Abstract
Background
Vitamin D deficiency may contribute to osteoporosis in nephrotic syndrome (NS).
Methods
A cross-sectional case–control study was performed to investigate 25 hydroxycholecalciferol [25(OH)D] status in 40 patients with NS in remission and 40 healthy controls. Serum levels of 25(OH)D, calcium, phosphate, alkaline phosphatase (ALP), and intact parathyroid hormone (PTH) were assayed. NS patients were segregated by age at onset, current age, type and duration of NS, months since relapse and current drug therapy.
Results
Levels of 25(OH)D showed a positive correlation with months elapsed since last NS relapse (r s = +0.4, p = 0.012) and were lower in NS patients within 3 months of relapse but similar to that of controls in patients in remission for >3 months [median 14.23 (interquartile range 12.19–17.63) vs. 19.75 (14.04–28.38) ng/ml, respectively; p = 0.039]. There was no correlation of 25(OH)D levels with other disease characteristics or drug therapy. ALP levels were also lowest after relapse (r s = +0.34, p = 0.036). Overall, 25(OH)D levels of <20 ng/ml occurred in 62.5 % of NS patients + controls, and correlated negatively with age (r s = −0.24, p = 0.037) but showed no significant correlation with calcium, phosphate, or PTH levels.
Conclusions
In our patients with NS, vitamin D stores remained low for 3 months after NS relapse but showed an increase with longer remission time to control levels. Vitamin D stores were not influenced by disease characteristics or therapy. Longitudinal studies are required to confirm these findings and evaluate the effect of vitamin D on bones, particularly in frequent relapsers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D is a vital component of bone metabolism and calcium homeostasis, and its deficiency is known to cause rickets, osteomalacia and hypocalcemia. Additionally, its deficiency has been associated with several risks across the human lifespan, such as diabetes, hypertension, inflammatory bowel disease, malignancies, and asthma. Maintenance of adequate levels of vitamin D is recommended, not only to maintain good bone health, but also to provide for its non-osseous functions [1–3].
Nephrotic Syndrome (NS) is a common relapsing childhood kidney disease, and previous studies have shown that bone mineral density is reduced relatively early in this disorder [4–6]. Daily treatment with steroids is the standard initial therapy for this condition [7, 8], but steroids are known to cause osteoporosis by inhibiting osteoblasts and increasing bone resorption [9–12].
Low levels of 25-hydroxycholecalciferol [25(OH)D] have been documented in NS patients during relapse [13, 14] due to the loss of both 25(OH)D and its binding protein in urine at this time [15–17]. However, since most NS relapses are short-lasting [18, 19], these low levels do not reflect the steady state of body stores. The evidence on vitamin D levels during remission of NS remains mixed [13, 14, 20, 21], but since vitamin D deficiency may contribute to osteoporosis in NS, this issue merits further clarification. Additionally, despite there being differences in vitamin D status in different regions and populations, to our knowledge there are no prior reports of vitamin D levels in children with NS from tropical countries.
We performed a cross-sectional study on patients with NS in remission and on healthy controls to assess: (1) whether NS patients under standard therapy [8] have vitamin D deficiency compared to controls, (2) whether vitamin D status differs according to NS disease characteristics and therapy, and (3) the relationship between vitamin D status and serum calcium, phosphate, alkaline phosphatase (ALP), and parathyroid hormone (PTH) in both groups.
Body stores of vitamin D are reflected by circulating 25(OH)D levels which are stable for 3–4 weeks in the absence of proteinuria. In contrast 1,25-dihydroxycholecalciferol [1,25(OH)2D] has a short half-life and is rapidly influenced by calcium and PTH levels. Therefore, as per convention, we estimated the steady state of vitamin D in the body by measuring 25(OH)D levels [1, 22].
Methods
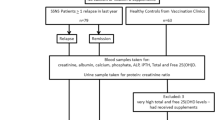
Patients with onset of NS between 1 and 10 years of age who had one or more episodes of NS treated with steroids in the past year were identified during outpatient visits. A medical chart review was performed to ascertain date of diagnosis, dates of relapses, treatment details and calcium and vitamin D supplementation. NS cases were stratified by age at onset, current age, duration of NS, current steroid therapy and current calcium or vitamin D supplementation. “Infrequent relapses” were defined as ≤3 relapses during the last 12 months, “frequent relapses” as ≥4 relapses during the same time, “steroid dependent” as ≥2 relapses while on steroid therapy or within 14 days of stopping steroids, and “steroid resistance” as failure to achieve remission within 4 weeks of treatment with prednisolone 60 mg/m2/day [8]. Patients with first episodes or infrequent relapses were categorized as Type 1 and those with frequent relapses, steroid dependence or steroid resistance as Type 2. Consecutive patients who were in remission for >1 month were included. Patients were required to have had tests that revealed the patient to be negative or trace for protein on dipstix urinalysis and normal for serum creatinine and serum albumin at study entry; those patients who were hospitalized due to any cause or suffering from any other chronic illness were excluded.
A control group matched for age and gender was selected from patients attending the immunization clinic in the same hospital. Patients who had any acute or chronic illness and/or required hospitalization(s) in the past year and those on any chronic therapy, including those on calcium or vitamin D supplements, were excluded since this would have implied that they had some medical complaints. For inclusion in the study, the serum albumin and creatinine levels of the patients in the control group had to be in the normal range.
Parental informed consent was obtained, and the study received ethical approval from the institution’s review board.
Blood samples were collected from both groups for serum albumin, creatinine, calcium, phosphate, ALP, 25(OH)D, and PTH measurements. All samples were obtained between 10 a.m. and 12 noon from June to September 2012 and preserved, if not tested the same day, at −20 °C until analysis.
Low levels of 25(OH)D were defined as “insufficient” if <20 ng/ml and “deficient” if <10 ng/ml, as in previous studies [20, 23]. Standard laboratory normal ranges were used for calcium, phosphate, ALP, and PTH.
Methods of assay
All parameters were estimated on automated platforms in the clinical chemistry laboratory of the Institute of Child Health, Kolkata. Serum creatinine, albumin, calcium, inorganic phosphate and ALP were measured on the Roche Integra 400 Plus chemistry analyzer (Roche Diagnostics, Manheim, Germany). Total circulating 25(OH)D and intact PTH were assayed on the Roche immunoassay platform (Cobas e411; Roche Diagnostics) using electrochemiluminiscence and validated for reproducibility using immunoassay quality control (QC) materials obtained from Bio-Rad (Irvin, CA, USA). Satisfactory coefficent of variation (CV %) data were obtained for 25(OH)D (9.8 %), and PTH (6.1 %) based on calculations on QC data run at significant levels of measurement.
Sample size calculations
Data from the two most recent studies examining 25(OH)D levels in NS remission compared to controls were used to estimate the sample size required. For achieving a two-tailed α of 0.05, the study by Weng et al. [20] indicated a sample size of 31 in each group would be sufficient for 80 % power and 41 for 90 % power. The study by Bykili et al. [21] suggested sample sizes of ten for 80 % power and 13 for 90 % power. Since a power of >80 % is considered satisfactory, we chose a round number of 40 children for inclusion in each group.
Statistical analysis
Normal distributions were summarized as means with 95 % confidence interval and Student’s unpaired t test was used for analyzing inter- and intra-group differences. Discrete data are presented as medians with interquartile range (IQR) and the Mann–Whitney U test was used for inter- and intra-group differences. The difference between categorical data was analyzed using Fisher’s exact test. Spearman’s rank correlation analysis was used to test for correlations (r s) between variables. In addition, multiple linear regression analysis was used within the NS group to cross-check results with 25(OH)D as the dependent variable and current age, duration of disease, time since last relapse, and alternate day prednisolone and vitamin D3 doses as independent variables. All analysis was performed in SPSS ver. 11.0 software (SPSS, Chicago, IL). A p value of <0.05 was considered to be statistically significant.
Results
Baseline characteristics of subjects
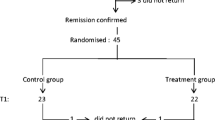
Forty NS patients who fulfilled the inclusion criteria were enrolled in the study. In this patient cohort, the median interval from last relapse was 2.5 (IQR 1–6.25) months, 26 (65 %) were males, median age at onset of NS was 2.55 (IQR 2–3) years, and median age at study entry was 6.25 (IQR 4–10) years. Two children had had their first episode of NS, 19 had infrequent relapses, eight had frequent relapses, 12 were steroid dependent, and three were initially steroid resistant but maintained remission after the addition of other immunosuppression, such as mycophenolate mofetil (MMF) or cyclosporine A (CSA), to their therapeutic regimen. Thus, there were 21 (52.5 %) and 19 (47.5 %) Type 1 and Type 2 patients, respectively. Twenty-one (52.5 %) patients were on alternate-day prednisolone at study entry (mean dose 0.82, 95 % CI 0.58–1.06 mg/kg/alternate day), and 20 (50 %) patients were on other immunosuppressive agents (levamisole: 9, MMF: 6, tacrolimus: 3, CSA: 2). Fifteen patients (37.5 %) were on calcium and vitamin D supplementation (mean daily doses; elemental calcium: 333, 95 % CI 266–401 mg; vitamin D3: 209, 95 % CI 175–243 IU).
Forty healthy controls were selected according to the inclusion criteria, of whom 25 (62.5 %) were males. the mean age of the controls was 5.0 (IQR 3–7.25) years, which was not statistically different from that of the NS group.
Difference between NS group and controls
The levels of 25(OH)D were not statistically different between the NS group and the control group (Table 1). 25(OH)D levels were <10 ng/ml in seven NS and eight control patients and <20 ng/ml in 27 NS and 23 control children (p = 0.49). There was no difference in serum calcium and phosphate levels in the two groups (Table 1).
The serum PTH and ALP levels were significantly lower in the NS group than in the control group (Table 1).
Within the NS group
Within the NS group, there was no significant difference in the levels of 25(OH)D, PTH, calcium, phosphate, or ALP when patients were segregated by sex, type of NS, current steroid treatment, and other immunosuppressive treatment. 25(OH)D levels also did not differ in 15 patients on calcium and vitamin D supplementation compared to those not on supplements [median 20 (IQR 12.9–24.25) vs. 16 (IQR 12.8–19) ng/ml, respectively; p = 0.3].
The levels of 25(OH)D showed a strongly positive correlation with months elapsed since last relapse (r s = +0.4, p = 0.012). Children who had an NS episode within the previous 3 months (n = 22) had a median 25(OH)D level of 14.23 (IQR 12.19–17.63) ng/ml compared to 19.75 (IQR 14.04–28.38) ng/ml in those who were in remission for >3 months (n = 18) (p = 0.039) (Fig. 1). ALP levels also correlated positively with time since last relapse, (r s = +0.34, p = 0.036), being lower in patients within 3 months of relapse (Fig. 2).
There was no significant correlation of 25(OH)D levels with current age, age at onset of NS, duration of NS or drug therapy using Spearman’s rank correlation analysis. These results were cross-checked with a multiple linear regression analysis (r 2 = 0.34, p = 0.013) which also confirmed that time since last relapse was the only independent variable that influenced 25(OH)D levels (β coefficient 0.526, p = 0.006).
Correlations in both groups taken together
The serum levels of 25(OH)D showed a significant negative correlation with patient age (r s = −0.24, p = 0.037) when both NS and control groups were taken together (Fig. 3).
There was no significant correlation between 25(OH)D levels and sex, serum PTH, calcium, or phosphate levels. Of the 15 patients, with 25(OH)D levels of <10 ng/ml, only two patients in the NS group and one patient in the control group had PTH levels above the normal upper laboratory limit of 60 pg/ml, with the highest of these being 86 pg/ml.
Discussion
This is the first study that clarifies vitamin D status in NS remission, and the results demonstrate that 25(OH)D levels correlate only with time since last relapse. 25(OH)D levels were significantly lower in patients within 3 months post-relapse (i.e., those with an NS episode within the previous 3 months), but similar to control values in patients who were in remission for >3 months. To our knowledge this is also the first report of vitamin D stores in NS patients from a tropical country. As such, the results are relevant since the main source of vitamin D is sunlight and exposure, both of which vary from region to region.
Childhood NS is characterized by recurrent proteinuria during relapses. More than 90 % of patients respond to steroids and are maintained in remission with or without alternate-day steroid therapy and steroid-sparing drugs [18, 19]. However, osteoporosis is frequently reported as a complication and may persist to cause long-term morbidity [4, 9, 24]. Treatment with steroids is a likely cause of osteoporosis [9, 25], but vitamin D deficiency may be an added causative factor, and concomitant supplementation with vitamin D and calcium has been shown to reduce (but not abolish) decreases in bone mineral density (BMD) during standard NS therapy [6].
During relapse of NS, vitamin D binding protein (DBP), which has a molecular weight of less than that of albumin, is lost in the urine together with 25(OH)D [15–17]. The serum levels of 25(OH)D correlate negatively with the degree of proteinuria and DBP urinary loss at this time [15]. However, since the majority of pediatric NS patients are in relapse for a very short duration of time, in our study we measured the steady state of 25(OH)D body stores during remission, after resolution of proteinuria for >1 month.
Previous studies estimating 25(OH)D levels in NS remission have reported mixed results. Early studies by Freundlich et al. [13] and Huang et al. [14] showed that low levels of 25(OH)D during relapse normalized quickly post-remission, after the resolution of proteinuria and loss of DBP by urinary leakage. In contrast, Bykilli et al. [21] showed that although 25(OH)D levels rise after remission has been achieved with steroid therapy, they are still lower than those in controls at 3 months. The study by Weng et al. [20] also revealed significantly low levels of 25(OH)D in NS remission compared to controls after a median of 3.5 months from last relapse, but there was no correlation with time interval since last relapse.
Our study shows that 25(OH)D levels in NS remission correlate only with time post-relapse—and not with age at onset, duration of disease, nature of NS, or maintenance alternate-day steroid therapy—suggesting that urinary loss during relapse is the likely cause of low 25(OH)D levels in NS. Our data also show that levels become similar to those of controls after 3 months of remission. At face value, this finding would suggest that there is therefore no need for vitamin D supplementation in steroid-sensitive nephrotic syndrome patients and that supplementation, if given at all, should be considered for the general population since the levels in controls were also lower than what is considered to be optimal [2]. However, our study has several limitations. First, it is cross-sectional study and the data need to be confirmed with longitudinal studies, particularly in patients with frequent relapses, as it is possible that such patients do not have sufficient time for their 25(OH)D levels to rise in between relapses. Unfortunately our study only included eight patients with “frequently relapsing NS”, and this number is too low for separate analysis. Second, we did not include measurements of BMD, and it is possible that low 25(OH)D levels lasting for even a few months post-relapse may contribute to the osteoporosis that has been shown to occur early in steroid-treated NS [4, 5] and respond at least partially to vitamin D therapy [6].
Although calcium supplementation is recommended in NS treatment guidelines for patients on long-term steroids [8], no recommendations exist regarding the administration of vitamin D in this disorder. The degree of supplementation that some of our patients received did not result in any significant benefit in terms of raising 25(OH)D levels, a finding which concurs with those reported in most other studies and thereby indicating that much higher doses (probably >1,000 IU daily) are required to correct the deficiency [1, 2]. However, this notion also warrants further research.
Vitamin D deficiency has been reported to have reached pandemic proportions in many countries, including those with adequate sunshine, which is the main source of the vitamin [1, 26, 27]. Our results also demonstrate that in the combined dataset including both NS and control groups, more than 60 % of children had 25(OH)D levels of <20 ng/ml. Data on adult populations have indicated that optimum levels of 30 ng/ml may be required to provide protection for the diverse osseous and non-osseous conditions for which vitamin D deficiency is a risk factor [2], but pediatric guidelines regarding supplementation based on nationwide data are still to be developed.
The overall negative correlation of 25(OH)D levels with age found in our study has been described previously [20]. The age range of our patients was between 2 and 15 years and, therefore, this effect may be secondary to increasing demand of the growing skeleton to achieve peak bone mass.
Vitamin D deficiency is believed to cause a drop in intestinal calcium absorption and hypocalcemia, leading to PTH secretion, increased conversion of 25(OH)D to 1,25(OH)2D, calcium reabsorption and phosphate loss from renal tubules, and reduction in bone mineralization [1, 28]. However, we and other authors have found no correlation of 25(OH)D levels with serum calcium, phosphate, or PTH [20, 21]. Although 15 children in our study had extremely low 25(OH)D levels of <10 ng/ml, only three had minor increases in serum PTH above the normal laboratory range. Serum calcium and phosphate levels were all normal in our study cohort. Thus, more sensitive markers are required to estimate the true effect of vitamin D deficiency and insufficiency as currently defined and to assess the effect of intervention. Such markers may include the measurement of BMD, bone biopsies, or other non-osseous parameters.
The significance of the lower median levels of PTH and ALP in the NS group (although well within normal laboratory range) compared to the controls is as yet unknown based on data available from our study and may be an effect of small sample size. Low ALP levels in NS has also been documented in other studies, and one hypothesis is that it is due to an inhibitory action of steroids on osteoblasts [21, 25]. This mechanism is supported by the fact that ALP levels correlated with time elapsed since last relapse in our study, being lowest in patients just after relapse and consequently after daily steroid treatment. ALP levels therefore cannot be used to screen for vitamin D deficiency in NS.
In conclusion, this is the first time that a study has shown that vitamin D stores, as measured by serum 25(OH)D, remain low for about 3 months after NS relapse and subsequently increase to control levels with longer duration of remission. We also found that vitamin D stores are not influenced by NS disease characteristics or therapy. Further long-term longitudinal studies are needed to confirm these findings and to assess the effect of vitamin D deficiency and supplementation on bone mineral content, particularly in patients with frequent NS relapses. Vitamin D insufficiency was found to be common in both NS and control patients, and large population-based studies are required to form guidelines for vitamin D management in the general pediatric population.
References
Rathi N, Rathi A (2011) Vitamin D and child health in the 21st century. Indian Pediatr 48:619–625
Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B (2006) Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18–28
Kulie T, Groff A, Redmer J, Hounshell J, Schrager S (2009) Vitamin D: an evidence-based review. J Am Board Fam Med 22:698–706
Feber J, Gaboury I, Ni A, Alos N, Arora S, Bell L, Blydt-Hansen T, Clarson C, Filler G, Hay J, Hebert D, Lentle B, Matzinger M, Midgley J, Moher D, Pinsk M, Rauch F, Rodd C, Shenouda N, Siminoski K, Ward LM, Canadian STOPP Consortium (2012) Skeletal findings in children recently initiating glucocorticoids for the treatment of nephrotic syndrome. Osteoporos Int 23:751–760
Koşan C, Ayar G, Orbak Z (2012) Effects of steroid treatment on bone mineral metabolism in children with glucocorticoid-sensitive nephrotic syndrome. West Indian Med J 61:627–630
Bak M, Serdaroglu E, Guclu R (2006) Prophylactic calcium and vitamin D treatments in steroid-treated children with nephrotic syndrome. Pediatr Nephrol 21:350–354
Lombel RM, Gipson DS, Hodson EM (2012) Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol 28:415–426
Indian Pediatric Nephrology Group, Indian Academy of Pediatrics, Bagga A, Ali U, Banerjee S, Kanitkar M, Phadke KD, Senguttuvan P, Sethi S, Shah M (2008) Management of steroid sensitive nephrotic syndrome: revised guidelines. Indian Pediatr 45:203–214
Gulati S, Godbole M, Singh U, Gulati K, Srivastava A (2003) Are children with idiopathic nephrotic syndrome at risk for metabolic bone disease? Am J Kidney Dis 41:1163–1169
Canalis E (2003) Mechanisms of glucocorticoid-induced osteoporosis. Curr Opin Rheumatol 15:454–457
O’Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS (2004) Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145:1835–1841
Freundlich M, Jofe M, Goodman WG, Salusky IB (2004) Bone histology in steroid-treated children with non-azotemic nephrotic syndrome. Pediatr Nephrol 19:400–407
Freundlich M, Bourgoignie JJ, Zilleruelo G, Abitbol C, Canterbury JM, Strauss J (1986) Calcium and vitamin D metabolism in children with nephrotic syndrome. J Pediatr 108:383–387
Huang JP, Bai KM, Wang BL (1992) Vitamin D and calcium metabolism in children with nephrotic syndrome of normal renal function. Chin Med J (Engl) 105:828–832
Grymonprez A, Proesmans W, Van Dyck M, Jans I, Goos G, Bouillon R (1995) Vitamin D metabolites in childhood nephrotic syndrome. Pediatr Nephrol 9:278–281
Chan YL, Mason RS, Parmentier M, Savdie E, Lissner D, Posen S (1983) Vitamin D metabolism in nephrotic rats. Kidney Int 24:336–341
Barragry JM, France MW, Carter ND, Auton JA, Beer M, Boucher BJ, Cohen RD (1977) Vitamin D metabolism in nephrotic syndrome. Lancet 2:629–632
Greenbaum LA, Benndorf R, Smoyer WE (2012) Childhood nephrotic syndrome—current and future therapies. Nat Rev Nephrol 8:445–458
Sinha A, Bagga A (2012) Nephrotic syndrome. Indian J Pediatr 79:1045–1055
Weng FL, Shults J, Herskovitz RM, Zemel BS, Leonard MB (2005) Vitamin D insufficiency in steroid-sensitive nephrotic syndrome in remission. Pediatr Nephrol 20:56–63
Biyikli NK, Emre S, Sirin A, Bilge I (2004) Biochemical bone markers in nephrotic children. Pediatr Nephrol 19:869–873
Papandreou D, Malindretos P, Karabouta Z (2010) Possible health implications and low vitamin D status during childhood and adolescence: an updated mini review. Int J Endocrinol 2010:472173
Belostotsky V, Mughal MZ, Berry JL, Webb NJ (2008) Vitamin D deficiency in children with renal disease. Arch Dis Child 93:959–962
Hegarty J, Mughal MZ, Adams J, Webb NJ (2005) Reduced bone mineral density in adults treated with high-dose corticosteroids for childhood nephrotic syndrome. Kidney Int 68:2304–2309
Wetzsteon RJ, Shults J, Zemel BS, Gupta PU (2009) Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res 24:503–513
Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J, IOF Committee of Scientific Advisors (CSA) Nutrition Working Group (2009) Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 20:1807–1820
Harinarayan CV, Joshi SR (2009) Vitamin D status in India–its implications and remedial measures. J Assoc Phys India 57:40–48
Lips P (2006) Vitamin D physiology. Prog Biophys Mol Biol 92:4–8
Funding
None.
Competing Interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banerjee, S., Basu, S. & Sengupta, J. Vitamin D in nephrotic syndrome remission: a case–control study. Pediatr Nephrol 28, 1983–1989 (2013). https://doi.org/10.1007/s00467-013-2511-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-013-2511-y