Abstract
Background
Chronic kidney disease (CKD) is a major health problem, and the risk of CKD and hypertension in children born low birth weight (LBW) is under-recognized. We hypothesized that children born with LBW would have a higher prevalence of reduced kidney function and hypertension.
Methods
Using the National Health and Nutrition Examination Survey (NHANES), we conducted a cross-sectional study to evaluate whether LBW (< 2500 g), very low birth weight (VLBW < 1500 g), and large birth weight (BW) (> 4000 g) were associated with kidney disease using 4 different estimating equations. We used the Counahan–Barratt, updated Schwartz, CKiD-U25, and full age spectrum creatinine-based GFR estimating equations to evaluate associations between a history of LBW/VLBW/large BW and reduced kidney function (eGFR < 90 mL/min/1.73 m2) in children. We also assessed blood pressure (BP) using the old and new pediatric hypertension guidelines.
Results
Our analysis included 6336 children (age 12–15 years) in NHANES representing over 13 million US individuals. Using the updated Schwartz, the prevalence of reduced kidney function was 30.1% (25.2–35.6) for children born with LBW compared to 22.4% (20.5–24.3) in children with normal BW. Equations yielded different estimates of prevalence of reduced kidney function in LBW from 21.5% for Counahan–Barratt to 35.4% for CKiD-U25. Compared to those with normal BW, participants with LBW and VLBW had a 7.2 and 10.3% higher prevalence of elevated BP and a 2.4 and 14.6% higher prevalence of hypertension, respectively.
Conclusions
Children born with LBW are at higher risk of reduced kidney function and hypertension than previously described.
Graphical Abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Children born with low birth weight (LBW) have a higher risk of chronic kidney disease (CKD), a life altering health problem. The mortality rate for children with kidney failure receiving dialysis is 30–150 times higher than that for the general pediatric population, and their life expectancy is approximately 50 years less than that of a healthy child [1]. Given the high morbidity and mortality, focus on prevention and early detection is vital to affect the global burden of kidney disease.
A prior study used the National Health and Nutrition Examination Survey (NHANES) data to evaluate prevalence of CKD in adolescents with a history of being born with low birth weight (LBW < 2500 g) or very low birth weight (VLBW < 1500 g)[2]; however the use of the Counahan–Barratt equation to calculate estimated glomerular filtration rate (eGFR) and older 2004 pediatric hypertension guidelines underestimates the risk of CKD and hypertension and is not applicable to clinical practice today [3]. The updated Schwartz equation developed from the National Institutes of Health (NIH)-funded Chronic Kidney Disease in Children (CKiD) study is more sensitive, more widely used, and validated in children with normal kidney function [4,5,6,7]. The updated Schwartz, however, overestimates measured GFR at lower GFR values [7, 8]. Newer developed equations attempt to reduce this bias in estimating GFR. The CKiD under 25 (U25) equation [9] uses an age-and sex-dependent constant (K), and the full age spectrum equation (FAS) adjusts the serum creatinine to the median creatinine for age and sex within a healthy subpopulation [8]. Similarly, the newer 2017 pediatric hypertension guidelines by the American Academy of Pediatrics (AAP) more accurately estimate the prevalence of hypertension in children, since children who were overweight or obese are no longer included in the development of normative data tables [10]. We undertook this study to evaluate associations between a history of LBW and kidney disease as assessed by the newer developed eGFR equations and hypertension using the 2017 pediatric guidelines [2, 11]. To our knowledge, this is the first large study using these tools to identify kidney disease in the general pediatric population.
Methods
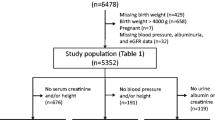
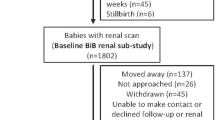
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative, population-based study in the United States conducted in 2-year cycles by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) [12]. NHANES methods for sampling have been well described previously [13]. We combined data from 9 cycles of NHANES and analyzed 6336 children (age 12–15 years) who participated in the NHANES from 1999 to 2016. Birth weight (BW), based on parental recollection, was collected up to age 15, and creatinine was available in those 12 and older. Among 8172 eligible participants, 487 with missing birth weight and 308 with missing height/weight were excluded. Participants were also excluded if they were missing outcome variables or were pregnant (n = 9) at the time of assessment (Fig. 1). This cross-sectional study was approved by the Institutional Review Board of Montefiore/Einstein (#2019–10,964). Demographic, questionnaire, examination, and laboratory data were obtained from the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).
Flowchart of participants. There were 8172 eligible 12- to 15-year-old participants. Those missing critical data or outcome variables were excluded: birth weight: n = 487, height/weight: n = 308, blood pressure: n = 252, and eGFR: n = 780 participants. Participants were also excluded if they were pregnant n = 9 at the time of assessment
Primary exposure
Low birth weight (LBW) was defined as BW < 2500 g, very low birth weight (VLBW) < 1500 g, and large BW > 4000 g according to the World Health Organization definitions [14] reported in the early childhood section of NHANES. These were each compared to children with normal BW of 2500–4000 g [15].
Primary outcome: kidney function
Two CKD outcomes were assessed defined by reduced eGFR: 1) < 90 mL/min per 1.73 m2) [15] as defined by the KDIGO guidelines for stage 2 CKD and 2) < 75 mL/min/1.73 m2 utilized in a prior publication on kidney function in adolescents with LBW [2]. We calculated eGFR using four creatinine-based equations for comparison, the updated Schwartz, Counahan–Barratt equation, CKiD U25, and FAS equations. Serum creatinine was measured using the Jaffe rate method in all participants using different analyzers in different survey years and was re-calibrated as recommended [16].
Secondary outcome: blood pressure
Hypertension was defined as a systolic or diastolic blood pressure (BP) of \(\ge\) 95th percentile for age, sex, and height using the 2017 American Academy of Pediatrics (AAP) “Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents” [10] or the 2004 National High Blood Pressure Education Program (NHBPEP) Working Group on Children and Adolescents’ “Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents” guidelines [17]. Elevated BP \(\ge\) 90th percentile for age, sex, and height using the 2017 American Academy of Pediatrics (AAP) hypertension guidelines was compared to the 2004 pre-hypertension \(\ge\) 90th percentile cut-offs. Blood pressure was taken manually in the right upper extremity for three consecutive measurements after the participant was seated quietly for 5 min. The mean was used for analyses.
Of note, to clinically diagnose CKD and hypertension, eGFR and blood pressure readings over time from separate encounters are required; therefore, this study is a surrogate for disease since there was only one encounter.
Covariates
Demographic information was collected by questionnaire and included self-reported age, sex, and race/ethnicity. The poverty income ratio (PIR) was calculated by dividing the participant’s household income specific to family size over a poverty threshold for that year defined by the US Census. We categorized PIR as below the poverty threshold, at or above poverty level, and > 200% above poverty level [18]. Maternal smoking during pregnancy status was self-reported by the parent/guardian and obtained from the early childhood questionnaire. Body mass index (BMI) (kg/m2) was calculated from participants’ measured weight (kg) and height (cm) and categorized as normal weight, overweight, and obese, defined using the CDC guidelines in percentile cutoffs for age and sex [19]. Albuminuria was obtained from a spot urine collection measuring both albumin and creatinine.
Statistical analysis
The complex NHANES survey design was accounted for by using the appropriate sampling weights and svyset commands in STATAv16 (StataCorp, College Station, TX, USA). This accounts for unequal probabilities, adjustments for non-response, and matched population estimates from the Census Bureau. A p-value \(\le\) 0.05 (2-sided) was considered statistically significant. Continuous variables were described using means and standard errors, and Student’s t-test was used for comparisons of normal birth weight vs. LBW. Categorical variables were described using percentages, and chi-squared test was used for comparisons. Using the different estimating equations and hypertension guidelines, we calculated the prevalence of reduced kidney function and elevated BP/hypertension. We assessed the association of the primary outcome of reduced kidney function with a history of LBW, VLBW and large BW. We also assessed the association of the secondary outcome, hypertension with a history of LBW, VLBW, and large BW. We used weighted multivariable logistic regression models adjusted for age, sex, race/ethnicity, poverty income ratio, BMI category, maternal smoking during pregnancy, survey year, and hypertension or reduced kidney function if assessing primary versus secondary outcome. These were selected given their individual associated risk of kidney disease and potential for confounding. Subgroup (or stratified) analyses were done by sex (i.e., male vs. female) and race/ethnicity (i.e., non-Hispanic White, non-Hispanic Black, and Mexican Americans). Other ethnicities did not have sufficient representation within groups to yield accurate results. Interaction by sex and race/ethnicity were assessed by placing a multiplicative term with LBW/VLBW/large BW and the outcome of interest. A p-value for interaction < 0.1 was considered significant. Sensitivity analysis was conducted using different definitions of reduced kidney function with eGFR < 90 mL/min/1.73 m2, including 1) low eGFR or the presence of albuminuria > 30 mg/g, and 2) the use of the same equations with additional information about cystatin C among birth weight groups. Cystatin C was corrected to IFCC standards for use in the U25 combined and cystatin C-based eGFR equations. Agreements among eGFR equations were assessed using kappa statistics.
Results
Participant characteristics
A total of 6336 children aged 12 to 15 years old representing 13,751,336 US individuals were included in the analysis (Fig. 1). The mean age was 13.5 years; 52.4% were male, 59.3% non-Hispanic White, 13.8% Black, 13.0% Mexican American, 6.9% Hispanic, and 7.0% other race. Characteristics of study participants are shown in Table 1. Of children born with LBW, 25.1% were Black, 27.9% were poor, and 23.5% were exposed to maternal smoking during pregnancy. Of those born with VLBW, 33.0% were Black, 42.1% were poor, and 20% were exposed to maternal smoking. Most children with VLBW were male (61.7%).
Associations between birth weight and reduced kidney function
Using the updated Schwartz equation, the prevalence of reduced kidney function (eGFR < 90 mL/min/1.73 m2) was 30.1% (25.2–35.6) for children born with LBW compared to 22.4% (20.5–24.3) in children with normal birth weight (Table 1). These estimations were lower using the Counahan–Barratt equation, 21.5% (16.7–27.2), and FAS equations, 22.2% (18.1–26.9), and higher using the CKiD-U25 equation, 35.4% (30.3–40.8). Among children with obesity, 30.8% (20.8–43.0) of those born with LBW had reduced kidney function (data not shown). Black children had the highest prevalence, 37.4 (30.7–44.6), of reduced kidney function (Fig. 2). There was no difference in income ratio among children with reduced kidney function (data not shown).
Prevalence of reduced kidney function, elevated BP, and hypertension in subgroups of adolescents born with LBW. Subgroup (or stratified) analyses were done by sex (male vs. female) and race/ethnicity (non-Hispanic White, non-Hispanic Black, and Mexican Americans). Among racial subgroups, Black children had the highest prevalence of reduced kidney function
Compared to children with normal birth weight, the odds ratio for the presence of reduced kidney function (eGFR < 90 mL/min/1.73 m2) using the updated Schwartz was 1.49 (1.11–2.00) for children born with LBW when adjusted for covariates (Table 2). The corresponding adjusted OR was 1.42 (1.00–2.01) using the Counahan–Barratt equation, 1.23 (0.95–1.60) using the CKiD-U25 equation, and 1.50 (1.15–1.96) using the FAS equation. Children with VLBW showed similar results although only the FAS equation remained significant 2.17 (1.02–4.64) (Table 3). Children with large BW showed conflicting results (Table 4).
Subgroup analysis
None of the eGFR equations showed a significant interaction with race/ethnicity or sex using cutoff of p < 0.1. Subgroup analyses are shown in Fig. 2 and Table S1.
Sensitivity analysis
The associations between LBW and reduced kidney function (eGFR < 90 mL/min/1.73 m2) were robust to different definitions of kidney dysfunction, including the use of albuminuria or cystatin C-based equations. Defining the outcome with reduced kidney function or albuminuria, the updated Schwartz showed an odds ratio of 1.46 (1.11–1.93), the Counahan–Barratt 1.40 (1.02–1.91), the CKiD-U25 1.25 (0.98–1.60), and the FAS 1.47 (1.14–1.89) for reduced kidney function in children with LBW. Using combined creatinine and cystatin C equations, estimates for GFR were lower than the creatinine-based equations, and prevalence of reduced eGFR (< 90 mL/min/1.73 m2) was higher (Table S2). The creatinine–cystatin C-based CKiD Eq. (2012) showed an odds ratio of 2.82 (0.92–8.60), the CKiD-U25 combined Eq. 1.87 (0.76–4.58), and the FAS combined Eq. 2.95 (0.98-8.86) for reduced kidney function in children with LBW. Agreement was greater than 80% between creatinine-based equations and between creatinine-based and combined equations except between the updated Schwartz and U25 combined equation at 74.5%. Agreements between creatinine and cystatin C-based equations were less than 80%.
Associations between birth weight and elevated BP/hypertension
The prevalence of abnormal BP above the elevated BP cutoff (> 90th percentile) was 19.6% (14.8–25.5) and 22.7% (9.9–43.9) for children born with LBW and VLBW respectively, compared to 12.4% (11.1–13.8) in children with normal birth weight (Table 1). The prevalence of hypertension (> 95th percentile) was 6.2% (3.7–10.3) and 18.4% (6.7–41.5) for children born with LBW and VLBW, respectively, compared to 3.8% (3.1–4.5) in children with normal birth weight. Using the 2004 NHLBI hypertension guidelines, the prevalence of pre-hypertension and hypertension was lower.
Using the newer 2017 AAP hypertension guidelines, the OR for the presence of elevated BP or hypertension was 1.79 (95% CI 1.00–3.20) for children born with LBW compared to normal birth weight when adjusted for covariates (Table 5). The increased OR of hypertension, 6.24 (1.99–19.57), was significant in children born with VLBW compared to normal birth weight when adjusted for covariates (Table 6). This was not significant in children born with large BW (Table 7).
Subgroup analysis
Elevated blood pressure and hypertension showed no significant interaction with race/ethnicity or sex using cutoff of p < 0.1. Analyses are shown in Fig. 2 and Table S1.
Discussion
In this study of 6336 NHANES participants representing over 13 million children in the United States, we found a higher prevalence of kidney dysfunction in children born with LBW/VLBW compared to those born with normal birth weight using all four GFR estimating formulas. This association was robust with different definitions of kidney dysfunction, including using microalbuminuria in the definition and using cystatin C-based formulas. We also found differences in prevalence of kidney dysfunction among the four formulas, with the newer U25 showing the highest prevalence and the Counahan–Barratt showing the lowest prevalence. Children with large BW had a lower prevalence of reduced kidney function. We also found a statistically significant association of elevated BP/hypertension in children with LBW and of hypertension in children with VLBW. Given the high prevalence of reduced kidney function and the long-term morbidity and mortality of CKD, our findings suggest that children born with LBW or VLBW may benefit from screening for kidney disease and elevated BP.
Children born with LBW have a higher risk of early-onset kidney disease due to low nephron endowment. The completion of nephrogenesis occurs at approximately 36 weeks gestational age; thus, children who are born prior to 36 weeks have a lower nephron number than those born at term [20]. Similarly, in children affected by intrauterine growth restriction or who are small for gestational age, nephron mass is decreased [21]. A study looking at glomerular volume and density on kidney biopsy showed lower glomerular density and glomerular enlargement in children born with LBW compared to age matched controls with normal birth weight [22]. It is predicted that children with LBW have a higher single nephron glomerular filtration leading to podocyte loss and post-adaptive sclerosis [23]. Secondary focal segmental glomerulosclerosis (FSGS) has been reported in children born with VLBW [24]. This process of adaptive sclerosis is significant because kidney biopsy findings of FSGS suggest a poor prognosis and a child has a higher likelihood to progress to kidney failure [25]. LBW has also been associated with worse outcomes in various forms of kidney diseases including nephrotic syndrome, lupus nephritis, and membranous nephropathy [26].
Our results are consistent with the increased odds of CKD seen in prior studies of adults born with LBW and studies of children born with LBW [21, 27]. A prospective Norwegian study of mostly White individuals with equal access to specialist healthcare showed a 1.41 adjusted odds of CKD in children born with LBW [21]. A systematic review of 31 cohort or case–control studies found a 70% higher risk of adulthood CKD for infants with LBW [28]. Other studies also demonstrate a higher prevalence of kidney disease in adults born with LBW [2, 29, 30]. In our study, the high percentage of children born with VLBW with obesity, another cause of secondary FSGS, may play a role in the higher prevalence of reduced kidney function. Maternal factors including smoking during pregnancy also play a significant role in birth outcomes for children [31, 32]. Preeclampsia may also significantly affect birth outcomes [33]. NHANES did not collect information on maternal preeclampsia; however, future studies may consider the use of markers that suggest preeclampsia in mothers and children of preeclamptic mothers.
Black children with LBW or large BW had the highest prevalence of reduced kidney function among subgroups. APOL1 kidney risk variants in individuals of recent African ancestry may play a role in some of the increased prevalence of kidney disease [34]. The development of kidney dysfunction, however, is more complex as social inequities are closely entangled in the health outcomes of Black Americans. In this context, studies that claim that largely genetic differences explain the increased risk of Black patients suffering from chronic disease become difficult to credit [35]. Sex showed less congruent results among equations. This difference currently has unclear credence, although according to Pottel, the FAS equation performs better than the updated Schwartz in female children [8]. The Birth Weight and Nephron Number Working Group has provided recommendations for action in caring for children born with LBW; however, there are no clinical practice guidelines for monitoring kidney function in this cohort [36].
Estimates of GFR varied using the different equations. A measured GFR comparison was not available in this US population to parse out these results. It is important to remember that these equations were developed in different settings, some with mostly European participants and some with US participants, which may explain some of the differences. Most of the equations were developed in children and adolescents with underlying kidney disease; therefore, their use in the general population is being validated. A recent European study with both measured and estimated GFR showed that the U25 equation underestimated GFR significantly compared to measured GFR values primarily when utilizing cystatin C measurements [37]. However, they recommend using the U25 equation if screening for CKD. In our Supplemental Table S2, when using the U25 equation with both cystatin C and creatinine, half of the cohort of adolescents in the general population had an eGFR < 90 ml/min/1.73 m2. The primary objective of our study was to evaluate the prevalence of kidney dysfunction in participants born with low birth weight, a group at high risk for kidney dysfunction; therefore we used the U25 equation. The prevalence of kidney dysfunction estimated by all the equations in the general pediatric population in NHANES requires confirmation with measured GFR studies.
Hypertension is a marker of kidney disease in children. The 2017 pediatrics hypertension guidelines address the need for a pertinent birth history in evaluation of hypertension and for children with VLBW to be included among those screened for hypertension younger than age 3 but should more be done as they approach adolescence [38]. Barker was the first to show association between LBW and hypertension [39]. Similarly, our study showed a significant association of hypertension in children born with VLBW and of elevated BP/hypertension in those born with LBW. Children born with VLBW had a 6.24 adjusted odds ratio, and over 18% had hypertension. Brenner discussed that decreased filtration surface area leads to renal sodium retention and elevated BP, although studies show mixed results regarding this mechanism [40, 41].
Although our study focused on children with LBW, our results also demonstrate large BW as a risk factor for hypertension and kidney dysfunction, although this was not sustained in adjusted models. (Table 1, 4 and 7) Various studies also show this U-shaped relationship with birth weight and hypertension, and even more pronounced with obesity [42,43,44]. The data, however, is conflicting, and there is limited evidence for kidney dysfunction in children born with large BW [45,46,47]. Proposed mechanisms for the development of hypertension in children born with large BW are sustained activation of the sympathetic nervous system and the renin–angiotensin–aldosterone system (RAAS) promoting sodium and fluid retention and increased glomerular capillary pressure [45, 48]. Kidney dysfunction may also be attributed to some of these mechanisms by way of glomerular hyperfiltration. In addition, hyperleptinemia, elevated insulin growth factor-1, and decreased adiponectin levels associated with insulin resistance and visceral obesity have been linked to blood pressure [48,49,50,51,52,53].
Limitations to this study include that birth weight was collected from parental recollection. Gestational age was not available and may have added further support to our findings. Gestational diabetes status of mothers of infants born with large BW was also not available. We utilized BMI in our multivariate model; however, mid-upper arm or waist circumference per height may be better indicator of adiposity in children [53, 54]. Similarly, creatinine is not the best estimate of eGFR in children given that body composition/muscle mass and diet can cause these values to falsely represent kidney function and therefore is not an exact measure such as the research based on iohexol or iothalamate studies. Cystatin C-based GFR equations typically give an estimation of kidney function that is independent of muscle mass and diet [55, 56]. Pediatric eGFR equations also may underestimate GFR since they were derived in children with CKD [56]. Among children with normal birth weight, 22.4% showed decreased eGFR. It is unclear how this translates to disease in adulthood. It is also unclear if longitudinally the variables assessed in this study should be used in children to assess future morbidity. Given that there are no large-scale studies using measured GFR in healthy children and a study of this nature may be difficult to justify, these GFR equations are currently the best estimates we have. In addition, hypertension and kidney disease are defined by longitudinal measurements which we do not have in a cross-sectional study.
This is an observational study, so causality cannot be inferred. Although the study design tries to account for any bias by oversampling special populations, this is not as perfect of a model as having a truly representative population. Lastly, there is a longstanding history of mistrust of the medical community among minority populations, so involvement in research study remains a hurdle.
Our study has several strengths including utilizing a dataset that is a surrogate for the United States population to assess kidney function on a national scale. It utilizes LBW as a marker for abnormal kidney development [57]. It demonstrates the association of low birth weight with higher risk for kidney disease as early as adolescence. Further, by utilizing newer GFR estimating equations, it highlights the under-recognized burden of kidney dysfunction and emphasizes the importance of keeping track of this special group of children. Finally, it again demonstrates racial disparities in adolescent healthcare.
In conclusion, children born with LBW have a significantly higher prevalence of reduced kidney function compared to children with normal birth weight. This is higher than previously reported when using newer estimating equations, at 30% using the updated Schwartz equation, and up to 35% using the U25 equation. Children with VLBW have an even higher risk, suggesting the need for a guided approach in this population. A significant disparity is also seen between Black and White children. Being born with VLBW was associated with a higher risk of hypertension, while being born with LBW was associated with elevated BP. Our study highlights the significant need for more attention to be paid to children born with LBW or VLBW who are at risk of kidney disease and hypertension prior to adulthood, both of which heighten their risk of worse cardiovascular outcomes.
Data Availability
All the NHANES data used in this study is publically available on https://www.cdc.gov/nchs/nhanes/index.htm.
Abbreviations
- CKD:
-
Chronic kidney disease
- eGFR:
-
Estimated glomerular filtration rate
- BP:
-
Blood pressure
- LBW:
-
Low birth weight
References
Becherucci F, Roperto RM, Materassi M, Romagnani P (2016) Chronic kidney disease in children. Clin Kidney J 9:583–591
Khalsa DDK (2016) Prevalence of chronic kidney disease risk factors among low birth weight adolescents. Pediatr Nephrol 31:1509–1516
Bell CS, Samuel JP, Samuels JA (2019) Prevalence of Hypertension in children. Hypertension 73:148–152
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Schwartz GJ, Work DF (2009) Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4:1832–1843
Lewis TV, Harrison DL, Gildon BL, Carter SM, Turman MA (2016) Applicability of the Schwartz equation and the chronic kidney disease in children bedside equation for estimating glomerular filtration rate in overweight children. Pharmacotherapy 36:598–606
Staples A, LeBlond R, Watkins S, Wong C, Brandt J (2010) Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol 25:2321–2326
Pottel H, Hoste L, Dubourg L, Ebert N, Schaeffner E, Eriksen BO, Melsom T, Lamb EJ, Rule AD, Turner ST, Glassock RJ, De Souza V, Selistre L, Mariat C, Martens F, Delanaye P (2016) An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 31:798–806
Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ (2020) Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int 99:948–956
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM, SUBCOMMITTEE ON SCREENING AND MANAGEMENT OF HIGH BLOOD PRESSURE IN CHILDREN (2017) Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140:e20171904
Judd E, Calhoun DA (2015) Management of hypertension in CKD: beyond the guidelines. Adv Chronic Kidney Dis 22:116–122
Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. Hyattsville, MD, U.S. Department of Health and Human Services, Ctr Dis Control Prev 1999–2016 https://wwwn.cdc.gov/nchs/nhanes/Default.aspx
Levy RV, Brathwaite KE, Sarathy H, Reidy K, Kaskel FJ, Melamed ML (2021) Analysis of active and passive tobacco exposures and blood pressure in US children and adolescents. JAMA Netw Open 4:e2037936
United Nations Children’s Fund (UNICEF), World Health Organization (WHO). UNICEF-WHO Low birthweight estimates: Levels and trends 2000–2015. Geneva: World Health Organ; May 2019 Licence: CC BY-NC-SA 3.0 IGO https://www.unicef.org/reports/UNICEF-WHO-low-birthweight-estimates-2019
KDIGO (2012) Clinical practice guideline for the evaluation and management of chronic kidney disease, kidney disease improving global outcomes. Kidney Int Suppl 3:1–150
Selvin JME, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J (2007) Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis 50:918–926
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Sarathy H, Henriquez G, Abramowitz MK, Kramer H, Rosas SE, Johns T, Kumar J, Skversky A, Kaskel F, Melamed ML (2016) Abdominal obesity, race and chronic kidney disease in young adults: results from NHANES 1999–2010. PLoS One 11:e0153588
Centers for Disease Control and Prevention, National Center for Health Statistics. June 16, 2017 Clinical Growth Charts, Children 2 to 20 years, Data Table of BMI-for-age Charts. https://www.cdc.gov/growthcharts/clinical_charts.htm
Charlton JR, Springsteen CH, Carmody JB (2014) Nephron number and its determinants in early life: a primer. Pediatr Nephrol 29:2299–2308
Gjerde A, Reisæter AV, Skrunes R, Marti HP, Vikse BE (2020) Intrauterine growth restriction and risk of diverse forms of kidney disease during the first 50 years of life. Clin J Am Soc Nephrol 15:1413–1423
Koike K, Ikezumi Y, Tsuboi N, Kanzaki G, Haruhara K, Okabayashi Y, Sasaki T, Ogura M, Saitoh A, Yokoo T (2017) Glomerular density and volume in renal biopsy specimens of children with proteinuria relative to preterm birth and gestational age. Clin J Am Soc Nephrol 12:585–590
Luyckx VA, Brenner BM (2005) Low birth weight, nephron number, and kidney disease. Kidney Int Suppl 97:S68-77
Hodgin JB, Rasoulpour M, Markowitz GS, D’Agati VD (2009) Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 4:71–76
D’Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA, Cohen AH, Gipson DS, Gassman JJ, Radeva MK, Moxey-Mims MM, Friedman AL, Kaskel FJ, Trachtman H, Alpers CE, Fogo AB, Greene TH, Nast CC (2013) Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol 8:399–406
Konstantelos N, Banh T, Patel V, Vasilevska-Ristovska J, Borges K, Hussain-Shamsy N, Noone D, Hebert D, Radhakrishnan S, Licht CPB, Langlois V, Pearl RJ, Parekh RS (2019) Association of low birth weight and prematurity with clinical outcomes of childhood nephrotic syndrome: a prospective cohort study. Pediatr Nephrol 34:1599–1605
Sanderson KR, Chang E, Bjornstad E, Hogan SL, Hu Y, Askenazi D, Fry RC, O’Shea TM (2020) Albuminuria, Hypertension, and Reduced Kidney Volumes in Adolescents Born Extremely Premature. Front Pediatr 8:230
White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, Haysom L, Craig JC, Salmi IA, Chadban SJ, Huxley RR (2009) Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis 54:248–261
Abitbol CL, Moxey-Mims M (2016) Chronic kidney disease: kow birth weight and the global burden of kidney disease. Nat Rev Nephrol 12:199–200
Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ (2000) Low birth weights contribute to high rates of early-onset chronic renal failure in the southeastern United States. Arch Intern Med 160:1472–1476
Ratnasiri AWG, Parry SS, Arief VN, DeLacy IH, Halliday LA, DiLibero RJ, Basford KE (2018) Recent trends, risk factors, and disparities in low birth weight in California, 2005–2014: a retrospective study. Matern Health Neonatol Perinatol 4:15
Ratnasiri AWG, Lakshminrusimha S, Dieckmann RA, Lee HC, Gould JB, Parry SS, Arief VN, DeLacy IH, DiLibero RJ, Basford KE (2020) Maternal and infant predictors of infant mortality in California, 2007–2015. PLoS One 15:e0236877
Johnson JD, Louis JM (2020) Does race or ethnicity play a role in the origin, pathophysiology, and outcomes of preeclampsia? An expert review of the literature. Am J Obstet Gynecol 226:S876–S885
Friedman DJ, Pollak MR (2021) APOL1 Nephropathy: from genetics to clinical applications. Clin J Am Soc Nephrol 16:294–303
Hoffman KM, Trawalter S, Axt JR, Oliver MN (2016) Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A 113:4296–4301
Luyckx VA, Perico N, Somaschini M, Manfellotto D, Valensise H, Cetin I, Simeoni U, Allegaert K, Vikse BE, Steegers EA, Adu D, Montini G, Remuzzi G, Brenner BM, writing group of the Low Birth Weight and Nephron Number Working Group (2017) A developmental approach to the prevention of hypertension and kidney disease: a report from the Low Birth Weight and Nephron Number Working Group. Lancet 390:424–428
Nyman U, Björk J, Berg U, Bökenkamp A, Dubourg L, Goffin K, Grubb A, Hansson M, Larsson A, Littmann K, Åsling-Monemi K, Pottel H, Delanaye P (2022) The modified CKiD study estimated gfr equations for children and young adults under 25 years of age: performance in a European multicenter cohort. Am J Kidney Dis 80:807–810
Flynn JT (2017) Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics (Evanston) 140:e20171904
Barker DJ, Osmond C (1988) Low birth weight and hypertension. BMJ 297:134–135
Brenner BM, Garcia DL, Anderson S (1988) Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1:335–347
Benz K, Schlote J, Daniel C, Kopp C, Dahlmann A, Schröder A, Cordasic N, Klanke B, Hilgers K, Titze J, Amann K (2018) Mild salt-sensitive hypertension in genetically determined low nephron number is associated with chloride but not sodium retention. Kidney Blood Press Res 43:1–11
Pocobelli G, Dublin S, Enquobahrie DA, Mueller BA (2016) Birth weight and birth weight for gestational age in relation to risk of hospitalization with primary hypertension in children and young adults. Matern Child Health J 20:1415–1423
Leong NM, Mignone LI, Newcomb PA, Titus-Ernstoff L, Baron JA, Trentham-Dietz A, Stampfer MJ, Willett WC, Egan KM (2003) Early life risk factors in cancer: the relation of birth weight to adult obesity. Int J Cancer 103:789–791
Kuciene R, Dulskiene V, Medzioniene J (2018) Associations between high birth weight, being large for gestational age, and high blood pressure among adolescents: a cross-sectional study. Eur J Nutr 57:373–381
El-Atat FA, Stas SN, McFarlane SI, Sowers JR (2004) The relationship between hyperinsulinemia, hypertension and progressive renal disease. J Am Soc Nephrol 15:2816–2827
MagnussonÅ LH, Loft A, Oldereid NB, Pinborg A, Petzold M, Romundstad LB, Söderström-Anttila V, Bergh C (2021) The association between high birth weight and long-term outcomes-implications for assisted reproductive technologies: a systematic review and meta-analysis. Front Pediatr 9:675775
Ferrannini E, Natali A, Capaldo B, Lehtovirta M, Jacob S, Yki-Järvinen H (1997) Insulin resistance, hyperinsulinemia, and blood pressure: role of age and obesity. European Group for the Study of Insulin Resistance (EGIR). Hypertension 30:1144–1149
de Silva AA, de Carmo JM, Li X, Wang Z, Mouton AJ, Hall JE (2020) Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can J Cardiol 36:671–682
Giapros V, Evagelidou E, Challa A, Kiortsis D, Drougia A, Andronikou S (2007) Serum adiponectin and leptin levels and insulin resistance in children born large for gestational age are affected by the degree of overweight. Clin Endocrinol (Oxf) 66:353–359
Luo ZC, Nuyt AM, Delvin E, Audibert F, Girard I, Shatenstein B, Cloutier A, Cousineau J, Djemli A, Deal C, Levy E, Wu Y, Julien P, Fraser WD (2012) Maternal and fetal IGF-I and IGF-II levels, fetal growth, and gestational diabetes. J Clin Endocrinol Metab 97:1720–1728
Yan-Jun L, Tsushima T, Minei S, Sanaka M, Nagashima T, Yanagisawa K, Omori Y (1996) Insulin-like growth factors (IGFs) and IGF-binding proteins (IGFBP-1, -2 and -3) in diabetic pregnancy: relationship to macrosomia. Endocr J 43:221–231
Snitker S, Pratley RE, Nicolson M, Tataranni PA, Ravussin E (1997) Relationship between muscle sympathetic nerve activity and plasma leptin concentration. Obes Res 5:338–340
Darendeliler F, Poyrazoglu S, Sancakli O, Bas F, Gokcay G, Aki S, Eskiyurt N (2009) Adiponectin is an indicator of insulin resistance in non-obese prepubertal children born large for gestational age (LGA) and is affected by birth weight. Clin Endocrinol (Oxf) 70:710–716
Bassali R, Waller JL, Gower B, Allison J, Davis CL (2010) Utility of waist circumference percentile for risk evaluation in obese children. Int J Pediatr Obes 5:97–101
Mian AN, Schwartz GJ (2017) Measurement and estimation of glomerular filtration rate in children. Adv Chronic Kidney Dis 24:348–356
Fadrowski JJ, Neu AM, Schwartz GJ, Furth SL (2011) Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol 6:1427–1435
Mañalich R, Reyes L, Herrera M, Melendi C, Fundora I (2000) Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int 58:770–773
Acknowledgements
We thank the NHANES participants and the staff for their contributions to this study.
Funding
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases (T32DK007110-47S1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brathwaite, K.E., Levy, R.V., Sarathy, H. et al. Reduced kidney function and hypertension in adolescents with low birth weight, NHANES 1999–2016. Pediatr Nephrol 38, 3071–3082 (2023). https://doi.org/10.1007/s00467-023-05958-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-05958-2