Abstract
Background
Peritonitis is the leading cause of peritoneal dialysis (PD) discontinuation. However, few data concern risk factors of peritonitis development and catheter removal caused by treatment failure in pediatric patients.
Methods
This single-center, retrospective study analyzed data from pediatric patients who underwent chronic PD between March 2002 and June 2022. The incidence rates of peritonitis by the person-year method were calculated, and they were stratified by patient age groups. Risk factors for peritonitis development and catheter removal were also analyzed by multivariate analysis using logistic regression model.
Results
Ninety patients were enrolled, and 62 peritonitis episodes were observed in 41 (46%) patients. The incidence rate of peritonitis was 0.21 episodes per patient-year, which was the highest in children aged under 2 years old (0.26 episodes per patient-year). Moreover, 44 (71%) cases were successfully cured by antibiotics alone, although 17 (27%) cases required catheter removal, and 4 (6%) cases transitioned to chronic hemodialysis because of peritoneal dysfunction. One patient died. The risk factor for peritonitis development and catheter removal caused by treatment failure was PD insertion at under 2 years old (odds ratio = 2.5; P = 0.04) and Pseudomonas aeruginosa (odds ratio = 11.0; P = 0.04) in the multivariate analysis. P. aeruginosa was also a risk factor for difficulty in re-initiating PD (P = 0.004).
Conclusions
The incidence rate of peritonitis was the highest in children under 2 years old. P. aeruginosa peritonitis is a risk factor for catheter removal and peritoneal dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peritoneal dialysis (PD) is the most common kidney replacement therapy in children with end-stage kidney disease. In pediatric patients, PD allows for home dialysis, gradual water and solute removal, and less food restriction, compared with hemodialysis (HD). However, PD-associated peritonitis is a serious complication. Sometimes, it might lead to catheter removal, peritoneal dysfunction, transition to HD, and death [1,2,3]. If PD re-initiation is difficult, they must switch to chronic HD. However, HD is a difficult modality, especially for infants. Thus, in pediatric patients, peritonitis must be prevented until kidney transplantation.
According to international guidelines, the incidence rate of peritonitis should be less than 0.40 episodes per patient-year [1]. Recently, with appropriate preventive measures, its incidence rate has decreased [4, 5]. Appropriate catheter placement, exit-site care, and PD training program for clean operation are important in preventing peritonitis. In previous reports, risk factors for peritonitis include young age, intraperitoneal insert other than PD catheter, and early PD initiation [6,7,8,9,10,11]. Antibiotic therapy is the first-line treatment, and catheters must be removed if antibiotics are ineffective. If the therapeutic effect is poor, PD could not be re-initiated because of peritoneal adhesions.
Fungi are a risk factor for PD discontinuation [1, 8, 12, 13]. The ISPD guidelines recommend catheter removal as an initial treatment for fungal peritonitis, and the catheter removal rate in fungal peritonitis is high [1]. However, few studies have focused on risk factors for PD discontinuation, other than fungal peritonitis. Moreover, risk factors for the incapability to re-initiate PD caused by peritoneal adhesion are not clearly known.
Therefore, this study aimed to investigate the incidence rate of peritonitis and determine the risk factors for peritonitis development in pediatric patients with PD in our institute. Moreover, we evaluated risk factors for treatment failure defined as catheter removal.
Materials and methods
Patients
This single-center, retrospective, observational study enrolled pediatric patients who received PD at our hospital between March 2002 and June 2022. The exclusion criteria were age over 20 years at the time of PD initiation and not on automatic PD machines such as infants in the neonatal intensive care unit. In most cases, the PD catheter was straight and double cuffed, and the downward exit was made downward on the left side.
Definitions
Peritonitis was diagnosed when at least two of the following factors were present: (1) clinical features consistent with peritonitis, that is, abdominal pain and/or cloudy dialysis effluent; (2) dialysis effluent white cell count > 100/µL with > 50% polymorphonuclear leukocytes; and (3) positive dialysis effluent culture (bacteria or fungi). Its definition is similar to those of the ISPD guidelines [1].
Treatment of peritonitis
If peritonitis was suspected based on elevated dialysis effluent white cell counts or cloudy dialysis effluent, empirical antibiotics covering both gram-positive and gram-negative bacteria were administered intraperitoneally or intravenously. As soon as the results of the dialysate culture were known, the antibiotics were changed based on sensitivity. In cases without clinical improvement within 5 days on appropriate antibiotics (persistent fever with elevated dialysis effluent white cell count and positive dialysate culture), the catheter was removed [1].
If patients required dialysis during the catheter removal period, they received transient HD. The catheter was reinserted after the peritonitis subsided (negative dialysate culture), usually at 4 weeks after removal. When injection and drainage of the dialysate were difficult because of peritoneal adhesion, patients transitioned to chronic HD, and the PD catheter was removed.
Study design
During the study period, the characteristics of all patients on PD from the medical charts were investigated (i.e., sex, primary disease, and age at PD initiation), and these were compared between the peritonitis group and the non-peritonitis group. For the peritonitis group, we investigated age at peritonitis onset, period from PD initiation to onset, cause of peritonitis, white blood test and C-reactive protein in the blood test at diagnosis, white cell counts in ascites at diagnosis, causative pathogens, treatment, and outcomes. Data collected during the observation period of all patients with PD were analyzed, and the incidence rates of peritonitis were calculated by the person-year method. The incidence rates were compared between the three age groups (< 2, 2–6, and > 6 years). Children under 2 years old have immature immune systems, and a previous study reported that the incidence rate was the highest in children under 2 years old [8]. We adopted 6 years old, which is the age at the start of school.
Risk factors for peritonitis development were also analyzed. Extrarenal complications (respiratory, circulatory, neurological, and gastrointestinal diseases), intraperitoneal devices other than PD catheter (gastrostomy, colostomy, vesicocutaneous fistula, etc.), and age under 2 years old at PD introduction were adjusted as risk factors. The prognosis of all patients and risk factors for catheter removal were reviewed. Age at peritonitis onset, exit-site/tunnel infection, early PD initiation (PD initiation < 14 days after PD catheter insertion), white cell count in the dialysis effluent at diagnosis, and Pseudomonas aeruginosa were adjusted as risk factors. The incapability to re-initiate PD was also analyzed.
Statistical analyses
All statistical analyses were performed using JMP Pro 16 (SAS Institute Japan, Tokyo, Japan). Values were expressed as medians with interquartile range for continuous variables and as a percentage for categorical variables. The Mann–Whitney U test and Fisher exact test were used for the comparison of continuous and categorical variables, respectively. Multivariate analysis of risk factors for developing peritonitis and catheter removal was performed using logistic regression analysis. A two-sided P-value of < 0.05 was considered to indicate statistical significance.
Ethics
This study was conducted according to the principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects of the Ministry of Health, Labor, and Welfare, Japan, and was approved by the institutional ethics committee of the National Center for Child Health and Development (Approval no. 2022–169). Informed consent was waived for the retrospective study.
Results
Characteristics of patients and peritonitis
Of the 90 PD patients, 41 patients experienced 62 episodes of peritonitis. Table 1 shows the clinical characteristics of the patients. During the study period, the median age at PD initiation was 0.5 [0.1–5.9] years in the peritonitis group and 3.2 [0.4–9.7] years in the non-peritonitis group (P = 0.03).
Table 2 shows the clinical characteristics of 62 episodes of peritonitis. The incidence of peritonitis was 0.21 episodes per patient-year. The most common cause of peritonitis was exit-site/tunnel infection (15%), followed by surgical procedures (catheter insertion, replacement, or reposition; 9%) and exit-site leakage (6%). Two episodes were caused by surgical procedures and leaks, and one episode was caused by exit-site infection and surgical procedures.
Incidence rates of peritonitis stratified by patient age
The incidence rates stratified by patient age are shown in Table 3. It was the highest in children under 2 years of old (0.26 per patient-year).
Pathogen of peritonitis
Gram-positive bacteria were noted in 30 (48%) cases, of which Staphylococcus aureus was the most common (n = 13, 21%), followed by Enterococcus spp. (n = 6, 10%; E. faecalis, n = 4; E. faecium, n = 1; E. raffinosus, n = 1 case). Gram-negative bacteria were found in 22 (36%) cases, of which Klebsiella spp. (n = 7, 11%: K. pneumoniae, n = 4; K. oxytoca, n = 3) and P. aeruginosa (n = 7, 11%) were the most common. Fungi were found in 2 (3%) cases, non-tuberculous mycobacteria in 1 (2%), and culture negative in 10 (16%). Multiple bacteria were detected in 3 cases (one with E. faecium and Clostridium beijerinckii, one with S. lentus and E. faecalis, and one with S. aureus and Serratia marcescens).
Clinical course and outcomes of peritonitis
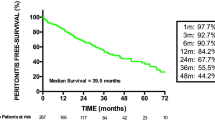
Figure 1 shows the clinical courses of all peritonitis cases. All patients were treated with antibiotics immediately after diagnosis. Forty-four cases (71%) were cured with antibiotics alone. Seventeen episodes (27%) required catheter removal because of treatment failure (Table 4). After the inflammation subsided, 16 patients (26%) received reinsertion of the catheter, except for one patient who continued HD because of scheduled kidney transplantation. Although PD could be re-initiated in 12 patients (19%), re-initiation was not possible in 4 patients (6%) because of poor drainage by abdominal adherence, small abdominal cavity or high peritoneal permeability, and transitioned to chronic HD. One patient (2%) died of sepsis. No patients suffered from encapsulating peritoneal sclerosis.
Risk factors for peritonitis
Multivariate analysis using logistic regression showed that PD initiation at age under 2 years old was a significant risk factor for peritonitis development (odds ratio [OR] = 2.5; 95% confidence interval [CI] = 1.1–5.9; P = 0.04) (Table 5).
Risk factors for catheter removal
Multivariate analysis using logistic regression showed that P. aeruginosa (OR = 11.0; 95% CI = 1.0–124.9; P = 0.04) was a significant risk factor for catheter removal (Table 6).
Outcomes of peritonitis caused by P. aeruginosa
Table 7 shows the comparison of outcomes between peritonitis caused by P. aeruginosa and that other than P. aeruginosa. Of the seven episodes of P. aeruginosa peritonitis, 6 (86%) did not respond to antibiotics and required catheter removal. After the inflammation subsided, PD re-initiation was tried in all these six patients, although dialysis was not possible in three because of peritoneal adhesions and transition to HD. The bivariate analysis showed that P. aeruginosa was a risk factor for the difficulty in re-initiating PD (P = 0.004).
Discussion
This single-center, retrospective, observational study investigated the incidence of PD-associated peritonitis, risk factors for peritonitis development, and risk factors for poor prognosis in patients undergoing PD. Of the 90 patients, 41 (46%) had 62 episodes of peritonitis, with an incidence of 0.21 episodes per patient-year. The younger the PD initiation age, the higher the incidence of peritonitis. Moreover, 27% of all episodes required catheter removal, 6% resulted in transition to chronic HD, and 2% of the patients died. One patient who died had congenital nephrotic syndrome combined with WEST syndrome and chronic lung disease. He developed peritonitis at the age of 8.7 years. The causative agent was unknown, and he died of sepsis on the 23rd day after the onset of peritonitis. Multivariate analysis identified age under 2 years old at PD initiation as a significant risk factor for peritonitis development. In addition. P. aeruginosa was a significant risk factor for PD catheter removal and difficulty in PD re-initiation after catheter removal.
The peritonitis guidelines by ISPD recommend that the incidence of peritonitis should be less than 0.40 episodes per patient-year [1]. In the present study, the incidence of peritonitis was lower in our cohort than in those reported in the SCOPE study and studies conducted in China, Brazil, and Saudi Arabia, with 0.30, 0.29, 0.43, and 0.6 episodes per patient-year, respectively [1, 5, 14, 15]. A previous study in Japan also reported a lower value (0.17 episodes per patient-year) [16]. In our center, standardized instruction in exit-site care and dialysis machine operation for caregivers may have contributed to the low incidence of peritonitis.
The incidence of peritonitis is higher in younger patients [4, 7, 8]. In this study, a similar result was obtained. Possible reasons include immature immunity, fragile skin and tendency to leak easily, high failure risk in clean operation because of the inability to keep rested, use of a single-cuff catheter, and difficulty in securing the distance to the exit site. The prevention of peritonitis through exit care, training in hygiene techniques, response to unclean operations, etc., is important in young patients.
The risk factors for peritonitis development include young age, short stature, PD initiation under 2 years old, intraperitoneal devices such as gastrostomy and colostomy, and early PD initiation after PD catheter insertion [4,5,6,7, 9,10,11,12, 17, 18]. Induction of PD under 2 years old was also a risk factor in this study, but the younger the age of induction, the longer the duration of PD treatment and naturally the higher the risk of developing peritonitis. In our study cohort, patients with heart disease, lung disease, neurological disease, and gastrointestinal disease were included, although the rates of peritonitis and its prognosis were similar between these groups. There were no patients with primary immunodeficiency, such as asplenia. Whether the way of antibiotics, such as intravenously or intraperitoneal, affects the prognosis is difficult to evaluate. In patients under 2 years of age, 4 were administered intravenously, 7 intraperitoneally, 3 both (intravenous administration was changed to intraperitoneal administration due to persistently positive culture), and 3 unknown (medical records are outdated and cannot be evaluated). There was no poor prognosis for intraperitoneal administration, and poor prognosis for intravenous administration (catheter removal in 3 cases, and difficulty in restarting PD in 1 case). However, in two cases of intravenous administration, the catheter was immediately removed due to fungal infection, making evaluation difficult.
In this study, P. aeruginosa was a risk factor not only for catheter removal but also for difficulty in re-initiating PD. As kidney replacement therapy for children, HD is technically difficult, especially for infants, because arteriovenous shunt cannot be created for them. Infants require vascular catheter placement and long-term hospitalization for HD management. As the difficulty in re-initiating PD is the most critical problem for children, strategy for P. aeruginosa peritonitis is crucial for childhood PD management. No study has examined the proportion of poor prognosis cases of P. aeruginosa peritonitis in pediatric patients with PD. In adult cases with P. aeruginosa peritonitis, catheter removal rates of 26%–44%, difficulty resuming PD rates of 11%–35%, and mortality rates of 3%–12% were reported [19, 20], [21]. In this study, the rates of both catheter removal and difficulty resuming PD were higher than those in previous studies, which may be because our study analyzed pediatric patients, and they may have immature immunologic defense. The poor prognosis of P. aeruginosa peritonitis was speculated to be due to biofilm formation, which increases antimicrobial resistance and makes it physically difficult for antimicrobials to penetrate [1], [21]. We believe that the poor prognosis of P. aeruginosa peritonitis was not due to the patient factors, as all cases of P. aeruginosa peritonitis in our study were first-peritonitis, not recurrence, and there was no difference in the primary disease or age at the time of peritonitis. ISPD recommends the administration of effective antibiotics with different mechanisms, and if no improvements were observed after 5 days, the catheter must be removed. The protection of peritoneal function is more important than catheter maintenance, and rapid catheter removal should be considered in patients with poor response to antibiotic therapy. In P. aeruginosa peritonitis, early catheter removal has a higher PD resumption rate and a lower mortality rate than delayed catheter removal [20], [22]. Given the poor prognosis of P. aeruginosa peritonitis, treatment with two antibiotics should be started, and if there is little improvement in 5 days, the catheter should be removed immediately.
This study has a few limitations. First, it is a single-center study, and the number of patients is relatively small. Second, some neonatal cases were excluded because they could not be transitioned to automated PD. Third, the protocol of antibiotic selection and time to catheter removal in the case of treatment failure were not restricted. However, all these choices were determined by kidney specialists and infectious disease experts. Fourth, fungal peritonitis, a known risk factor for catheter removal, could not be evaluated because of the small number of cases.
Conclusion
In the present study, we found that the incidence rate of peritonitis was the highest in children aged under 2 years old. P. aeruginosa peritonitis is a risk factor for catheter removal and peritoneal dysfunction. The treatment strategy for P. aeruginosa peritonitis is crucial for the long-term preservation of peritoneal function in childhood PD.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available because permission for their publication was not obtained from the participants or approved by the ethics committee. However, they are available from the corresponding author on reasonable request.
References
Li PK, Chow KM, Cho Y, Fan S, Figueiredo AE, Harris T, Kanjanabuch T, Kim YL, Madero M, Malyszko J, Mehrotra R, Okpechi IG, Perl J, Piraino B, Runnegar N, Teitelbaum I, Wong JK, Yu X, Johnson DW. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;42:110–53. https://doi.org/10.1177/08968608221080586.
Szeto CC, Chow KM. Gram-negative peritonitis – the Achilles heel of peritoneal dialysis? Perit Dial Int. 2007;27(Suppl 2):S267-271.
Brown MC, Simpson K, Kerssens JJ, Mactier RA, Scottish Renal Registry. Peritoneal dialysis-associated peritonitis rates and outcomes in a national cohort are not improving in the post-millennium (2000–2007). Perit Dial Int. 2011;31:639–50. https://doi.org/10.3747/pdi.2010.00185.
Neu AM, Richardson T, De Souza HG, Mahon AR, Keswani M, Zaritsky J, Munshi R, Swartz S, Sethna CB, Somers MJG, Warady BA, SCOPE Collaborative Participants. Continued reduction in peritonitis rates in pediatric dialysis centers: results of the standardizing care to improve outcomes in pediatric end stage renal disease (SCOPE) Collaborative. Pediatr Nephrol. 2021;36:2383–91. https://doi.org/10.1007/s00467-021-04924-0.
Zhai Y, Zhou Q, Fang X, Shen X, Chen J, Zhang J, Miao Q, Guo W, Cao Q, Rao J, Shen Q, Xu H. Reduction in peritonitis rates: 18-year results from the most active pediatric peritoneal dialysis center in China. Pediatr Nephrol. 2021;37:2437–48. https://doi.org/10.1007/s00467-022-05450-3.
Keswani M, Redpath Mahon AC, Richardson T, Rodean J, Couloures O, Martin A, Blaszak RT, Warady BA, Neu A. Risk factors for early onset peritonitis: the SCOPE collaborative. Pediatr Nephrol. 2019;34:1387–94. https://doi.org/10.1007/s00467-019-04248-0.
Chadha V, Schaefer FS, Warady BA. Dialysis-associated peritonitis in children. Pediatr Nephrol. 2010;5:425–40. https://doi.org/10.1007/s00467-008-1113-6.
Sethna CB, Bryant K, Munshi R, Warady BA, Richardson T, Lawlor J, Newland JG, Neu A. Risk factors for and outcomes of catheter-associated peritonitis in children: the SCOPE Collaborative. Clin J Am Soc Nephrol. 2016;11:1590–6. https://doi.org/10.2215/CJN.02540316.
Zaritsky JJ, Hanevold C, Quigley R, Richardson T, Wong C, Ehrlich J, Lawlor J, Rodean J, Neu A, Warady BA, SCOPE Investigators. Epidemiology of peritonitis following maintenance peritoneal dialysis catheter placement during infancy: a report of the SCOPE collaborative. Pediatr Nephrol. 2017;33:713–22. https://doi.org/10.1007/s00467-017-3839-5.
Chan EYH, Borzych-Duzalka D, Alparslan C, Harvey E, Munarriz RL, Runowski D, Vidal E, Coccia PA, Jankauskiene A, Principi I, Serdaroglu E, Szczepanska M, Tse Y, Vazquez A, Weaver DJ, Schaefer F, Warady BA; International Pediatric Peritoneal Dialysis Network. Colostomy in children on chronic peritoneal dialysis. Pediatr Nephrol. 2020;35:119–26. https://doi.org/10.1007/s00467-019-04372-x.
Jellouli M, Ferjani M, Abidi K, Hammi Y, Boutiba I, Naija O, Zarrouk C, Ben Abdallah T, Gargah T. Peritonitis in pediatric patients receiving peritoneal dialysis. Nephrol Ther. 2015;11:558–63. https://doi.org/10.1016/j.nephro.2015.06.005.
Munshi R, Sethna CB, Richardson T, Rodean J, Al-Akash S, Gupta S, Neu AM, Warady BA. Fungal peritonitis in the standardizing care to improve outcomes in pediatric end stage renal disease (SCOPE) Collaborative. Pediatr Nephrol. 2018;33:873–80. https://doi.org/10.1007/s00467-017-3872-4.
Alsuhaibani M, Aldosari E, Rahim KA, Alzabli S, Alshahrani D. Fungal peritonitis in children on peritoneal dialysis at a tertiary care centre. BMC Nephrol. 2020;21:400. https://doi.org/10.1186/s12882-020-02014-1.
Ponce D, de Moraes TP, Pecoits-Filho R, Figueiredo AE, Barretti P. Peritonitis in children on chronic peritoneal dialysis: the experience of a large national pediatric cohort. Blood Purif. 2018;45:118–25. https://doi.org/10.1159/000484344.
AlZabli SM, Alsuhaibani MA, BinThunian MA, Alshahrani DA, Al Anazi A, Varghese S, Rose V, Rahim K. Peritonitis in children on peritoneal dialysis: 12 years of tertiary center experience. Int J Pediatr Adolesc Med. 2021;8:229–35. https://doi.org/10.1016/j.ijpam.2020.09.001.
Hoshii S, Wada N, Honda M, Japanese Study Group of Pediatric Peritoneal Dialysis. A survey of peritonitis and exit-site and/or tunnel infections in Japanese children on PD. Pediatr Nephrol. 2006;21:828–34. https://doi.org/10.1007/s00467-006-0004-y.
Al Mokali K, Al Sannaa Z, Al Mutairi F, Ahmed AE. Factors influencing occurrence of peritonitis in Saudi children on peritoneal dialysis. BMC Pediatr. 2020;20:42. https://doi.org/10.1186/s12887-020-1936-2.
Kempf C, Holle J, Berns S, Henning S, Bufler P, Müller D. Feasibility of percutaneous endoscopic gastrostomy insertion in children receiving peritoneal dialysis. Perit Dial Int. 2022;42:482–8. https://doi.org/10.1177/08968608211057651.
Lu W, Kwan BC, Chow KM, Pang WF, Leung CB, Li PK, Szeto CC. Peritoneal dialysis-related peritonitis caused by Pseudomonas species: insight from a post-millennial case series. PLoS ONE. 2018;13: e0196499. https://doi.org/10.1371/journal.pone.0196499.
Siva B, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW. Pseudomonas peritonitis in Australia: predictors, treatment, and outcomes in 191 cases. Clin J Am Soc Nephrol. 2009;4:957–64. https://doi.org/10.2215/CJN.00010109.
Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–9. https://doi.org/10.1016/s0966-842x(00)01913-2.
Szeto CC, Chow KM, Leung CB, Wong TY, Wu AK, Wang AY, Lui SF, Li PK, et al. Clinical course of peritonitisdue to Pseudomonas species complicating peritoneal dialysis: a review of 104 cases. Kidney Int. 2001;59(6):2309–15. https://doi.org/10.1046/j.1523-1755.2001.00748.x.
Acknowledgements
The authors thank Enago Crimson Interactive Pvt. Ltd. for English proofreading and editing a draft of this manuscript.
Funding
The authors did not receive any external funding for this study.
Author information
Authors and Affiliations
Contributions
All authors were physicians treating the patients in this report. Misaki Akiyama prepared the manuscript and performed data collection and analysis. Kentaro Nishi, Mai Sato, and Masao Ogura edited and reviewed the manuscript. Shuichi Ito revised the manuscript. Koichi Kamei oversaw the work as the corresponding author and revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
Koichi Kamei has received research funding from the Public Foundation of Vaccination Research Center, and the Taiju Life Social Welfare Foundation; donations from Chugai Pharmaceutical Co. Ltd., Teijin Pharma Ltd., Kyowa Kirin Co. Ltd., Shionogi & Co. Ltd., Daiichi Sankyo Co. Ltd., Mitsubishi Tanabe Pharma Co. Ltd, and Otsuka Pharmaceutical Co. Ltd.; and lecture fees from Terumo Co. Ltd., Baxter Ltd., and Zenyaku Kogyo Co., Ltd. Shuichi Ito has received research funding from Zenyaku Kogyo Co., Ltd., and Teijin Pharma Ltd.; and honoraria from Astrazeneca PLC and Alexion Pharmaceuticals Inc.
Ethics approval
This study was approved by the Ethics Committee of the National Center for Child Health and Development (Approval no. 2022–169).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Akiyama, M., Kamei, K., Nishi, K. et al. Frequency and prognosis of peritoneal dialysis-associated peritonitis in children. Clin Exp Nephrol 28, 692–700 (2024). https://doi.org/10.1007/s10157-024-02482-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-024-02482-x