: Nauka, 1987.
Brink, R.A. and Cooper, D.C., The antipodals in relation to abnormal endosperm behaviour in Hordeum jubatum × Secale cereale hybrid, Genetics, 1944, vol. 29, pp. 391–406.
Chaban, I.A., Lazareva, E.M., Kononenko, N.V., and Polyakov, V.Yu., Antipodal complex development in the embryo sac of wheat, Russ. J. Dev. Biol., 2011, vol. 42, no. 2, pp. 79–91.
Diboll, A.G., Fine structural development of the megagametophyte of Zea mays following fertilization, Am. J. Bot., 1968, vol. 55, pp. 797–806.
Diboll, A.G. and Larson, D.A., An electron microscopic study of the mature megagametophyte in Zea mays, Am. J. Bot., 1966, vol. 53, pp. 391–402.
Engell, K., Embryology of barley. IV. ultrastructure of the antipodal cells of Hordeum vulgare L. cv. Bomi before and after fertilization of the egg cell, Sex Plant Reprod., 1994, vol. 7, pp. 333–346.
Jensen, G.H., Studies on the morphology of wheat, Washington 110 Univ. Agric. Exp. Stat. Bull., 1918, vol. 150, pp. 3–30.
Kaltsikes, P.J., Early seed development in hexaploid triticale, Can. J. Bot., 1973, vol. 51, pp. 2291–2300.
Lazareva, E.M. and Chentsov, Yu.S., Localization of fibrillarin, 53 kDa protein and Ag-Nor proteins in the nuclei of giant antipodal cells of the wheat Triticum aestivum, Tsitologiya, 2004, vol. 46, pp. 125–135.
Maeda, E. and Miyake, H., Ultrastructure of antipodal cells of rise (Orysa sativa) after anthesis, as related to nutrient transport in embryo sac, Jap. J. Crop Sci., 1996, vol. 65, pp. 340–351.
Maeda, E. and Miyake, H., Ultrastructure of antipodal cells of rise (Orysa sativa) before anthesis with special reference to concentric configuration of endoplasmic reticula, Jap. J. Crop Sci., 1997, vol. 66, pp. 488–496.
Monneron, A. and Bernhard, W., Fine structural organization of the interphase nucleus in some mammalian cells, J. Ultrastruct. Res., 1969, vol. 27, pp. 266–288.
Morrison, J.W., Fertilization and postfertilization development in wheat, Can. J. Bot., 1954, vol. 33, pp. 168–176.
Petrova, T.F., Solov’yanova, O.B., and Chentsov, Yu.S., The ultrastructure of the giant polytene chromosomes in barley antipodal cells, Tsitologiya, 1985, vol. 17, pp. 499–503.
Poddubnaya-Arnol’di, V.A., Tsitoembriologiya pokrytosemennykh rastenii. Osnovy i perspektivy (Cytoembryology of Angiosperms: Fundamentals and Prospects), Moscow: Nauka, 1976.
Reynolds, E.S., The use of lead citrate at high ph as an electron-opaque stain in electron microscopy, J. Cell Biol., 1963, vol. 17, no. 1, p. 208.
Rigin, B.V. and Orlova, I.N., Pshenichno-rzhanye amfidiploidy (Wheat–Rye Amphidiploids), Leningrad: Kolos, 1974.
Terada, S., Embryological studies of Oryza sativa L., J. College Agricult. Hokkaido, 1928, vol. 9, pp. 245–260.
You, R. and Jensen, W., Ultrastructural observations of the mature megagametophyte and the fertilization in wheat (Triticum aestivum), Can. J. Bot., 1985, vol. 63, pp. 163–178.
Zhang, W.C., Yan, W.M., and Lou, C.H., The structural changes during the degeneration process of antipodal complex and its function to endosperm formation in wheat caryopsis, Acta Biol. Cracov., Ser. Bot., 1988, vol. 30, pp. 457–462.
Zhimulev, I.F., Morphology and structure of polytene chromosomes, Adv. Genet., 1996, vol. 34, pp. 1–359.
Zhimulev, I.F., Khromosomy. Struktura i funktsii (Chromosome: The Structure and Function), Novosibirsk: Sib. Otd. Ross. Akad. Nauk, 2009.
Zybina, E.V., Tsitologiya trofoblasta (Ttrophoblast Cytology), Leningrad: Nauka, 1986.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Shulskaya
Rights and permissions
About this article
Cite this article
Doronina, T.V., Chaban, I.A. & Lazareva, E.M. Structural and Functional Features of the Wheat Embryo Sac’s Antipodal Cells during Differentiation. Russ J Dev Biol 50, 194–208 (2019). https://doi.org/10.1134/S1062360419040039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360419040039