Abstract
Background
Peritoneal dialysis (PD) is the preferred chronic dialysis modality amongst pediatric patients. Peritonitis is a devastating complication of PD. Adult data demonstrates early onset peritonitis (EP) is associated with higher rates of subsequent peritonitis and technique failure. Limited data exists regarding EP in the pediatric population, here defined as peritonitis occurring within 60 days of catheter insertion.
Methods
PD catheter insertion practices and EP episodes were examined from the Standardizing Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) collaborative database.
Results
There were 98 episodes of EP amongst 1106 PD catheters inserted. Multivariable analysis demonstrated a significant association between early use of the PD catheter and EP (P = 0.001). Age less than 1 year at the time of catheter insertion (P < 0.001), first catheter placed (P < 0.001) for the patient, use of a plastic adapter (P = 0.003), placement of sutures at the exit site (ES) (P = 0.032), and dressing change prior to 7 days post-operatively (P < 0.001) were all significantly associated with early PD catheter use. Concurrent placement of a hemodialysis catheter was associated with a decreased risk for early PD catheter use (P = 0.010).
Conclusions
In this large cohort of pediatric PD recipients, 8.4% of PD catheters were associated with the development of EP. The finding of an association between early use of the PD catheter and EP represents a potentially modifiable risk factor to reduce infection rates within this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peritoneal dialysis (PD) is the most frequently utilized renal replacement modality amongst children requiring chronic dialysis [1]. Peritonitis is a devastating complication of PD, the most severe outcomes of which include technique failure resulting in permanent transition to hemodialysis (HD) and death [2, 3].
The adult literature has demonstrated that early onset peritonitis (EP) (variably defined as occurring between 3 and 24 months post-PD initiation) is associated with higher rates of subsequent peritonitis episodes and technique failure as compared to late-onset peritonitis [4, 5]. A paucity of data exists evaluating EP, here defined as peritonitis occurring within 60 days of catheter insertion, within the pediatric population. Given the impact identified in adults, EP in pediatrics warrants exploration to identify risk factors, especially potentially modifiable ones, which could influence the development of peritonitis.
The SCOPE collaborative is a multicenter quality improvement initiative in the USA aimed at reducing peritonitis rates amongst the pediatric chronic dialysis population through the use of standardized care bundles for catheter insertion, training, and follow-up care [6]. This study aims to evaluate the application of the insertion care bundle in SCOPE participating dialysis centers to identify risk factors associated with EP.
Design and methods
Cohort definition
The SCOPE collaborative design, catheter care bundle characteristics, and impact of the quality improvement activities on peritonitis rates have been previously described [6]. All PD catheters inserted at or after enrollment during the study period defined as October 1, 2011, through November 30, 2017, were included in this analysis. Multiple catheter insertions in a single patient during the study period were counted as separate events. PD catheters placed for treatment of acute kidney injury were excluded; chronic PD catheters placed prior to the launch of the Collaborative were also excluded from this analysis. Relapsing episodes of peritonitis were not counted as separate episodes, as per the International Society for Peritoneal Dialysis (ISPD) [7]. PD catheter insertion bundle compliance (Table 1) and peritonitis episodes were prospectively collected. The Declaration of Helsinki was followed and the collaborative protocol was approved by the Institutional Review Board at each participating center. Informed consent was obtained where required by the institution’s Institutional Review Board.
Data collection
Patient demographics were collected at the time of enrollment. Catheter characteristics, provider insertion bundle compliance, and peritonitis episodes were prospectively collected for all PD catheter placements during the collaborative. While patients enrolled in the collaborative may have been followed for a longer period of time, this analysis was restricted to the first 60-day post-PD catheter insertion, regardless of whether PD was actually initiated.
Peritonitis events were reported by each center. Centers were encouraged to use the definition of peritonitis endorsed by the ISPD, i.e., an effluent white blood cell count greater than 100/mm3 with at least 50% polymorphonuclear leukocytes, with or without the presence of a positive culture [7].
Statistical analysis
Patient demographics and clinical characteristics at insertion were summarized using frequencies for categorical variable and medians with interquartile range (IQR) for continuous variables. We compared demographic and clinical characteristics between (1) catheters with versus without EP and (2) catheters in which dialysis was initiated early (within 14 days of insertion) versus catheters for which dialysis was initiated more than 14 days after insertion using a chi-square test for association.
To assess risk factors associated with EP and early PD catheter use, we used a multivariable generalized linear mixed model (GLMM) approach assuming an underlying binomial distribution and a logit link function. To account for correlation of insertion and care practices of catheters seen at the same center, we fit a PD-center-specific random effect. The risks of EP and early PD catheter use were summarized using adjusted odds ratios (OR) with 95% confidence intervals (CI). Only covariates demonstrating statistical significance in a univariate analysis were included in the GLMM adjustment. Covariates included in the GLMM assessing risks of EP and early PD catheter use can be found in Tables 2 and 4, respectively.
All analyses were performed using SAS (v. 9.4; SAS Institute, Inc., Cary, NC). P values less than 0.05 were considered statistically significant.
Results
Patient characteristics
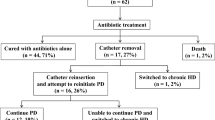
There were 1106 unique catheter insertions, 93 of which were associated with a total of 98 episodes of EP within the study period. Eighty-eight catheters were associated with 1 episode of EP, 5 catheters were associated with 2 episodes, none of which were relapsing. Of the 98 episodes of EP, 34.7% were culture-negative, 28.6% gram-positive, and 19.4% gram-negative in origin. Table 2 compares patient and catheter characteristics of those with and without EP. In the univariate analysis, sex, race, underlying etiology of ESRD, and catheter placement technique (open vs. laparoscopic) were not significantly different between the two groups. However, age at the time of catheter insertion, dressing change prior to 7-day post-catheter insertion, and early use of the PD catheter were all significantly associated with EP.
Risk factors for early peritonitis
Results of the multivariable GLMM assessing factors associated with EP demonstrated that, of all the aforementioned variables, only early use of the PD catheter was significantly associated with EP (OR 1.9; 95% CI 1.2 to 3.1; P = 0.001) (Table 3).
Timeline to peritonitis
The timeline in days to the first episode of EP was not significantly different between PD catheters used early (within 14 days of insertion) as compared with those used after 14 days of insertion. Median time to the first episode of EP was 23.5 days (IQR [10, 35]) for early use PD catheters versus 25 days (IQR [14, 39]) for catheters used after 14 days of insertion (P = 0.423).
Early PD catheter use
Given the strong association between early PD catheter use and EP, a secondary analysis was conducted to further evaluate early catheter use (Table 4). Of the 1065 catheters inserted for which initiation of catheter use was reported, 345 (32.4%) were used for dialysis within 14 days of insertion. Similar to the development of EP, age at the time of catheter insertion was significantly associated with early catheter use in the unadjusted analysis. Additionally, primary diagnosis, catheter orientation, number of catheter cuffs, tunnel configuration, adaptor type, sutures placed at the ES, and dressing change prior to 7-day post-catheter insertion were also significantly associated with early use of the PD catheter.
Multivariable analysis (Table 5) demonstrated that age < 1 year at the time of PD catheter insertion was a significant risk factor for early use (OR 2.5; 95% CI 1.7 to 3.6; P < 0.001). Primary diagnosis of polycystic kidney disease (OR 2.5; 95% CI 1.3 to 4.9; P = 0.009), first catheter placed (OR 2.2; 95% CI 1.5 to 3.2; P < 0.001), plastic adapter (OR 1.7; 95% CI 1.2 to 2.4; P = 0.003), sutures placed at the ES (OR 1.8; 95% CI 1.0 to 2.9; P = 0.032), and dressing change prior to 7 days post-operatively (OR 2.0; 95% CI 1.5 to 2.8; P < 0.001) were all significantly associated with early PD catheter use. Single cuff and upward orientation both approached, but did not meet statistical significance. Placement of a hemodialysis catheter at the time of PD catheter insertion was associated with a decreased risk for early PD catheter use (OR 0.5; 95% CI 0.3 to 0.8; P = 0.010).
Discussion
In this large cohort of pediatric, chronic PD patients, 8.4% of catheters were associated with the development of EP. Adult data has identified a variety of patient-specific risk factors associated with EP including male gender, higher BMI, hypoalbuminemia, and a preceding ES infection [8,9,10]. In our study, the multivariable model revealed that the occurrence of EP was significantly higher in those children in whom the PD catheter was used for dialysis earlier than the current International Society for Peritoneal Dialysis (ISPD) recommended wait time of 14 days following insertion [7]. This finding is noteworthy as it demonstrates a potentially modifiable risk factor to mitigate the development of EP.
It is equally important to discuss known risk factors for peritonitis which did not demonstrate a significant association with EP. Age less than 1 year was associated with an increased risk of EP in the unadjusted analysis; however, once adjusted for other covariates including early PD catheter use, the association between age and EP no longer existed, despite the fact that peritonitis rates are known to be highest in this age group [11]. This is not to negate the association between young age and the development of peritonitis on the whole. Rather, it highlights the finding that in terms of the critical window within which EP is defined, only early use of the catheter confers a significant risk.
Noteworthy is the recognition that most prior published analyses regarding peritonitis in children have defined their study periods based on dialysis initiation and the follow-up of patients over a prolonged period of time. Our study is unique in that the window of exposure began at the time of catheter placement, extending 60 days. This therefore allows for the inclusion of the critical window between insertion and dialysis initiation, irrespective of when the latter occurs. As a result, the association between known risk factors for peritonitis, such as young patient age, in this study may be slightly different than the association between age and risk of peritonitis noted elsewhere. The same rationale can be applied to other known risk factors for peritonitis, such as upward orientation of the catheter, which did not demonstrate an association with EP.
Prior publications examining the outcome of early PD catheter use in pediatrics are limited. Patel et al. [12] found that acute PD starts (within 48 h of insertion) were associated with a greater frequency of dialysate leakage, while infections were more frequently found in the comparative delayed (mean, 20 days ± 10 days) start group. That study was limited by a small sample size of 33 patients, as well as a broad definition of “delayed” start (range 5–44 days). The adult literature is difficult to generalize to pediatrics given inherent differences in age and associated comorbidities, in addition to conflicting results between studies. Povlsen et al. [13] found that acute PD catheter use (< 24 h following insertion) was associated with an increase in mechanical complications and therefore surgical intervention, as compared with catheters associated with a conventional start (> 12 days following insertion), within a 3-month study period. However, no significant difference in infectious complications was noted. Contradicting this study was the investigation by Pai et al. [14], in which no difference in mechanical complications or infection rates were found when comparing catheters associated with acute (< 14 days post-insertion) and conventional (> 14 days post-insertion) start times, respectively. Our study is unique in that it clearly demonstrates an infectious risk associated with early PD catheter use in a large pediatric cohort.
Our data demonstrated that early use of the catheter was also inversely associated with concurrent placement of a HD catheter, the latter procedure in effect decreasing the risk of early catheter use. It is interesting to note that concurrent placement of a HD catheter was not found to be significantly associated with a decreased risk of peritonitis within 60 days of catheter placement. This analysis is limited by the relatively small number of concurrent HD and PD catheter placements (N = 112). Although SCOPE has more recently expanded to collect data on children maintained on hemodialysis, the dataset available for the current analysis does not identify patients who had an HD catheter placed prior to PD placement. Thus, this compromises our ability to evaluate the impact of HD catheter placement on the early use of a PD catheter, and/or the development of EP.
Early PD catheter use was subsequently found to be significantly associated with age less than 1 year at the time of insertion, use of a plastic adapter, and sutures placed at the exit site. Recognizing that despite the association between early PD catheter use and EP, clinical situations arise where a 14-day wait period following PD catheter insertion is not possible. It is therefore prudent to make every effort to follow best practices in these settings. Such practices include placement of a double-cuffed catheter, lateral- or downward-oriented catheter ES without sutures at the ES, and the use of a titanium rather than plastic adapter and/or gastrostomy tube (G-tube) insertion prior to or at the time of PD catheter placement when feasible [15], to avoid compounded risks for peritonitis.
The current ISPD recommendation of a wait period of 14 days following PD catheter insertion is a clinical recommendation meant to allow time for the catheter ES to form a seal and inhibit dialysate leakage. It is reasonable to assume that in some instances, a PD catheter ES may reach this state prior to 14 days in which case “early use” could be considered appropriate. Alternatively, should an ES take considerably longer than 14 days to form an adequate seal, initiation of PD at 15 days post-catheter placement would be considered compliant with the SCOPE catheter insertion bundle and the ISPD recommendations however may not be clinically appropriate, potentially leading to catheter leak and subsequent peritonitis. Moving forward, the collaborative will collect the exact date PD was initiated, the date the ES was visualized and deemed healed, and information regarding dialysate leakage within 30-day post-PD catheter insertion. This will ultimately allow for a more informed recommendation on the preferred timing of PD initiation relative to catheter insertion.
The results of this study should be considered in light of several limitations. Firstly, the enrollment of patients at each participating SCOPE center is voluntary; therefore, selection bias may exist. However, SCOPE participants are highly motivated towards infection prevention and therefore it is presumed that most patients at each center are enrolled. Secondly, although centers were encouraged to follow ISPD guidelines in terms of the definition of peritonitis, there may be institutional variability in the evaluation and diagnosis of an infection event. Finally, due to the relatively low number of EP episodes and small sample size, our study may not be sufficiently powered to detect other meaningful associations between provider practices and EP episodes. For example, although we considered a sub-analysis of peritonitis episodes within 30 days of catheter insertion, as these episodes are more likely most reflective of surgical/intra-operative practices, we found that there were an insufficient number of episodes within this time frame for meaningful analysis.
In summary, this study demonstrates a significant association between early use of the PD catheter for dialysis and the development of peritonitis within 60 days of catheter insertion, thereby further supporting the current ISPD recommendation regarding avoidance of PD catheter use for 14 days post-insertion [7]. The fact that these data identify a provider-driven risk factor for peritonitis highlights a potentially modifiable practice to reduce infection rates amongst the chronic pediatric dialysis population.
References
Alexander SR, Warady BA (2004) The demographics of dialysis in children. In: Warady BA, Schaefer FS, Fine RN, Alexander SR (eds) Pediatric dialysis. Kluwer, Dordrecht, pp 35–46
Chadha V, Schaefer FS, Warady BA (2010) Dialysis-associated peritonitis in children. Pediatr Nephrol 25:425–440
The North American Pediatric Renal Trials and Collaborative Studies. NAPRTCS 2011 annual dialysis report (2011)
Feng S, Wang Y, Qiu B, Wang Z, Jiang L, Zhan Z, Jiang S, Shen H (2016) Impact of early-onset peritonitis on mortality and technique survival in peritoneal dialysis patients. Springerplus. 5(1):167
Harel Z, Wald R, Bell C, Bargman JM (2006) Outcome of patients who develop early-onset peritonitis. Adv Perit Dial 22:46–49
Neu AM, Miller MR, Stuart J, Lawlor J, Richardson T, Martz K, Rosenberg C, Newland J, McAfee N, Begin B, Warady BA, Collaborative Participants SCOPE (2014) Design of the standardizing care to improve outcomes in pediatric end stage renal disease collaborative. Pediatr Nephrol 29:1477–1484
Warady BA, Bakkaloglu S, Newland J, Cantwell M, Verrina E, Neu A, ChadhaV YHK, Schaefer F (2012) Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int 32(Suppl 2):S32–S86
See EJ, Johnson DW, Hawley CM, Pascoe EM, Darssan D, Clayton PA, Borlace M, Badve SV, Sud K, Boudville NC, Cho Y (2017) Early peritonitis and its outcome in incident peritoneal dialysis patients. Perit Dial Int
Tian Y, Xie X, Xiang S, Yang X, Lin J, Zhang X, Shou Z, Chen J (2017) Risk factors and outcomes of early-onset peritonitis in chinese peritoneal dialysis patients. Kidney Blood Press Res 42(6):1266–1276
Wu H, Huang R, Yi C, Wu J, Guo Q, Zhou Q, Yu X, Yang X (2016) Risk factors for early-onset peritonitis in southern Chinese peritoneal dialysis patients. Perit Dial Int 36(6):640–646
Sethna CB, Bryant K, Munshi R, Warady BA, Richardson T, Lawlor J, Newland JG, Neu A (2016) SCOPE investigators: risk factors for and outcomes of catheter-associated peritonitis in children: the SCOPE collaborative. Clin J Am Soc Nephrol 11(9):1590–1596
Patel UD, Mottes TA, Flynn JT (2001) Delayed compared with immediate use of peritoneal catheter in pediatric peritoneal dialysis. Adv Perit Dial 17:253–259
Povlsen JV, Ivarsen P (2006) How to start the late referred ESRD patient urgently on chronic APD. Nephrol Dial Transplant 21(Suppl 2):ii56–ii59
Pai MF, Yang JY, Chen HY, Hsu SP, Chiu YL, Wu HY, Tsai WC, Peng YS (2016) Comparing long-term outcomes between early and delayed initiation of peritoneal dialysis following catheter implantation. Ren Fail 38(6):875–881
Zaritsky JJ, Hanevold C, Quigley R, Richardson T, Wong C, Ehrlich J, Lawlor J, Rodean J, Neu A, Warady BA (2017) Epidemiology of peritonitis following maintenance peritoneal dialysis catheter placement during infancy: a report of the SCOPE collaborative. Pediatr Nephrol 33(4):713–722
Acknowledgements
Participating Centers Contributing Data to the SCOPE Collaborative:
Akron Children’s Hospital, OH
American Family Children’s Hospital, WI
Ann & Robert H. Lurie Children’s Hospital of Chicago, IL
Arkansas Children’s Hospital, AR
Arnold Palmer Hospital for Children, FL
Boston Children’s Hospital, MA
Children’s Health, Dallas, TX
Children’s of Alabama, AL
Children’s Hospital Colorado, CO
Children’s Hospital Los Angeles, CA
Children’s Hospital of Wisconsin, WI
Children’s Mercy Kansas City, MO
Children’s National Health System, DC
Cincinnati Children’s Hospital Medical Center, OH
Cleveland Clinic Children’s, OH
Cohen Children’s Medical Center, NY
Connecticut Children’s Medical Center, CT
Cook Children’s Medical Center, TX
Dell Children’s Medical Center of Central Texas, TX
Doernbecher Children’s Hospital at Oregon Health & Science University, OR
Driscoll Children’s Hospital, TX
Golisano Children’s Hospital at the University of Rochester Medical Center, NY
Johns Hopkins Children’s Center, MD
Levine Children’s Hospital, NC
Lucile Packard Children’s Hospital at Stanford, CA
Mattel Children’s Hospital UCLA, CA
MUSC Children’s Hospital, SC
Nationwide Children’s Hospital, OH
Nemours/Alfred I. duPont Hospital for Children, DE
Nicklaus Children’s Hospital, FL
Phoenix Children’s Hospital, AZ
Primary Children’s Hospital, UT
Seattle Children’s Hospital, WA
SSM Cardinal Glennon Children’s Medical Center, MO
St. Louis Children’s Hospital, MO
Texas Children’s Hospital, TX
The Children’s Hospital at Montefiore, NY
The Children’s Hospital at OU Medical Center, OK
The Children’s Hospital of Philadelphia, PA
The Mount Sinai Kravis Children’s Hospital, NY
UCSF Benioff Children’s Hospital San Francisco, CA
University of California, Davis, CA
University of Iowa Stead Family Children’s Hospital, IA
University of Minnesota Masonic Children’s Hospital, MN
UPMC Children’s Hospital of Pittsburgh
Yale-New Haven Children’s Hospital, CT
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The Declaration of Helsinki was followed and the collaborative protocol was approved by the Institutional Review Board at each participating center. Informed consent was obtained where required by the institution’s Institutional Review Board.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keswani, M., Redpath Mahon, A.C., Richardson, T. et al. Risk factors for early onset peritonitis: the SCOPE collaborative. Pediatr Nephrol 34, 1387–1394 (2019). https://doi.org/10.1007/s00467-019-04248-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04248-0