Abstract
Background
Low birth weight (LBW) is a risk factor for chronic kidney disease (CKD) in later life and is becoming increasingly common in developed countries, including Japan. Furthermore, a serial decrease in birth weight has been associated with an increasing prevalence of CKD stage 2 in male Japanese adolescents. Sex-specific differences affect CKD susceptibility, and the association between birth weight and CKD in women, has not been elucidated. In this study, we investigated the sex-specific effect of LBW on renal function.
Methods
Annual cross-sectional data of 2417 Japanese adolescents (males 1736; females 681), aged 15–16 years, were evaluated over 8 years (2007–2014).
Results
Over the study period, mean birth weights decreased significantly in males (p < 0.01) and females (p < 0.05). Furthermore, both sexes showed significant decrease in estimated glomerular filtration rates corresponding to the birth weight reduction. The prevalence of CKD stage 2 also increased in males (from 26.0 to 32.4%, p < 0.01) and females (from 6.3 to 18.5%, p < 0.05). The incidence of CKD stage 2 was significantly related to history of LBW (males: odds ratio 1.73; 95% confidence interval 1.06–2.80; p < 0.05; females: odds ratio 3.29; 95% confidence interval 1.25–8.02; p < 0.05).
Conclusions
Our data revealed that renal function and birth weight have decreased over time, in healthy Japanese adolescents. In view of the recent declining trend demonstrated by birth weight in Japan, we speculate that the prevalence of CKD might increase in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) contributes to the global disease burden because it is an independent risk factor for cardiovascular diseases and end-stage renal disease (ESRD) [1]. There has been a substantial increase in the incidence of CKD in Japan and other developed countries [2, 3]. Causes of CKD are multifactorial, and the disorder is usually associated with non-communicable diseases such as obesity, diabetes, and hypertension. In addition to these disorders, intrauterine growth retardation (IUGR) is also emerging as a contributor to CKD [4], with low birth weight (LBW) (< 2500 g) acting as a surrogate marker of IUGR. Birth weight shows a linear correlation with the total number of functional nephrons, and LBW infants have to contend with reduced numbers of compensatory hypertrophied glomeruli [5, 6]. This can, in turn, predispose an individual to develop CKD during adulthood, according to the hyperfiltration theory [7]. Animal experiments have demonstrated a direct correlation between LBW on one hand and reduced nephron numbers and impaired renal function on the other [8].
LBW increases the risk of infant mortality and is linked to higher risk of development of non-communicable diseases such as hypertension, diabetes, and CKD, according to the developmental origins of health and disease theory [9,10,11]. LBW has a reported global incidence of 15–20%, and affects infants in both developing and developed countries [4, 12]. From the mid- to late-twentieth century, the mean birth weight had increased globally and has since, shown a declining trend, [13] with an increase in prevalence of LBW noted in most developed countries [12]. Recently, the prevalence of LBW in Japan, has been estimated to be ~ 10%, which is the highest among that in developed countries. Therefore, there is a concern that this increased prevalence will affect the rates of LBW-related diseases in Japanese patients [14, 15]. Considering this, we have previously reported that the increasing LBW prevalence in Japan is associated with an increasing prevalence of renal dysfunction in male adolescents [16].
The degree of susceptibility to development of CKD is known to be sex-specific [17, 18], and meta-analyses have shown that renal function declines more slowly in women than in men, when adjusted for risk factors such as blood pressure or serum lipid levels [19]. Some studies have shown that the effect of LBW on renal outcomes is weaker in women [20, 21], though there are conflicting observations regarding the association of LBW with the risk of CKD development in this population [22].
In this study, we investigated changes in estimated glomerular filtration rates (eGFRs) and birth weights in healthy, Japanese male and female adolescents (15–16 years of age), before occurrence of overt manifestations of metabolic disorders and/or hypertension. We also examined sex-specific differences while determining the effect of LBW on renal function in these adolescents.
Materials and methods
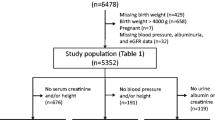
The study participants included 2417 Japanese high school students (1736 males, 681 females), from four private high schools in Tokyo, Saitama and Kanagawa, who attended annual school medical checkups conducted by the Health Center of Keio University, between 2007 and 2014. We excluded individuals with missing birth weight data from the study. The study protocol was approved by the review committee of Keio University (approval number 18-001) and was conducted in accordance with the Declaration of Helsinki.
The annual medical checkup of each participant was conducted, as previously reported [16]. Each participant’s height was measured, to the nearest centimeter, while standing without shoes. Body weights were determined to the nearest 100 g using an automatic device, while each participant was wearing light clothing. Body mass indices (BMIs) were calculated by dividing the participant’s weight (kg) by the square of his/her height (m). Blood pressure (BP) was measured, after the participant had remained seated for at least 3 min, using an automatic electronic sphygmomanometer. If a student’s blood pressure was > 140/90 mm Hg (until 2010) or > 140/85 mm Hg (2011–2014), the BP was measured again. These cutoff values were based on the Japanese Society of Hypertension Guidelines for the Management of Hypertension [23]. If > 1 measurement was taken, the average BP was used to denote the systolic and diastolic BPs. Urine samples were examined using dipstick tests. Blood samples were collected, after overnight fasting, to determine serum levels of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), glucose, uric acid (UA), and serum creatinine (Cr), using standard methods. The eGFRs were calculated using the following Cr-based equation, adjusted for Japanese children and adolescents [24]: eGFR = 110.2 × (reference serum Cr/patient serum Cr) + 2.93. While reference serum Cr level for males was calculated using the formula: − 1.259 × (height [m])5 + 7.815 × (height [m])4 − 18.57 × (height [m])3 + 21.39 × (height [m])2 − 11.71 × height (m) + 2.628, that for females was determined using: − 4.536 × (height [m])5 + 27.16 × (height [m])4 − 63.47 × (height [m])3 + 72.43 × (height [m])2 − 40.06 × height (m) + 8.778.
Based on derived eGFR values, each participant was classified into one of five stages, as defined by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines [25]. The birth weight, height, and gestational age, recorded at the time of each participant’s delivery, were obtained from routine obstetric records. The students were further divided into three groups, based on their birth weights. Only four female students demonstrated high birth weights and were included in the normal birth weight group.
Statistical analyses
The anthropometric and laboratory characteristics were summarized and analyzed annually. The data are expressed as means ± standard deviations (SDs) or as medians and interquartile ranges (IQRs) for TG levels. The prevalences of LBW, pre-term birth (< 37 weeks), and CKD stage 2 (60 mL/min/1.73 m2 ≤ eGFR < 90 mL/min/1.73 m2) were also evaluated annually. The annual trends of continuous variables were examined yearly, using trend-tests in linear regression models. Linear trends for proportions of students with LBW, pre-term birth, and mild renal dysfunction were estimated using the Cochran–Armitage test [26]. The odds ratio (OR) for CKD stage 2 was determined using multivariate logistic regression analyses. BP, TC, TG, glucose, and UA levels were expressed as values per unit SD, and being overweight was defined as current BMI at or above the 85th percentile of the recommended cutoff (sex-specific) BMI values for Japanese adolescents [27]. We examined the associations between eGFR and birth weight using simple linear regression analysis or multiple linear regression analysis as follows: Model 1; unadjusted; Model 2: adjusted for current BW/SD, mean BP/SD; Model 3: Model 2 + TC/SD, TG/SD; Model 4: Model 3 + UA/SD, Glu/SD. TG levels were log10-converted prior to inclusion in calculations. All analyses were performed using JMP 12 (SAS Institute, Cary, NC, USA) software, and each test was two-sided with a significance level of 5%.
Results
Characteristics
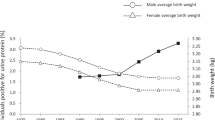
The trends for current body weight, birth weight, BP, and serum metabolic parameters are summarized in Table 1. Gestational ages of 2242 study participants were available. While current body-weight trends were similar in both male and female adolescents across the 8-year study period, the birth-weight trends showed a significant decline (males: 3159 ± 360 g in 2007 to 3088 ± 342 g in 2014, p < 0.05; females: 3114 ± 307 g in 2007 to 2988 ± 384 g in 2014, p < 0.01). Previously, we reported that the prevalence of LBW in males had increased significantly over 18 years [16]. However, in this study, the prevalence of LBW was similar across both sexes, over the 8-year study period. This occurred probably due to the shorter observational period (Table 2). None of the individual students had an eGFR < 60 mL/min/1.73 m2, reflecting a healthy study population. The mean eGFR of females was higher than that of males, every year. However, both sexes displayed a significant decrease in eGFR over time [mean eGFR for males was 99 ± 14 mL/min/1.73 m2 in 2007 and 96 ± 13 mL/min/1.73 m2 in 2014 (p < 0.01) while for females it was 107 ± 15 mL/min/1.73 m2 in 2007 and 100 ± 11 mL/min/1.73 m2 in 2014 (p < 0.01)] (Table 1). Accordingly, the prevalence of CKD stage 2 increased in both sexes between 2007 and 2014 (males: from 26.1 to 32.2% (p < 0.01); females: from 6.3 to 18.5% (p < 0.05) (Table 2). While the rate of urinary protein positivity reduced significantly in males, this decrease was not observed in the female study population (Table 2). Serum TG levels increased significantly from 2007 to 2014 (Table 1). Although, the mean BP of male participants was found to decrease over the study period, an opposite trend was observed in females.
Table 3 shows the trends for various parameters, according to birth weight categories. We found that in both male and female adolescents, birth weight was significantly associated with eGFR as well as with current height and body weight. We examined the prevalence of CKD stage 2 in relation to birth weight (Fig. 1) and observed that it was commoner in the lower than in the normal birth-weight group, for both sexes.
CKD stage 2 risk
The interactions between CKD stage 2 and various risk factors [10] were investigated using multiple logistic regression analyses. Being overweight, having a low birth weight, and levels of TC and UA were found to be risk factors for CKD stage 2 in males (Table 4, left panel). On the other hand, mean blood pressure, birth weight, and levels of TG, TC, and UA were the most predictive risk factors in females (Table 4, right panel). The ORs for CKD stage 2 were higher in the LBW groups (males: OR 1.73; 95% CI 1.06–2.80; p < 0.05; females: OR 3.29; 95% CI 1.25–8.02; p < 0.05).
Relationships between renal function and birth weight, BP, and metabolic parameters
We demonstrated the associations between eGFR levels and birth weight, current body weight, mean BP, and metabolic parameters in Table 5. In male and female adolescents, the unadjusted analysis revealed that birth weight was positively associated with eGFR. Specifically, each 1-kg decrease in birth weight was associated with a decrease in eGFR [coefficient 5.17 mL/min/1.73 m2 in males (p < 0.01) and 3.67 mL/min/1.73 m2 in females (p < 0.05)] (Table 5). After adjusting for the current body weight, BP, and metabolic parameters, a significant correlation was found to persist between birth weight and eGFR (males: coefficient 4.44; 95% CI 2.70–6.19; p < 0.01; females: coefficient, 2.97; 95% CI 0.01–5.93; p < 0.05). Conversely, eGFR levels were found to correlate inversely with metabolic parameters including TC and UA values.
Discussion
LBW is associated with the risk of development of non-communicable diseases, according to the developmental origins of health and disease hypothesis, and there has been concern that the serial increase in prevalence of LBW in Japan will affect the future incidence of CKD [4, 28]. We had previously published results of an 18-year study on the relationship between birth weight and renal function in male adolescents [16]. Since sex-specific differences may affect the influence of LBW on renal disease [21], we included both male and female adolescents in this study. In the present study, we focused on these differences and clarified the adverse effects of serial decrease in birth weight on renal function in both healthy male and female adolescents. We found that the mean birth weights of healthy Japanese adolescents recruited into the study between 2007 and 2014, showed a significant decreasing trend. Furthermore, this decrease in birth weight was associated with a similarly reducing trend with respect to eGFR values. Thus, we found LBW to be an independent risk factor for mild reduction in renal function, regardless of current body weight, blood pressure, and laboratory variables. This indicates that both male and female adolescents are vulnerable to the influence of LBW on long-term renal function.
The prevalence of LBW in Japan has been increasing during the last 3 decades and is among the top 10% among developed countries [15, 29]. In addition to smoking, older age at the time of conception, and multiple fertilization procedures, an increasing prevalence of underweight women of reproductive age (due to dieting and inadequate weight gain during pregnancy) may also contribute to this trend [15, 30]. In this study, mean birth weight declined significantly in both male and female adolescents between 2007 and 2014 (Table 1). However, while the LBW (i.e., birth weight < 2500 g) prevalence in males was not observed to change during this study period (Table 3), the prevalence of males who weighed < 3000 g at birth increased significantly in these 8 years (from 28.3% in 2007 to 38.9% in 2014, p < 0.05) and that of females showed an upward trend (from 35.0% in 2007 to 45.7% in 2014, p = 0.07). To elucidate the influence of this birth weight reduction on renal outcomes, we analyzed the annual checkup data of all adolescents, who were free of any overt manifestations of metabolic disorders and/or hypertension at this early age. We found that LBW was an independent risk factor for CKD stage 2 in both male and female adolescents and that birth weight < 3000 g was an independent risk factor in males (Table 4). Moreover, we derived a positive linear association between birth weight and eGFR values (Table 5). These data suggest that birth weight is a major determinant of renal function in later life for both sexes. The Japanese national survey showed an increase in the prevalence of proteinuria in high school students since 2000s and similar results were obtained in our previous study, using dipstick tests for urinalysis [16]. However, in this study, we did not observe a similar trend. It might be due to the comparatively short observation period or because of the small number of subjects included in this study. Furthermore, it has been reported that IUGR and LBW interfere with renal development and lead to a reduction in nephron numbers. This decrease results in development of glomerular hypertension and hyperfiltration, which might cause glomerular sclerosis. The sclerosis further reduces filtration area and worsens extent of hyperfiltration [5,6,7]. Although, we could not conduct confirmatory renal pathological examination in this study population of healthy adolescents, based on our findings, we believe that nephron numbers were reduced in those with history of LBW.
Sex-specific differences also affect susceptibility to CKD [17, 18], and the cumulative incidence of ESRD in Japan was shown to be higher in men than in women [17]. The rate of ESRD in women was also shown to be lower during their reproductive years, and rising thereafter [17]. Therefore, a woman’s postmenopausal status is likely to accelerate progression of CKD. In addition, eGFR values are higher in females than in males at younger ages [24, 31]. In the present study, eGFR values and incidences of CKD stage 2 were significantly lower in female than in male adolescents, which corroborated with findings of previous studies [31, 32]. The association between LBW and renal outcomes in females is reportedly insignificant in later life [20, 21]. Our study demonstrated that LBW is significantly associated with mild reduction in renal function in both female and male adolescents, but the impact of birth weight on eGFR values of females was weaker (coefficient 2.97; 95% CI 0.01–5.93; p < 0.05) than that of males (coefficient 4.44; 95% CI 2.70–6.19; p < 0.01) (Table 5). One possible explanation for weaker association between LBW and eGFR in female adolescents may be due to a protective effect of estrogen against renal injury [33, 34]. Some researchers demonstrated that estrogen prevented renal sclerosis and proliferation in mice and postulated that the hormone might have a role in delaying progression of CKD in humans [35, 36]. However, the influence of LBW may become more apparent with increasing age [37]. As an example, obesity in those with a history of LBW, also increases the risk of development of CKD [4, 21], and while the prevalence of obesity among Japanese men has increased, it has remained stable among Japanese women [38], making the former population more susceptible to renal dysfunction. Therefore, further longitudinal studies are needed to clarify the sex-specific differences in LBW-associated renal effects occurring in later life.
Serum TC and UA levels were also found to be associated with decreased eGFR values in this study. LBW is reportedly associated with high serum TC [39] and UA [40] levels, and the latter can lead to reduced nephron numbers and development of hypertension [41]. In our study, while serum TC and UA levels were found to correlate significantly with CKD stage 2 (Table 4), they did not demonstrate a parallel association with birth weight, over time. Therefore, the role of serum TC and UA in regulating renal function requires further investigation.
There are several limitations of this study. First, this study population was small and was collated from only four private high schools, which might limit generalization of results. Second, birth weight was found to be significantly associated with current body height (Table 3). The current height, which was also possibly influenced by the birth weight, was used for the calculation of eGFR (along with Cr level) of each participant. Therefore, it would have been more accurate to directly evaluate kidney function using an exogenous substance such as inulin, which would have been independent of the effects of a person’s physique. Finally, the cross-sectional nature of the study disallows determination of the cause of impaired renal function.
In conclusion, we found that the mean annual eGFRs in both male and female adolescents decreased over the period of the study and were associated with an observed, associated decrease in the birth weights of the study participants. These findings suggest that adolescents with history of LBW may be particularly vulnerable to CKD during their adult years. In Japan, the prevalence of obesity is increasing among men, but not among women [38]. Considering the recently observed trend of decreasing birth weight, and accounting for the weight changes that occur through later life, further study is needed to investigate the sex-specific differences in the prevalence of CKD.
References
Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major non-communicable diseases. Kidney Int. 2011;80(12):1258–70. https://doi.org/10.1038/ki.2011.368.
Nagata M, Ninomiya T, Doi Y, Yonemoto K, Kubo M, Hata J, et al. Trends in the prevalence of chronic kidney disease and its risk factors in a general Japanese population: the Hisayama Study. Nephrol Dial Transpl. 2010;25(8):2557–644. https://doi.org/10.1093/ndt/gfq062.
Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–47. https://doi.org/10.1001/jama.298.17.2038.
Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes—a global concern. Nat Rev Nephrol. 2015;11(3):135–49. https://doi.org/10.1038/nrneph.2014.251.
Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63(6):2113–222. https://doi.org/10.1046/j.1523-1755.2003.00018.x.
Manalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58(2):770–3. https://doi.org/10.1046/j.1523-1755.2000.00225.x.
Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49(6):1774–7. https://doi.org/10.1038/ki.1996.265.
Schreuder MF, Nyengaard JR, Fodor M, van Wijk A, de Delemarre-van Waal HA. Glomerular number and function are influenced by spontaneous and induced low birth weight in rats. J Am Soc Nephrol. 2005;16(10):2913–9. https://doi.org/10.1681/asn.2004100875.
Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300(24):2886–977. https://doi.org/10.1001/jama.2008.886.
White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54(2):248–61. https://doi.org/10.1053/j.ajkd.2008.12.042.
Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? Lancet. 2002;360(9334):659–65. https://doi.org/10.1016/s0140-6736(02)09834-3.
Organization for Economic Coperation and Development. 2019. OECD Family Database [database on the Internet]. https://www.oecd.org/els/family/CO_1_3_Low_birth_weight.pdf. Accessed 1 Feb 2019
Oken E. Secular trends in birthweight. Nestle Nutr Inst Workshop Ser. 2013;71:103–14. https://doi.org/10.1159/000342576.
Gluckman PD, Seng CY, Fukuoka H, Beedle AS, Hanson MA. Low birthweight and subsequent obesity in Japan. Lancet. 2007;369(9567):1081–2. https://doi.org/10.1016/s0140-6736(07)60524-8.
Takemoto Y, Ota E, Yoneoka D, Mori R, Takeda S. Japanese secular trends in birthweight and the prevalence of low birthweight infants during the last three decades: a population-based study. Sci Rep. 2016;6:31396. https://doi.org/10.1038/srep31396.
Kanda T, Takeda A, Hirose H, Abe T, Urai H, Inokuchi M, et al. Temporal trends in renal function and birthweight in Japanese adolescent males (1998–2015). Nephrol Dial Transpl. 2018;33(2):304–10. https://doi.org/10.1093/ndt/gfw428.
Iseki K. Gender differences in chronic kidney disease. Kidney Int. 2008;74(4):415–7. https://doi.org/10.1038/ki.2008.261.
Carrero JJ. Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res. 2010;33(5):383–92. https://doi.org/10.1159/000320389.
Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of non-diabetic renal disease a meta-analysis. J Am Soc Nephrol. 2000;11(2):319–29
Eriksson JG, Salonen MK, Kajantie E, Osmond C. Prenatal growth and CKD in older adults: longitudinal findings from the Helsinki birth cohort study, 1924–1944. Am J Kidney Dis. 2018;71(1):20–6. https://doi.org/10.1053/j.ajkd.2017.06.030.
Li S, Chen SC, Shlipak M, Bakris G, McCullough PA, Sowers J, et al. Low birth weight is associated with chronic kidney disease only in men. Kidney Int. 2008;73(5):637–42. https://doi.org/10.1038/sj.ki.5002747.
Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low birth weights contribute to the high rates of early-onset chronic renal failure in the southeastern United States. Arch Intern Med. 2000;160(10):1472–6. https://doi.org/10.1001/archinte.160.10.1472.
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37(4):253–390. https://doi.org/10.1038/hr.2014.20.
Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y, et al. Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol. 2014;18(4):626–33. https://doi.org/10.1007/s10157-013-0856-y.
Levey AS, Coresh J, Bolton K, Culleton B, Harvey KS, Ikizler TA, Johnson CA, Kausz A, Kimmel PL, Kusek J, Levin A. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. https://doi.org/10.1053/ajkd.2002.30943.
Asamoah-Odei E, Garcia Calleja JM, Boerma JT. HIV prevalence and trends in sub-Saharan Africa: no decline and large subregional differences. Lancet. 2004;364(9428):35–40. https://doi.org/10.1016/s0140-6736(04)16587-2.
Inokuchi M, Hasegawa T, Anzo M, Matsuo N. Standardized centile curves of body mass index for Japanese children and adolescents based on the 1978–1981 National Survey Data. Ann Hum Biol. 2006;33(4):444–53. https://doi.org/10.1080/03014460600802353.
Abitbol CL, Moxey-Mims M. Chronic kidney disease: low birth weight and the global burden of kidney disease. Nat Rev Nephrol. 2016. https://doi.org/10.1038/nrneph.2016.19.
Ministry of Health, Labour and Welfare. 2019. Vital statistics in Japan [database on the Internet]. https://www.mhlw.go.jp/english/database/db-hw/vs01.html. Accessed 1 Mar 2019
Tsukamoto H, Fukuoka H, Koyasu M, Nagai Y, Takimoto H. Risk factors for small for gestational age. Pediatr Int. 2007;49(6):985–90. https://doi.org/10.1111/j.1442-200X.2007.02494.x.
Xu R, Zhang L-X, Zhang P-H, Wang F, Zuo L, Wang H-Y. Gender differences in age-related decline in glomerular filtration rates in healthy people and chronic kidney disease patients. BMC Nephrol. 2010;11(1):20. https://doi.org/10.1186/1471-2369-11-20.
Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y, et al. Reference glomerular filtration rate levels in Japanese children: using the creatinine and cystatin C based estimated glomerular filtration rate. Clin Exp Nephrol. 2015;19(4):683–7. https://doi.org/10.1007/s10157-014-1042-6.
Blush J, Lei J, Ju W, Silbiger S, Pullman J, Neugarten J. Estradiol reverses renal injury in Alb/TGF-beta1 transgenic mice. Kidney Int. 2004;66(6):2148–54. https://doi.org/10.1111/j.1523-1755.2004.66005.x.
Negulescu O, Bognar I, Lei J, Devarajan P, Silbiger S, Neugarten J. Estradiol reverses TGF-beta1-induced mesangial cell apoptosis by a casein kinase 2-dependent mechanism. Kidney Int. 2002;62(6):1989–98. https://doi.org/10.1046/j.1523-1755.2002.00679.x.
Kattah AG, Smith CY, Gazzuola Rocca L, Grossardt BR, Garovic VD, Rocca WA. CKD in patients with bilateral oophorectomy. Clin J Am Soc Nephrol. 2018;13(11):1649–58. https://doi.org/10.2215/CJN.03990318.
Silbiger S, Neugarten J. Gender and human chronic renal disease. Gend Med. 2008;5(Suppl A):S3–S10. https://doi.org/10.1016/j.genm.2008.03.002.
Hoy WE, Rees M, Kile E, Mathews JD, McCredie DA, Pugsley DJ, et al. Low birthweight and renal disease in Australian aborigines. Lancet. 1998;352(9143):1826–7. https://doi.org/10.1016/s0140-6736(05)79888-3.
Nagai M, Ohkubo T, Murakami Y, Takashima N, Kadota A, Miyagawa N, et al. Secular trends of the impact of overweight and obesity on hypertension in Japan, 1980–2010. Hypertens Res. 2015;38(11):790. https://doi.org/10.1038/hr.2015.81.
Lawlor DA, Owen CG, Davies AA, Whincup PH, Ebrahim S, Cook DG, et al. Sex differences in the association between birth weight and total cholesterol. A meta-analysis. Ann Epidemiol. 2006;16(1):19–25. https://doi.org/10.1016/j.annepidem.2005.04.006.
Franco MC, Christofalo DM, Sawaya AL, Ajzen SA, Sesso R. Effects of low birth weight in 8- to 13-year-old children: implications in endothelial function and uric acid levels. Hypertension. 2006;48(1):45–50. https://doi.org/10.1161/01.HYP.0000223446.49596.3a.
Feig DI, Nakagawa T, Karumanchi SA, Oliver WJ, Kang DH, Finch J, et al. Hypothesis: uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney Int. 2004;66(1):281–7. https://doi.org/10.1111/j.1523-1755.2004.00729.x.
Funding
This work was supported by the Daiwa Securities Health Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have declared that no conflict of interest exists.
Ethical approval
The study protocol was approved by the review committee of Keio University (approval number 18-001) and was conducted in accordance with the Declaration of Helsinki.
Informed consent
General informed consent was obtained according to the local ethical committee guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Murai-Takeda, A., Kanda, T., Azegami, T. et al. Low birth weight is associated with decline in renal function in Japanese male and female adolescents. Clin Exp Nephrol 23, 1364–1372 (2019). https://doi.org/10.1007/s10157-019-01784-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-019-01784-9