Abstract
Background
Pediatric as well as adult patients with chronic kidney disease (CKD) are susceptible to cardiovascular disease (CVD) events, which increase their mortality. Dyslipidemia is thought to be one of the most important contributing risk factors for developing CVD. This study aimed to evaluate the prevalence of dyslipidemia and assess clinical and laboratory risk factors associated with dyslipidemia in East Asian pediatric patients with CKD.
Methods
From April 2011 to April 2016, 469 patients with CKD aged < 20 years were enrolled in KNOW-PedCKD (the KoreaN cohort study for Outcomes in patients With Pediatric Chronic Kidney Disease); 356 patients were included in the final analysis. Using the baseline data of the cohort cross-sectionally, a multivariable logistic regression analysis was performed to assess the risk factors for dyslipidemia; a subanalysis for each lipid abnormality was also done.
Results
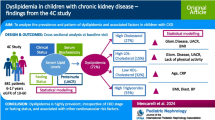
The prevalence of dyslipidemia was 61.5% (n = 219). For dyslipidemia, nephrotic range proteinuria and 25-hydroxyvitamin D deficiency significantly increased the adjusted odds ratio. In the subanalysis, glomerulonephropathy as the origin of CKD and nephrotic range proteinuria significantly increased the risks for high total cholesterol and high low-density lipoprotein cholesterol. Overweight or obese body mass index z-score, elevated proteinuria, hypocalcemia, and 1,25-dihydroxyvitamin D deficiency were significantly associated with low high-density lipoprotein cholesterol. Glomerular filtration rate stage 3b or higher and hyperphosphatemia significantly increased the risk for high triglycerides.
Conclusions
Long-term data accumulation and prospective analysis are needed to clarify the relationship between CKD progression and dyslipidemia and to find additional risk factors for dyslipidemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric as well as adult patients with chronic kidney disease (CKD) are susceptible to cardiovascular disease (CVD) events, which increase their mortality [1,2,3,4].
According to several studies, atherosclerosis can also begin in adolescents with normal kidney function in childhood. This atherosclerosis process can be accelerated in cases of nephrotic syndrome, proteinuria, and CKD due to abnormal lipid metabolism and other atherosclerosis risk factors [5]. Therefore, dyslipidemia is thought to be one of the most important contributing risk factors for developing CVD, and the prevalence of dyslipidemia in pediatric patients with CKD is known to be as high as 39–65% [6,7,8].
Although there have been many previous studies on dyslipidemia in adult patients with CKD, studies on pediatric patients with CKD are relatively scarce. For example, in the USA, some studies based on the Chronic Kidney Disease in Children (CKiD) cohort reported the prevalence of dyslipidemia and its associated factors; however, to our knowledge, there are few similar studies in East Asia thus far [8, 9].
The aims of this study were to evaluate the prevalence of dyslipidemia and to assess the clinical and laboratory risk factors associated with dyslipidemia in East Asian pediatric patients with CKD using the baseline results from KNOW-PedCKD (the KoreaN cohort study for Outcomes in patients With Pediatric Chronic Kidney Disease) [10].
Patients and methods
Study population
From April 2011 to April 2016, a total of 469 patients < 20 years of age with CKD were enrolled in KNOW-PedCKD. This cohort is from a nationwide, 10-year prospective and observational study conducted at seven major pediatric nephrology centers in Korea. It is included at ClinicalTrials.gov with the designation number NCT02165878 (submitted on June 11, 2014). The detailed study protocol of KNOW-PedCKD was previously reported [10]. This study was approved by the human subject institutional review boards at each participating center. Written informed consent was obtained from the patients or their parents or guardians if the patient was < 18 years of age. Of the 469 patients initially recruited, 356 were included in the final analysis. The authors excluded 11 patients who were either in violation of the inclusion criteria or who transferred to a hospital not participating in this study. Another three groups were then excluded: 21 patients aged 19–20 years whose glomerular filtration rate (GFR) prediction formula was quite different from that of pediatric patients, 59 patients whose baseline lipid profile was insufficient to diagnose dyslipidemia, and another 22 patients who were receiving lipid-lowering therapy that might have the potential to distort the results.
Data collection
The authors used the baseline data from the time the patients were enrolled in the cohort. Clinical data included the patient’s age, sex, height, weight, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), comorbidities (defined as heart failure, arrhythmia, urinary tract infection, diabetes, retinopathy, sensorineural hearing loss, developmental delay, and metabolic syndrome), type and duration of primary renal disease, left ventricular mass index (LVMI) measured by 2-dimensional echocardiography according to the American Society of Echocardiography pediatric guidelines [11], and laboratory values (total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), hemoglobin, parathyroid hormone (PTH), serum albumin, serum total calcium, serum phosphate, serum potassium, spot urine protein to creatinine ratio (Up/c), serum creatinine, serum 25-hydroxyvitamin D, serum 1,25-dihydroxyvitamin D, serum uric acid, and random plasma glucose). CKD was defined and classified according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria, and the estimated glomerular filtration rate (eGFR) was calculated by using the bedside Chronic Kidney Disease in Children study (CKiD) formula [12, 13].
In this study, dyslipidemia was defined as the presence of one or more of the following: TC ≥ 200 mg/dL, LDL-C ≥ 130 mg/dL, HDL-C < 40 mg/dL, and TG ≥100 mg/dL for ages 0–9 years and ≥ 130 mg/dL for ages 10–18 years. These cutoff values were according to both US and Korean studies on dyslipidemia in children and adolescents [14,15,16,17]. The BMI z-score was calculated and classified as follows: within normal range (< 1.04), overweight (1.04–1.65, corresponding to 85–95 percentiles), or obese (≥ 1.65, corresponding to the highest 5 percentiles) according to Korean national growth charts [18]. Left ventricular hypertrophy (LVH) was defined as LVMI ≥ 38 g/m2.7 [19]. Proteinuria was classified according to the Up/c as follows: normal to minimal (< 0.5), elevated (0.5–1.99), or nephrotic (≥ 2.0) [20]. Hypertension was defined as SBP or DBP ≥ the 95th percentile with respect to age, sex, and height [21]. Patients were classified as having anemia if their hemoglobin was < 5th percentile of hemoglobin distribution stratified by age and sex [22, 23]. Hypoalbuminemia and hyperkalemia were defined as serum albumin < 3.8 g/dL and serum potassium > 5.2 mmol/L, respectively [24]. Serum PTH was considered elevated if it was > 70, > 110, or > 300 pg/mL when the GFR was ≥ 30, 15–29, or < 15 mg/mL/1.73 m2, respectively [25]. Hypocalcemia was defined as serum total calcium < 8.8 mg/dL [25]. Hyperphosphatemia was defined as serum phosphate > 6 mg/dL for children < 13 years of age and > 5.5 mg/dL for children aged 13–18 years [25]. Deficiency in 25-hydroxyvitamin D was defined as a serum level < 20 ng/mL [26]. Deficiency in 1,25-dihydroxyvitamin D was defined as a serum level < 18 pg/mL [27]. Hyperuricemia was defined as serum uric acid > 6 mg/dL for girls of all ages and for boys ≤ 15 years, and > 7 mg/dL for boys > 15 years of age [28].
Statistical analysis
In descriptive statistics, mean ± standard deviation or median with interquartile range (IQR) was presented for continuous variables and frequency with percentages was presented for categorical variables. For performing multivariable logistic regression analysis, several clinical variables suspected to be risk factors for the prevalence of dyslipidemia were determined by backward elimination and used to generate an adjusted odds ratio (AOR) and its 95% confidence interval (CI). Not only for the composite dyslipidemia outcome but for each lipid abnormality (TC ≥ 200 mg/dL, LDL-C ≥ 130 mg/dL, HDL-C < 40 mg/dL, and TG ≥ 100 mg/dL for ages 0–9 years and ≥ 130 mg/dL for ages 10–18 years), four additional subanalyses were performed by logistic regression. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using the R software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria)
Results
Baseline characteristics of the study subjects
Clinical characteristics and laboratory values at the time of enrollment are described in Table 1. Median age was 10.8 years (IQR 5.3, 14.6), with males accounting for about 69% of the total 356 patients. Patients with a GFR between 30 and 60 mg/mL/1.73 m2 (stage 3) and < 30 (stage 4 to 5) accounted for 32.6% (n = 116) and 21.1% (n = 75) of the total population, respectively. The origin of CKD was glomerulonephropathy (GN) in 25.3% of patients (n = 90). The median duration of primary renal disease was 4.5 years (IQR 1.7, 8.7), and 54.8% of patients (n = 195) had comorbid disease. Of 351 patients with Up/c data, 15.7% of the patients (n = 55) had values in the nephrotic range, 30.5% (n = 107) had values in the elevated range, and 53.8% (n = 189) had values in the normal to minimal range. Most patients (87.9%) had a normal BMI z-score, but 7.0% (n = 25) were overweight (z-score ≥ 1.0 corresponding to the 85–95 percentiles), and 5.1% (n = 18) were obese (z-score ≥ 1.65 corresponding to the highest 5 percentiles). The prevalence of dyslipidemia was 61.5% (n = 219), and abnormalities in each lipid measurement were as follows: 25.0% (n = 89), 19.0% (n = 65), 15.2% (n = 54), and 15.2% (n = 54) of the patients showed high TC (≥ 200 mg/dL), high LDL-C (≥ 130 mg/dL), low HDL-C (< 40 mg/dL), and high TG (≥ 100 or ≥ 130 mg/dL for ages < 10 or ≥10 years), respectively.
Assessing risk factors for the prevalence of dyslipidemia and for each of the lipid abnormalities
The AOR with 95% CI of the selected risk factors for each outcome is described in Table 2. For the prevalence of dyslipidemia, nephrotic range Up/c and 25-hydroxyvitamin D deficiency were shown to have significantly increased AOR of 3.29 (95% CI 1.32–8.22) and 1.81 (95% CI 1.08–3.06), respectively.
In the case of high TC (≥ 200 mg/dL), GN compared with other origins of CKD, such as reflux nephropathy and anatomical lesion, showed significantly increased AOR of 2.24 (95% CI 1.15–4.36). Also, notably, nephrotic range Up/c was quite significantly associated with the outcome (AOR = 6.83, 95% CI 2.87–16.28, p value < 0.001).
For the risk factors of high LDL-C (≥ 130 mg/dL), it was found that they were very similar to those of high TC: GN origin of CKD and nephrotic range Up/c significantly contributed to the outcome, with AORs of 2.40 (95% CI 1.12–5.15) and 4.98 (95% CI 1.80–13.77), respectively.
Some different risk factors were shown to significantly increase the outcome of low HDL-C (< 40 mg/dL) with AORs as follows: overweight BMI z-score 4.24 (95% CI 1.38–13.01), obese BMI z-score 8.06 (2.32–27.97), hypocalcemia 2.95 (1.26–6.91), and 1,25-dihydroxyvitamin D deficiency 2.50 (1.20–5.20). For low HDL-C, elevated Up/c showed a significant relationship (AOR = 2.40, 95% CI 1.05–5.48) but nephrotic Up/c did not.
Unlike the other outcomes, high TG (≥ 100 mg/dL for ages 0–9 years, ≥ 130 mg/dL for ages 10–18 years) was shown to be significantly associated with higher GFR stage: AOR was calculated as 2.32 (95% CI 1.11–4.83) for stage 3b and 2.60 (1.23–5.49) for stage 4 to 5 as compared with reference (stage 1 to 2). In addition, hyperphosphatemia significantly increased the outcome as much as 3.41 times (95% CI 1.16–10.02).
Discussion
In CKD patients, dyslipidemia is known to be due to metabolic changes in postprandial lipoproteins and other triglyceride-rich lipoproteins, alterations in reverse cholesterol transport and lipoprotein structure, and increased levels of lipoprotein(a). It has been reported that CKD patients with a GFR of stage 3 or higher show hypertriglyceridemia, low HDL-C levels, and variable levels of LDL-C and TC, thus resulting in a relatively high prevalence (39–65%) of dyslipidemia compared with the general population. In this study, the prevalence of dyslipidemia in the KNOW-PedCKD population was 61.5%, which is in good agreement with previous findings. In our subanalysis of each of the lipid abnormalities, the groups with GFR stage 3b or higher showed significantly increased TG, but no significant association with the other three lipid abnormalities [2, 6, 7, 29,30,31].
In patients with nephrotic syndrome (NS), it is known that increased albuminuria is associated with dyslipidemia independent of changes in the GFR. It is suggested that impaired clearance and altered biosynthesis of serum lipid and lipoprotein can cause dyslipidemia: decreased activity of lipoprotein lipase, decreased expression of the LDL receptor, and structural and functional changes of the HDL particles can cause marked hypercholesterolemia, hypertriglyceridemia, and low-to-normal HDL-C in patients with NS [2, 32]. In this study, although not shown in the Results section, there were 14 patients with nephrotic syndrome, all of whom had GN origin of CKD and dyslipidemia. Similar to the previous studies of NS, the patients with GN origin of CKD showed significantly increased TC and LDL-C compared with results in patients with reflux/anatomical/other origins of CKD, and the nephrotic range proteinuria was significantly associated with increased TC, LDL-C, and TG [2, 32].
Pediatric and adult patients with dyslipidemia are known to have increased aortic intima–media thickness and early atherosclerosis. In addition, fatty streaks in adolescence can cause CVD problems during early adult life, even if patients have normal renal function [6, 33]. Consequently, it is expected that pediatric patients with CKD who have a high prevalence of dyslipidemia will have an increased risk of atherosclerosis (CVD) in their early adulthood as well as during adolescence. Therefore, it is important for researchers to focus on dyslipidemia and its associated risk factors in pediatric patients with CKD.
Recently, it was reported in The Third National Health and Nutrition Examination Survey (NHANES III) cohort that vitamin D deficiency was associated with an increased prevalence of proteinuria, high prevalence of CVD, and mortality. Other studies also reported that vitamin D deficiency was associated with traditional CVD risk factors such as metabolic syndrome, hypertension, insulin resistance, diabetes, and dyslipidemia. Also, vitamin D deficiency was thought to be associated with the higher risk of CVD seen in patients with CKD compared with individuals without CKD [34, 35]. In accordance with previous studies, results of this study showed that 25-hydroxyvitamin D deficiency and 1,25-dihydroxyvitamin D deficiency were significantly associated with increased dyslipidemia and decreased HDL-C, respectively. In addition, hypocalcemia and hyperphosphatemia were shown to be related to decreased HDL-C and increased TG levels, respectively. Although not directly related to the development of dyslipidemia, there have been some reports from large cohort studies that hypocalcemia and hyperphosphatemia increase the CVD-related mortality in dialysis patients. Therefore, further study on the association between these risk factor abnormalities and dyslipidemia in the course of developing CVD is needed [36, 37].
There were some limitations to this study. First, this was a cross-sectional study using baseline data, although it was originally designed as a 10-year prospective cohort. Thus, it was not possible to analyze outcome in relation to changes in major risk factors, such as GFR decline over time. Second, because fasting blood glucose sampling protocols were not clearly specified, random plasma glucose sampling was used instead, and this might affect the TG and TC levels. However, several recent studies of the effect of fasting on evaluation of lipid profiles reported that there is no significant difference in lipid profiles between the fasting and nonfasting states [8]. Third, in addition to the nonlinear variability by age and sex of the lipid measurements during childhood, the criteria for dyslipidemia in children and adolescents in Korea are not clearly established according to age and sex. Therefore, the authors used the dyslipidemia criteria of children and adolescents in the USA instead, which may be different from the actual situation in Korea. Also, the possibilities of confounding effects by age and sex still remain, although these were adjusted for in the multivariate analysis.
On the other hand, the study had some strengths. So, far as we know, this is the first and the largest multicenter study of dyslipidemia in pediatric patients with CKD in Korea, based on the KNOW-PedCKD cohort in which the participants will be followed up for 10 years. It was thought to be meaningful to construct baseline information on the cohort before conducting the full-length cohort study in the future. Furthermore, it is known that the common cause of CKD in pediatric patients is primary renal disease, and that the prevalence of coexisting medical conditions is lower than that in adult patients with CKD. Therefore, the dyslipidemic effect of CKD is delineated more clearly in pediatric patients than in adult patients [8].
In conclusion, long-term data accumulation and prospective analysis are needed to clarify the relationship between CKD progression and dyslipidemia and to find additional risk factors for dyslipidemia that were not identified in the present study. Further study on the use of medications known to affect dyslipidemia, such as steroids and cyclosporin, is also needed.
References
American Academy of Pediatrics (2007) Cardiovascular risk reduction in high-risk pediatric populations. Pediatrics 119:618–621
Ferro CJ, Mark PB, Kanbay M, Sarafidis P, Heine GH, Rossignol P, Massy ZA, Mallamaci F, Valdivielso JM, Malyszko J, Verhaar MC, Ekart R, Vanholder R, London G, Ortiz A, Zoccali C (2018) Lipid management in patients with chronic kidney disease. Nat Rev Nephrol 14:727–749
Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, Van De Kar NJ, Wolff ED, Davin JC, Heymans HSA (2002) Mortality and causes of death of end-stage renal disease in children: a Dutch cohort study. Kidney Int 61:621–629
Parekh RS, Carroll CE, Wolfe RA, Port FK (2002) Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr 141:191–197
Wanner C, Tonelli M, Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members (2014) KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int 85:1303–1309
Khurana M, Silverstein DM (2015) Etiology and management of dyslipidemia in children with chronic kidney disease and end-stage renal disease. Pediatr Nephrol 30:2073–2084
Mikolasevic I, Žutelija M, Mavrinac V, Orlic L (2017) Dyslipidemia in patients with chronic kidney disease: etiology and management. Int J Nephrol Renov Dis 10:35–45
Saland JM, Pierce CB, Mitsnefes MM, Flynn JT, Goebel J, Kupferman JC, Warady BA, Furth SL (2010) Dyslipidemia in children with chronic kidney disease. Kidney Int 78:1154–1163
Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, Wong C, Mak RH, Moxey-Mims M, Warady BA (2011) Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol 6:2132–2140
Kang HG, Choi HJ, Han KH, Kim SH, Cho HY, Cho MH, Shin JI, Lee JH, Lee J, Oh KH, Park YS, Cheong HI, Ahn C, Ha I-S (2016) KNOW-PedCKD (KoreaN cohort study for outcomes in patients with pediatric CKD): design and methods. BMC Nephrol 17:35
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 23:465–495
International Society of Nephrology (2013) Chapter 1: definition and classification of CKD. Kidney Int Suppl 3:19–62
Fadrowski JJ, Neu AM, Schwartz GJ, Furth SL (2011) Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol 6:1427–1435
Lim JS (2013) The current state of dyslipidemia in Korean children and adolescents and its management in clinical practice. Ann Pediatr Endocrinol Metab 18:1–8
Yang S, Hwang JS, Park HK, Lee HS, Kim HS, Kim EY, Lim JS (2012) Serum lipid concentrations, prevalence of dyslipidemia, and percentage eligible for pharmacological treatment of Korean children and adolescents; data from the Korea National Health and Nutrition Examination Survey IV (2007–2009). PLoS One. https://doi.org/10.1371/journal.pone.0049253
Yoon JM (2014) Dyslipidemia in children and adolescents: when and how to diagnose and treat? Pediatr Gastroenterol Hepatol Nutr 17:85–92
Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute (2011) Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 128 Suppl 5:S213–S256.
Kim JH, Yun S, Hwang S, Shim JO, Chae HW, Lee YJ, Lee JH, Kim SC, Lim D, Yang SW, Oh K, Moon JS (2018) The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr 61:135–149
De Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH (1992) Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 20:1251–1260
Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, Mitsnefes M, Kaskel F, Greenbaum LA, Mak RH, Flynn J, Moxey-Mims MM, Furth S (2015) Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the chronic kidney disease in children (CKiD) cohort. Am J Kidney Dis 65:878–888
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Kidney disease: improving global outcomes (KDIGO) anemia work group (2012) KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2:279–335
Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C (2005) Hematological and iron-related analytes--reference data for persons aged 1 year and over: United States, 1988-94. Vital Health Stat 11:1–156
Kopple JD (2001) National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis 37:S66–S70. https://doi.org/10.1053/ajkd.2001.20748
Langman CB, Salusky IB (2005) K/DOQI clinical practice guidelines for bone metabolism and disease in children with chronic kidney disease. Am J Kidney Dis 46(Suppl 1):S6–S121
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Pagana KD, Pagana TJ, Pagana TN (2019) Mosby’s diagnostic & laboratory test reference, 14th edn. Elsevier, St. Louis
Fathallah-Shaykh SA, Cramer MT (2014) Uric acid and the kidney. Pediatr Nephrol 29:999–1008
Kaysen GA (2006) Dyslipidemia in chronic kidney disease: causes and consequences. Kidney Int 70:S55–S58
Vaziri ND (2006) Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Ren Physiol 290:F262–F272
Saland JM, Ginsberg H, Fisher EA (2002) Dyslipidemia in pediatric renal disease: epidemiology, pathophysiology, and management. Curr Opin Pediatr 14:197–204
Agrawal S, Zaritsky JJ, Fornoni A, Smoyer WE (2018) Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol 14:57–70
Brady TM, Schneider MF, Flynn JT, Cox C, Samuels J, Saland J, White CT, Furth S, Warady BA, Mitsnefes M (2012) Carotid intima-media thickness in children with CKD: results from the CKiD study. Clin J Am Soc Nephrol 7:1930–1937
Ganji V, Zhang X, Shaikh N, Tangpricha V (2011) Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001-2006. Am J Clin Nutr 94:225–233
Kandula P, Dobre M, Schold JD, Schreiber MJ, Mehrotra R, Navaneethan SD (2011) Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol 6:50–62
Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, Kronenberg F, Marcelli D, Passlick-Deetjen J, Schernthaner G, Fouqueray B, Wheeler DC (2011) Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 26:1948–1955
Naves-Díaz M, Passlick-Deetjen J, Guinsburg A, Marelli C, Fernández-Martín JL, Rodríguez-Puyol D, Cannata-Andía JB (2011) Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant 26:1938–1947
Funding
KNOW-PedCKD (trial registration: NCT02165878-ClinicalTrials.gov) was funded by grants 2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, and 2016E3300201 awarded by the Research of Korea Centers for Disease Control and Prevention after peer review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the institutional review boards of the participating centers, namely Jeju University Hospital (Jeju, South Korea), Pusan National University Children’s Hospital (Yangsan, South Korea), Severance Children’s Hospital (Seoul, South Korea), Kyungpook National University Children’s Hospital (Daegu, South Korea), Seoul National University Children’s Hospital (Seoul, South Korea), Samsung Medical Center (Seoul, South Korea), and Asan Medical Center (Seoul, South Korea), Seoul National University Bundang Hospital (Seongnam, South Korea), and Hallym University Kangnam Sacred Heart Hospital (Seoul, South Korea). Children with CKD aged < 18 years were enrolled in this study after informed consent had been obtained from their parents or guardians.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baek, H.S., Kim, S.H., Kang, H.G. et al. Dyslipidemia in pediatric CKD patients: results from KNOW-PedCKD (KoreaN cohort study for Outcomes in patients With Pediatric CKD). Pediatr Nephrol 35, 1455–1461 (2020). https://doi.org/10.1007/s00467-020-04545-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04545-z